Abstract
The spotted asparagus beetle, Crioceris duodecimpunctata (L.) is an invasive host-specific pest of asparagus cultivations. To contribute to the understanding of the role of plant volatiles in host-finding by this species, behavioural and electrophysiological tests were carried out. Y-tube olfactometer bioassays, testing intact or mechanically-damaged cladophylls vs. clean air, revealed sexually-dimorphic responses with males being the only sex attracted to both plant materials. Electroantennographic (EAG) assays showed that antennae of both sexes can perceive a wide range of asparagus volatiles. Male and female EAG profiles were almost similar and (Z)-3-hexen-1-ol was by far the most EAG-active compound. (E)-2-hexenal, (±)-linalool, and 3-heptanone elicited the strongest EAG amplitude within the corresponding chemical groups. Eight of the most EAG-active compounds elicited dose-dependent responses indicating the sensitivity of male and female olfactory systems to changes in stimulus concentration. In a Y-tube olfactometer bioassay, (Z)-3-hexen-1-ol at the doses of 1, 10, and 50 μg did not elicit female attraction whereas a significant attraction at the 10 μg dose and a repellent effect at the 50 μg dose was induced in males. Sexual dimorphism of male behavioural response to host plant volatiles is discussed. This study provides a basis for future investigations that could contribute to the development of semiochemical-based monitoring and management strategies for this pest.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Host plant utilization by phytophagous insects depends on coordinated insect-plant interaction (Thorpe et al. 1947; Dethier 1947). Host plant location by phytophagous insects is mediated by numerous sensory inputs, including olfactory and gustatory cues as well as physical and visual information (Visser 1986; Pickett et al. 1998; Bruce et al. 2005; Van den Berg et al. 2008). In the Chrysomelidae family, orientation to host plant cues has been investigated intensively for the Colorado potato beetle, Leptinotarsa decemlineata (Say). Adult L. decemlineata attraction to volatiles emitted by undamaged potato plants was observed about one century ago (McIndoo 1926) and was even the first evidence that host plant odours attract insects (Dickens 2000a). Later on, the olfactory sensitivity of male and female Colorado potato beetle antennal receptors to plant volatiles was carefully investigated by electrophysiological experiments (Visser 1979; Ma and Visser 1978; Weissbecker et al. 1997; Dickens 1999) and adults’ attraction to a five-component blend comprising of (E)-2-hexen-1-ol, (Z)-3-hexen-1-ol, (±)-linalool, nonanal, and methyl salicylate was demonstrated (Dickens 2000b).
The spotted asparagus beetle, Crioceris duodecimpunctata (L.) (Coleoptera, Chrysomelidae), is a major monophagous pest of commercially grown Asparagus officinalis L. in most areas of production (LeSage et al. 2008; Morrison III and Szendrei 2014). Native to the Palearctic region (Drake and Harris 1932), mostly around the Mediterranean Sea (FGP Consortium 2014), C. duodecimpuntata was first detected outside its native range in 1881 in the United States near Baltimore, MD (Chittenden 1917). Subsequently, the pest spread north and westward across Canada and the United States (LeSage et al. 2008) where it can now be found wherever asparagus is commonly grown (Capinera 2001; Morrison III and Szendrei 2014).
C. duodecimpunctata overwinters as an adult and undergoes two generations per year (Pollini 1998; Capinera 2001). In the spring, adults emerge from overwintering shelters such us hollow asparagus stems and other crop debris and feed on asparagus spears. Subsequently, they deposit eggs singly on cladophylls and ramifications of thin asparagus branches. Larvae complete their development feeding on 2-5 asparagus berries (Fink 1913; Dingler 1935). Mature larvae fall off the plant and pupate in the soil. The newly emerged adults repeat this cycle from which overwintering adults will develop.
In the spring, adult feeding on emerging asparagus spears creates small pits in the epidermis (Capinera 2001; Morrison III and Szendrei 2014) resulting in severe direct and aesthetic damage to commercial production. Later in the season, the feeding activity of C. duodecimpunctata adults and larvae on leaves causes reduction of plant photosynthetic capability reducing crop production in the subsequent year (Capinera 2001). Moreover, larval feeding on berries prevents seed formation (Capinera 2001) and therefore could be a problem for breeders and seed producers (Morrison and Szendrei 2014).
