Abstract
The effect of EDTA-masking on the alpha-spectral characteristics corresponding to uranium analysis in ground- and seawater samples after cation-exchange and electrodeposition is systematically investigated. Addition of EDTA (1 mmol l−1) to the investigated samples results in dramatic increase of the spectra quality, including improvement of the separation efficiency up to 75% and 85% for ground and seawaters, respectively. For EDTA concentrations lower than 1 mmol l−1 the spectral resolution and counting efficiencies decline, whereas for higher concentrations the resolution increases but the separation efficiency decreases due to the U(VI) stabilization in solution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Analysis of uranium in ground- and seawaters is of particular interest with respect to uranium monitoring in water resources for drinking purposes [1], environmental issues [2,3,4,5,6], and uranium recovery [7]. Uranium analysis by alpha-spectroscopy after cation-exchange separation using Chelex®100 has been extensively described [8,9,10] and used for the uranium separation from tap-, ground- and seawaters prior analysis by liquid scintillation counting [11] and alpha-spectroscopy [12].
Regarding alpha-spectroscopic analysis the corresponding measurements have shown that in waters with increased concentration of interfering cations (e.g. Ca2+, Fe3+ etc.) the spectral resolution, as well as the counting efficiency and subsequently the detection limits were declined due to the deposition of interfering cations on the stainless steel disk along with the U(VI) cations [1,2,3,4,5,6, 13]. The deposition of interfering cations results in energy loss of the emitted alpha-particles by the deposited matrix materials and alpha-spectra of low resolution/quality, particularly for samples of increased salinity [14]. Usually organic acids (e.g. acetic, malonic, oxalic acid) are added to the studied waters in order to mask the interfering cations and avoid their transfer in the electrolyte solution and finally their deposition on the source disk [13]. In the present study the addition of EDTA in ground- and seawater samples to mask interfering cations (e.g. Ca2+, Fe3+ etc.) has been systematically studied and the optimum conditions were identified.
Experimental
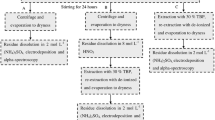
Ground- and seawater samples obtained from a local aquifer and coastal area have been repeatedly (seven times each) analysed with and without EDTA addition and the data obtained have been compared with one another. Prior to any treatment, all water samples were traced with 50 mBq 232U tracer (NPL Laboratories). The uranium separation and pre-concentration were performed by cation-exchange using Chelex®100 as described elsewhere [1,2,3,4,5, 8,9,10]. However, in this study similar samples have been investigated also after adding different amounts of 0.1 M EDTA solution (1, 2, 5, 10 and 20 ml) to 200 ml of the water samples to be analysed. The procedure of the uranium electrodeposition on stainless steel disks was carried out similar to previous studies [1,2,3,4,5].
The alpha-spectroscopic analysis of uranium was performed after pre-concentration and separation of uranium using Chelex®100 resin as described elsewhere [1,2,3,4,5, 8,9,10]. Alpha-spectroscopic measurements were performed by means of an a-spectrometer (Alpha Analyst Integrated Alpha Spectrometer, Canberra) equipped with passivated implanted planar silicon (PIPS) detectors and analysed as described elsewhere [1,2,3,4,5].
Results and discussion
Figure 1a, b present alpha-spectra of uranium extracted from ground- and seawater samples, respectively, which have been obtained by alpha-spectroscopy after separation and pre-concentration of uranium by cation exchange from test solutions of varying EDTA concentrations.
From the spectra it is obvious that increasing EDTA concentrations results in significant improvement of the alpha-spectra quality. The spectral resolution increases dramatically, particularly in the case of the seawater samples, due to the masking effect of EDTA, which strongly complexes the interfering matrix cations (e.g. Ca2+ and Fe3+) and stabilizes them in solution. This results in the preparation of uniform and almost massless sources, because the addition of EDTA to the solution minimizes the effect of interfering ions, present in environmental water samples [13, 15, 16]. The source discs are coated with a very thin layer of radionuclides and with no other material above to attenuate the alpha-radiation [14].
However, above a certain EDTA concentration (1 mmol l−1) the chemical yield is decreasing significantly, because EDTA at relatively high levels (> 1 mmol l−1) competes with the resin binding of U(VI), stabilizes U(VI) in solution and results in lower chemical/separation yields. The corresponding alpha-spectra have been quantitatively evaluated and the related data are graphically summarized in Fig. 2.