The life cycle of C. duodecimpunctata is similar to that of the sympatric Crioceris asparagi (L.), but damage to asparagus spears by C. duodecimpunctata is considered less important due to its later emergence in the season (Morrison III and Szendrei 2014). However, the adult flight periods, as well as the relative abundance of the two species in different areas, remain to be fully defined. For example, in the Foggia Province (Apulia, southern Italy), the most important area of green asparagus cultivation in Italy, only the presence of C. duodecimpunctata was noticed from the end of April to June, corresponding to the majority of the harvesting period, as well as a possible overlap of adult flight periods of the two C. duodecimpunctata generations (personal observations).
In this context, the identification of host-plant compounds to attract C. duodecimpunctata adults could greatly contribute to the development of semiochemical-based monitoring tools useful to improve the timing of control measures and to develop low-impact direct control means such as mass trapping and/or attract and kill methods.
To date, no studies have been carried out to investigate the capability of male and female C. duodecimpunctata to perceive and orient to host-plant odours. To contribute to the knowledge on the role of chemical signals in host location by C. duodecimpunctata adults, in this study Y-tube olfactometer bioassays and electroantennographic (EAG) tests were performed. Experiments were designed to investigate: 1) the behavioural response of males and females to odours emitted by intact or mechanically damaged host plant cladophylls; 2) the antennal capability of both sexes to perceive a range of asparagus volatile organic compounds (VOCs); and, 3) the behavioural response of males and females to the most EAG-active compound (Z)-3-hexen-1-ol.
Methods and Materials
Insects
During April to June 2018, adults of C. duodecimpunctata were collected from infested asparagus plants in privately owned lands near Borgo Mezzanone and Lesina (Foggia, Apulia Region, Italy). Permission to collect insects was obtained from the owners. The field-collected adults were transferred to rearing cages (40 × 40 × 60 cm) and maintained at 25 ± 2 °C, 60 ± 5% relative humidity (r.h.), and L16:D8 photoperiod. Insects were fed with A. officinalis stems and spears placed with the base into a glass beaker (500 mL) containing tap water (300 mL) and replaced every 2 days.
All individuals used in the experiments were actively feeding on plant materials and exhibiting mating activity. A small group of insects was also dissected and their mature reproductive status was confirmed by the presence of well-developed ovaries, ovarioles, and accessory glands (Bean et al. 2007; Gaffke et al. 2020).
Prior to EAG and behavioural tests, adult beetles were kept individually in transparent plastic containers (6 cm i.d. × 8 cm) covered with a fine mesh net (1 mm) without food supply and in the absence of asparagus odours for at least 30 min. Experimental insects were used only once. At the end of each experiment, the specimen was dissected and the sex determined by observing the genitalia with a stereomicroscope (SPZ series, Optika, Ponteranica, Italy).
Plant Materials
A. officinalis (cultivar Sunlim F1) stems (approximately 50 cm long) were collected in the field from the end of April to June and placed with the cut ends into a glass beaker containing tap water. Plant materials were used for behavioural bioassays no later than 24 h after cutting.
Chemicals
Twenty-three VOCs were selected among the most abundant identified from intact or mechanically damaged A. officinalis spears and cladophylls (Sun et al. 2001; Thibout et al. 2005; Morrison III et al. 2016) and to represent different chemical classes (Table 1). Compounds were purchased from Sigma-Aldrich (Milan, Italy). For EAG experiments, all compounds were dissolved in mineral oil (Sigma-Aldrich) and stored at −20 °C until needed.
Olfactometer Bioassays
The behavioural response of C. duodecimpunctata males and females to odours of host plant material was assessed in a glass Y-tube olfactometer (each arm 26 cm long at 75 °C angle, stem 30 cm long, 6.0 cm i.d.) similar to that previously described (Germinara et al. 2011). Each arm of the Y-tube was connected to a glass cylinder (9 cm long and 6.0 cm i.d.; 1 cm high, 2 cm i.d. screw-cup central opening) as an odour source container. The apparatus was put into an observation chamber (80 × 80 × 70 cm) and illuminated from above by two 28-W cool white fluorescent lamps providing uniform lighting (2500 lx) inside the tube. A purified (activated charcoal) and humidified airflow maintained at 60 mL/min by a flowmeter was pumped through each arm.
Two choice tests were conducted: (1) intact cladophylls versus clean air; (2) mechanically damaged cladophylls versus clean air. Intact test material (1.25 g) consisted of cladophylls (4-5 cm long) detached from the stems 30 min before the experiment and placed with the cut end in a glass vial containing water to maintain the physiological water content (Tasin et al. 2005). Mechanically damaged test material (1.25 g) consisted of cladophylls detached from plant stems and cut into pieces (1 cm long) using scissors 30 min before the experiment.
The behavioural response of adult beetles to different concentrations of the strongest antennal stimulant (Z)-3-hexen-l-ol was also tested. In this case, the odour chamber contained a filter paper (Whatman, cat. No 1001–110, Buckinghamshire, UK) disk (2 cm i.d.) (loaded with 1, 10, or 50 μg (10 μL of a 0.1 μg, 1 μg, or 5 μg/μL mineral oil solution, respectively) of (Z)-3-hexen-l-ol while the other chamber contained a similar filter paper disk loaded with 10 μL of mineral oil. A white cotton thread (8 cm long; 0.5 mm i.d) was passed through the centre of a disk using a needle. The disk was then inserted into the odor chamber through the central opening and suspended in the centre of the cross section by properly fixing the cotton thread ends between the screw cap and the central opening of the chamber.
Bioassays were run between 10.00 and 14.00 h at 25 ± 2 °C and 60 ± 5% r.h. Each experiment lasted 10 min. Individual insects, within 3 days of field collection, were released at the open end of the Y-tube stem. A choice was recorded when the insect moved 3 cm up an arm of the Y-tube and remained beyond the decision line (marked on both arms) for more than 30 s. The time spent by test insects in each arm was also recorded. After 3 individuals were tested, the olfactometer was cleaned with distilled water and acetone, dried (200 °C for 30 min) and test and control stimuli renewed. The position of the treatments in the arms were switched to avoid positional bias. For each test stimulus, at least 30 beetles of each sex, used once, were tested. Individuals used in these experiments were from cohorts of 40–50 adults collected every 3-4 days from the end of April to June.
Electroantennography (EAG)
The EAG technique was used to assess the antennal selectively and sensitivity of C. duodecimpunctata males and females to the selected 23 volatile organic compounds (VOCs).
An antenna was excised at the base and mounted between glass electrodes filled with Kaissling’s saline (Kaissling and Thorson 1980). The electrical continuity between the antennal preparation and an IDAC-4 amplifier (Syntech Laboratories, Hilversum, The Netherlands) connected to a PC equipped with the Software EAG Pro (Syntech Laboratories, Hilversum, The Netherlands) was maintained using AgCl-coated silver wires.
Just before EAG experiments, 10 μL of each test solution (100 μg/μL) was adsorbed onto a filter paper strip (1 cm, Whatman No. 1) placed in a Pasteur pipette (15 cm long), which served as an odour cartridge. Vapour stimuli (2.5 cm3) were blown by a disposable syringe for 1 s into a constant stream of charcoal-filtered humidified air (500 mL/min) flowing in a glass delivery tube (i. d. 8 mm) with the outlet positioned at approximately 1 cm from the antenna.
For each sex, antennal selectivity was assessed based on the EAG response to the 1 mg dose (10 μL of a 100 μg/μL mineral oil solution) of each test VOC. To evaluate antennal sensitivity, EAG dose-response curves were calculated on stimulation with 0.01, 0.1, 1, 10, and 100 μg doses (10 μL of 0.001, 0.01, 0.1, 1, 10, 100 μg/μL mineral oil solutions, respectively) of 8 compounds selected among the most EAG-active in different chemical classes.
Control (10 μL mineral oil) and standard (10 μL of 10 μg/μL (Z)-3-hexen-l-ol mineral oil solution) stimuli were applied at the beginning of the experiment and after each group of 6 test stimuli. To allow the recovery of the antennal responsiveness, stimuli were presented at 1-min intervals.