According to the data shown in Fig. 2 the optimum concentration regarding the chemical yield is given when the EDTA concentration is 1 mmol l−1 for both ground and seawater samples, which is significantly below the concentration of the interfering ions (e.g. Ca2+) in the respective waters [16]. Nevertheless, EDTA at the given concentration seems to effectively mask the interfering ions, without affecting dramatically the uranium binding by the iminodiacetic resin moieties [9].
Conclusion
Addition of EDTA at concentration levels around 1 mmol l−1 to environmental water samples results in dramatic increase of the alpha-spectra quality and chemical yields up to 75% and 85% for ground and seawaters, respectively.
For EDTA concentrations lower than 1 mmol l−1 the spectral resolution and counting efficiencies decline, whereas for higher concentrations the resolution increases but separation efficiency decreases due to the U(VI) stabilization in solution.
References
Philippou K, Pashalidis I (2017) Uranium analysis in drinking waters in Cyprus. J Radioanal Nucl Chem 312:361–365
Charalambous C, Aletrari M, Piera P, Nicolaidou-Kanari P, Efstathiou M, Pashalidis I (2013) Uranium levels in Cypriot groundwater samples determined by ICP-MS and α-spectroscopy. J Environ Radioact 116:187–192
Ioannidou A, Samaropoulos I, Efstathiou M, Pashalidis I (2011) Uranium in ground water samples of Northern Greece. J Radioanal Nucl Chem 289(2):551–555
Pashalidis I, Tsertos H (2004) Radiometric determination of uranium in natural waters after enrichment and separation by cation-exchange and extraction techniques. J Radioanal Nucl Chem 260:439–442
Philippou K, Pashalidis I (2018) Uranium monitoring in ground- and wastewaters. Desalin Water Treat 112:94–98
Efstathiou M, Aristarchou T, Kiliari T, Demetriou A, Pashalidis I (2014) Seasonal variation, chemical behavior and kinetics of uranium in an unconfined groundwater system. J Radioanal Nucl Chem 299:171–175
Rodríguez R, Avivar J, Ferrer L, Leal OL, Cerdà V (2015) Uranium monitoring tool for rapid analysis of environmental samples based on automated liquid-liquid micro-extraction. Talanta 134:674–680
Kiliari T, Pashalidis I (2010) Simplified alpha-spectroscopic analysis of uranium in natural waters after its separation by cation-exchange. Radiat Meas 45:966–968
Kiliari T, Pashalidis I (2012) Selective separation of actinyl(V, VI) cations from aqueous solutions by Chelex-100. Radiochim Acta 100:439–444
Kiliari T, Pashalidis I (2010) Alpha spectroscopic analysis of actinides (Th, U and Pu) after separation from aqueous solutions by cation-exchange and liquid extraction. J Radioanal Nucl Chem 284:547–551
Antoniou S, Tsiaili A, Pashalidis I (2008) Alpha radiometry of uranium in surface and ground waters by liquid scintillation counting after separation of the radionuclide by cation exchange. Radiat Meas 43:1294–1298
Efstathiou M, Kiliari T, Pashalidis I (2011) Uranium analysis in cypriot groundwaters by total alpha-radiometry and alpha-spectroscopy. Radiat Meas 46:626–630
Puphal KW, Olsen DR (1972) Electrodeposition of alpha-emitting nuclides from a mixed oxalate–chloride electrolyte. Anal Chem 44(2):284–289
Kressin IK (1977) Electrodeposition of plutonium and americium for high resolution α spectrometry. Anal Chem 49(6):842–846
Skoog DA, West DA, Holler FJ, Crouch SR, Analytical chemistry, an introduction, 7th edn. ISBN 0-03-020293-0
Georghiou G, Pashalidis I (2007) Boron in groundwaters of Nicosia (Cyprus) and its treatment by reverse osmosis. Desalination 215:104–110
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Paschalidou, P., Pashalidis, I. Alpha-spectroscopic analysis of uranium in ground- and seawater samples after EDTA-masking of interfering cations. J Radioanal Nucl Chem 321, 973–975 (2019). https://doi.org/10.1007/s10967-019-06639-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-019-06639-x