In antennal selectivity experiments, each compound was tested on 6 antennae of different males and females. In antennal sensitivity experiments, test compounds were assessed on 3 antennae of different specimens of each sex.
Data Analyses
The absolute EAG response (mV) to each stimulus was subtracted by the mean response to the two nearest controls (mineral oil) to compensate for solvent and mechanosensory artifacts (Raguso and Light 1998). The subsequent EAG value was corrected according to the reduction of the EAG amplitude to the standard stimulus to compensate for the decrease of the antennal responsiveness during the experiment (Otter et al. 1991).
The corrected male and female EAG responses to the 1 mg dose of each compound were compared to a “0” value using the Wilcoxon rank sum test and considered measurable if significant at P = 0.05. The mean EAG responses of males and females to different test compounds of each chemical group were subjected to analysis of variance (ANOVA) followed by the Tukey’s HSD (Honestly Significant Difference) test (P = 0.05) or to Student’s t test for mean comparison. Prior to these analyses, values were √ x-transformed and tested for homogeneity of variance using Levene’s test. The mean male and female EAG responses to each test stimulus were compared using the Student’s t test (P = 0.05) for independent samples. In EAG dose-response curves, the activation threshold was taken as the first dose at which the mean response was higher than a “0” value using the Shapiro-Wilk test for normality followed by the one-sample Student’s t test (P = 0.05) (Germinara et al. 2017); the saturation level was considered to be the lowest dose at which the mean response was equal to or less than the previous dose (Germinara et al. 2009). Significant differences between the number of beetles choosing the treatment or control arm of the olfactometer were compared using χ2 tests. The differences between the time spent by beetles in each arm were analyzed by paired sample t-tests. Statistical analyses were performed using SPSS (Statistical Package for the Social Sciences) version 10.0.7 for Windows (SPSS Inc., Chicago, IL, USA).
Results
Behavioural Response to Plant Material
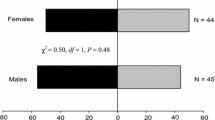
In Y-tube behavioural bioassays, males presented with intact cladophylls vs. clean-air control exhibited a significant preference for the odour stimulus (χ2 = 13.37, df = 1, P < 0.001) and spent significantly (t = 3.97, df = 32, P < 0.001) more time in the treatment arm (Table 2). When males were given a choice between mechanically damaged cladophylls and clean air, there was no preference (χ2 = 2.13, df = 1, P = 0.14) but they spent significantly (t = 3.67, df = 33, P < 0.001) more time in the treatment arm. Odours from intact and mechanically damaged cladophylls did not elicit a significant attraction in female beetles measured either as first choice or as time spent in the treatment arm when clean air was the alternative.
Antennal Selectivity
The mean EAG responses of C. duodecimpunctata males and females to different test stimuli are shown in (Table 1). At the 1 mg dose, all test compounds elicited measurable EAG responses (P < 0.05 in all Wilcoxon rank sum test) ranging from 0.12 ± 0.05 mV for α-pinene to 4.22 ± 0.16 mV for (Z)-3-hexen-1-ol in males, and from 0.06 ± 0.03 mV for hexadecene to 3.52 ± 0.24 mV for (Z)-3-hexen-1-ol in females. Among all compounds tested, the highest EAG responses were evoked by (Z)-3-hexen-1-ol, 1-octen-3-ol, 3-heptanone, (E)-2-hexenal, heptanal, nonanal, hexanal, and (±)-linalool in both sexes. The weakest stimulants were α-pinene and β-pinene in males and hexadecene and p-cymene in females. The mean male EAG response to (Z)-3-hexen-1-ol was significantly (t = 2.39, df = 10, P = 0.038) higher than that of females. For the remaining compounds, male and female EAG responses did not differ significantly (t = 0.03–1.60, df = 10, P = 0.14-0.99).
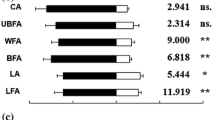
In both sexes, significant differences were found among the EAG responses to test compounds in the chemical groups of aliphatic alcohols (male, t = 4.625, df = 10, P = 0.001; female, t = 6.625, df = 10, P < 0.001), terpenes (male, F = 17.238; df = 9, P < 0.001; female, F = 8.013; df = 9, P < 0.001), and others (male, F = 8.589; df = 5, P < 0.001; female, F = 16.886; df = 5, P < 0.001). As regards aliphatic aldehydes, there were significant differences among the EAG responses of males (F = 2.787; df = 4, P = 0.048) but not of females (F = 1.809; df = 4, P = 0.159). In both sexes, (E)-2-hexenal, (Z)-3-hexenol, (±)-linalool, and 3-heptanone elicited the strongest EAG amplitude within the corresponding chemical groups (Fig. 1).
Antennal Sensitivity
The antennal sensitivity of adult C. duodecimpunctata antennae toward increasing concentrations of 8 EAG-active compounds is reported in Fig. 2. For all compounds, the amplitude of the EAG response increased with dose. For both sexes, the activation threshold was recorded at the 0.1 μg dose for (Z)-3-hexen-1-ol, (E)-2-hexenal, heptanal, nonanal, and (±)-linalool and at the 1 μg dose for the remaining compounds. Male and female EAG responses increased from 100 to 1000 μg doses of all compounds, indicating that no saturation of olfactory receptors occurred at the 100 μg dose. Mean male EAG response was significantly higher than that of female to 0.1 (t = 4.756; df = 4; P = 0.009) 10 (t = 3.087; df = 4; P = 0.037) and 1000 μg (t = 3.401; df = 4; P = 0.027) of (Z)-3-hexen-1-ol, 100 μg (t = 11.420; df = 4; P < 0.001) of (E)-2-hexenal, 10 μg (t = 4253; df = 4; P = 0.013) of heptanal, and 100 μg of (±)-linalool (t = 3.101; df = 4; P = 0.036).
Behavioural Response to (Z)-3-Hexen-1-Ol
The behavioural responses of adult beetles to increasing doses of the most EAG-active compound (Z)-3-hexen-1-ol vs. mineral oil are reported in Table 3. The 1 μg dose did not elicit significant responses in both sexes. At the 10 μg dose, males exhibited a significant preference for the test compound (χ2 = 7.53, df = 1, P < 0.01) and spent significantly more time in the treatment arm (t = 3.96, df = 28, P < 0.001). At the 50 μg dose, males did not show any preference but they spent significantly more time in the control arm (t = 2.63, df = 17, P = 0.013). The 10 and 50 μg doses of (Z)-3-hexen-1-ol did not elicit a significant response in females measured either as first choice or time spent in the treatment arm.
Discussion
In Y-tube olfactometer bioassays, adults of C. duodecimpunctata exhibited sexually-dimorphic behavioural responses to the odours emitted by cladophylls of the host plant A. officinalis. In the absence of visual stimuli, males were significantly attracted by odours of cladophylls while females were not. In more detail, male preference towards intact cladophylls was significant both in terms of first choice and time spent in the treatment arm while towards mechanically damaged cladophylls males only spent significantly more time in the treatment arm. In these latter experiments, a higher odour concentration in the olfactometer due to tissue breakdown may have initially interfered with the insect orientation towards the host odour source making the first choice not significant. Similar sexually-dimorphic behavioural responses to plant volatiles have been reported for L. decemlineata whose males oriented preferentially to specific blends of host plant volatiles whilst females showed little preference (Dickens 2000a; Dickens 2006). This behaviour in L. decemlineata was considered consistent with the presence of a male-produced aggregation pheromone (Dickens et al. 2002) and it was suggested that pioneer males initiate colonisation, locating host plants using odour signals and then produce an aggregation pheromone to which colonising beetles of both sexes respond (Dickens 2006; Landolt and Phillips 1997).
Interestingly, a similar host-finding behaviour has been proposed for the asparagus fly, Plioreocepta poeciloptera (Schrank) (Diptera: Tephritidae). In this monophagous pest, males are attracted to the host plant in the first day after emergence and begin to release sex pheromone the following day when the combination of male pheromone and the host plant volatiles, mainly green asparagus volatiles, attract inexperienced virgin females which respond to the male pheromone only in the presence of plant volatiles (Thibout et al. 2005). To date, the use of intraspecific semiochemicals by C. duodecimpunctata adults has not been investigated; however, male-produced aggregation pheromones that attract both sexes over long distances have been identified in different chrysomelids including the closely-related species Oulema melanopus L. in the Criocerinae subfamily (Rao et al. 2002).
Despite differences between sexes in the behavioural response to host plant odours, EAG selectivity assays revealed a high similarity between the male and female response profiles to compounds tested in this study. The EAG responses of males and females to different compounds did not differ significantly, except for (Z)-3-hexen-1-ol which elicited higher EAG responses in males than in females. The similarity in antennal responses of male and female insects to plant odours has been reported for several phytophagous pests and could arise from the use of the same chemical stimuli present in the habitat they share (Li et al. 1992). However, sexually dimorphic EAG responses are more likely due to a different number of receptor neurons tuned to individual compounds in male and female antennae as a result of different roles played by the same compound in the ecology of each sex (Germinara et al. 2009). Among all compounds tested, (Z)-3-hexen-1-ol was by far the most EAG-active. In both sexes, the response to this compound was about 2-fold higher than that recorded for the second most EAG-active compound, 1-octen-3-ol.
All 8 compounds tested in EAG sensitivity assays elicited dose-dependent responses in males and females with only a few significant differences, at certain doses, between sexes proving the insect olfactory system to be sensitive to change in odour concentration. In both sexes, the lowest activation threshold was recorded for (Z)-3-hexen-1-ol, (E)-2-hexenal, heptanal, nonanal, and (±)-linalool suggesting the insects’ capability to detect them from a longer distance. Overall, EAG experiments clearly showed that both C. duodecimpunctata sexes were able to perceive a wide range of plant volatiles strongly suggesting a role of these chemical cues not only in male but also in the female ecology. Future experiments should address whether EAG-active plant volatiles could enhance or synergy female long distance attraction to a putative male-produced aggregation pheromone (Landolt and Phillips 1997; Reddy and Guerrero 2004; Dickens 2006).
According to the results of electrophysiological tests, the behavioural response of C. duodecimpunctata males and females to the most EAG-active compound, (Z)-3-hexeno-1-ol was evaluated in further Y-tube olfactometer bioassays. Sexual-dimorphic responses similar to those seen with host plant materials were observed. In the experimental conditions adopted, none of the three (Z)-3-hexen-1-ol doses tested elicited a significant preference in females. On the contrary, males were significantly attracted, both at first choice and time spent in the treatment arm, by the 10 μg dose of (Z)-3-hexen-1-ol but they were repelled by the 50 μg. (Z)-3-hexen-1-ol is a general green leaf volatile (GLV) (Paré and Tumlinson 1999) contributing to the “green odour” of the leaves of numerous plant species that plays different roles in insect-plant interactions (Wei and Kang 2011). The GLVs may be produced constitutively or induced by leaf damage and may be caused by abiotic or biotic elicitation (Visser 1986; Loughrin et al. 1996; Turlings et al. 1998; Ruther et al. 2002; Graus et al. 2004; Chamberlain et al. 2006). GLVs have been identified as major components of A. officinalis including (Z)-3-hexen-1-ol, which was identified from healthy plants and shown to be attractive to the asparagus miner, Ophiomyia simplex Loew (Diptera: Agromyzidae) (Morrison III et al. 2016). Among chrysomelids, different behavioural activities have been reported for (Z)-3-hexen-1-ol, including differential responses to variation in concentrations as in this study. In Cassida denticollis Suffrian (Coleoptera: Chrysomelidae), (Z)-3-hexen-1-ol enhanced the ability of larvae to distinguish stems of host plants (Müller and Hilker 2000). Adults of L. decemlineata were attracted to a five-component blend comprising low doses of the green leaf volatiles (E)-2-hexen-1-ol and (Z)-3-hexen-1-ol (Dickens 1999), but volatile blends containing relatively high amounts of the same green leaf volatiles were unattractive or repellent (Dickens 2000a).
It is interesting to note that while the response of L. decemlineata adults is due to ratio of GLVs in the blend rather than individual components, which were individually inactive (Ma and Visser 1978; Visser 1979; Dickens 2000a), in C. duodecimpunctata, (Z)-3-hexen-1-ol alone elicited significant behavioural responses in males. This attraction, to be confirmed in further field studies, along with the observation that (Z)-3-hexen-1-ol is released by healthy asparagus plants (Morrison III et al. 2016) strengthens the idea that this compound plays a key role at least in the male host-searching behaviour.
In conclusion, this study demonstrated that C. duodecimpunctata adults can detect a variety of A. officinalis volatiles with a particular antennal sensitivity to (Z)-3-hexen-1-ol in both sexes. However, behavioural bioassays also revealed sexually-dimorphic responses to odours of plant materials and to (Z)-3-hexen-1-ol with males being the only responsive sex. Further investigations should test the behavioural activity of combinations of GLVs with other asparagus volatiles towards females to find out possible attractive blends and identify a putative male-produced aggregation pheromone in C. duodecimpunctata. The kairomonal activity of (Z)-3-hexen-1-ol has potential practical interest mainly to develop effective monitoring and control tools of this pest.
Data Availability
Data and material are reported in the manuscript.
Code Availability
Not applicable.
References
Bean DW, Wang T, Bartelt RJ, Zilkowski BW (2007) Diapause in the leaf beetle Diorhabda elongata (Coleoptera: Chrysomelidae), a biological control agent for tamarisk (Tamarix spp.). Environ Entomol 36:531–540. https://doi.org/10.1603/0046-225X(2007)36[531:DITLBD]2.0.CO;2
Bruce TJ, Wadhams LJ, Woodcock CM (2005) Insect host location: a volatile situation. Trends Plant Sci 10(6):269–274. https://doi.org/10.1016/j.tplants.2005.04.003
Capinera JL (2001) Order Coleoptera-beetles, weevils. White Grubs and Wireworms. Handbook of vegetable pests, Academic, San Diego
Chamberlain K, Khan ZR, Pickett JA, Toshova T, Wadhams LJ (2006) Diel periodicity in the production of green leaf volatiles by wild and cultivated host plants of stemborer moths, Chilo partellus and Busseola fusca. J Chem Ecol 32:565–577. https://doi.org/10.1007/s10886-005-9016-5
Chittenden FH (1917) The asparagus beetles and their control. United States Department of Agriculture, Washington D.C
Dethier VG (1947) Chemical insect attractants and repellents. Blakiston, Philadelphia
Dickens JC (1999) Predator-prey interactions: olfactory adaptations of generalist and specialist predators. Agr Forest Entomol 1(1):47–54. https://doi.org/10.1046/j.1461-9563.1999.00007.x
Dickens JC (2000a) Orientation of Colorado potato beetle to natural and synthetic blends of volatiles emitted by potato plants. Agri Forest Entomol 2(3):167–172. https://doi.org/10.1046/j.1461-9563.2000.00065.x
Dickens JC (2000b) Sexual maturation and temporal variation of neural responses in adult Colorado potato beetles to volatiles emitted by potato plants. J Chem Ecol 26:1265–1279. https://doi.org/10.1023/A:1005492229377
Dickens JC, Oliver JE, Hollister B, Davis JC, Klun JA (2002) Breaking a paradigm: male-produced aggregation pheromone for Colorado potato beetle. J Exp Biol 205:1925–1933
Dickens JC (2006) Plant volatiles moderate response to aggregation pheromone in Colorado potato beetle. J App Entomol 130:26–31. https://doi.org/10.1111/j.1439-0418.2005.01014.x
Dingler M (1935) Über unsere beiden Spargelkäter (Crioceris duodecimpunctata L. und Crioceris asparagi L.). Z Angew Entomol 21(3):415–442
Drake CJ, Harris HM (1932) Asparagus insects in Iowa. Circular, Ames
Fink DE (1913) The asparagus miner and the twelve-spotted asparagus beetle. Cornell University Agricultural Experiment Station, Ithaca
Gaffke MA, Sing SE, Millar JG, Dudley TL, Bean WD, Peterson RKD, Weaver DK (2020) An herbivore-induced plant volatile from saltcedar (Tamarix spp.) is repellent to Diorhabda carinulata (Coleoptera: Chrysomelidae). Environ Entomol 49:1063–1070. https://doi.org/10.1093/ee/nvaa079
Germinara GS, De Cristofaro A, Rotundo G (2009) Antennal olfactory responses to individual cereal volatiles in Theocolax elegans (Westwood) (Hymenoptera, Pteromalidae). J Stored Prod Res 45(3):195–200. https://doi.org/10.1016/j.jspr.2009.02.002
Germinara GS, De Cristofaro A, Rotundo G (2011) Chemical cues for host location by the chestnut gall wasp, Dryocosmus kuriphilus. J Chem Ecol 37(1):49–56. https://doi.org/10.1007/s10886-010-9893-0
Germinara GS, Ganassi S, Pistillo MO, Di Domenico C, De Cristofaro A, Di Palma AM (2017) Antennal olfactory responses of adult meadow spittlebug, Philaenus spumarius, to volatile organic compounds (VOCs). PLoS One 12(12). https://doi.org/10.1371/journal.pone.0190454
Graus M, Schnitzler JP, Hansel A, Cojocariu C, Rennenberg H, Wisthaler A, Kreuzwieser J (2004) Transient release of oxygenated volatile organic compounds during light-dark transitions in grey poplar leaves. Plant Physiol 135(4):1967–1975. https://doi.org/10.1104/pp.104.043240
Kaissling KE, Thorson J (1980) Insect olfactory sensilla: structural, chemical and electrical aspects of the functional organisation. In: Satelle DB et al (eds) Receptors for neurotransmitters, hormones and pheromones in insects. Elsevier/North-Holland Biomedical Press, New York, pp 261–282
Landolt PJ, Phillips TW (1997) Host plant influences on sex pheromone behavior of phytophagous insects. Annu Rev Entomol 42:371–391. https://doi.org/10.1146/annurev.ento.42.1.371
Li Y, Dickens JC, Steiner WWM (1992) Antennal olfactory responsiveness of Microplitis croceipes (Hymenoptera: Braconidae) to cotton plant volatiles. J Chem Ecol 18:1761–1773. https://doi.org/10.1007/BF02751101
LeSage L, Dobesberger EJ, Majka CG (2008) Introduced leaf beetles of the maritime provinces, 6: the common asparagus beetle, Crioceris asparagi (L.), and the twelve-spotted asparagus beetle, Crioceris duodecimpunctata (L.) (Coleoptera: Chrysomelidae). P Entomol Soc Wash 110(3):602–621. https://doi.org/10.4289/07-075.1
Loughrin JH, Potter DA, Hamilton-Kemp TR, Byers ME (1996) Role of feeding-induced plant volatiles in aggregative behavior of the japanese beetle (Coleoptera: Scarabaeidae). Environ Entomol 25(5):1188–1191. https://doi.org/10.1093/ee/25.5.1188
Ma WC, Visser JH (1978) Single unit analysis of odour quality coding by the olfactory antennal receptor system of the Colorado beetle. Entomol Exp App 24(3):520–533. https://doi.org/10.1111/j.1570-7458.1978.tb02813.x
McIndoo NE (1926) An insect olfactometer. J Econ Entomol 19(3):545–571. https://doi.org/10.1093/jee/19.3.545
Morrison WR III, Szendrei Z (2014) The common asparagus beetle and spotted asparagus beetle (Coleoptera: Chrysomelidae): identification, ecology, and management. J Integr Pest Manag 5(3):B1–B6. https://doi.org/10.1603/IPM14004
Morrison WR III, Ingrao A, Ali J, Szendrei Z (2016) Identification of plant semiochemicals and evaluation of their interactions with early spring insect pests of asparagus. J Plant Interact 11(1):11–19. https://doi.org/10.1080/17429145.2015.1133848
Müller C, Hilker M (2000) The effect of a green leaf volatile on host plant finding by larvae of a herbivorous insect. Naturwissenschaften 87(5):216–219. https://doi.org/10.1007/s001140050706
Otter CD, Tchicaya T, Schutte AM (1991) Effects of age, sex and hunger on the antennal olfactory sensitivity of tsetse flies. Physiol Entomol 16(2):173–182. https://doi.org/10.1111/j.1365-3032.1991.tb00554.x
Paré PW, Tumlinson JH (1999) Plant volatiles as a defense against insect herbivores. Plant Physiol 121(2):325–332. https://doi.org/10.1104/pp.121.2.325
Pickett JA, Wadhams LJ, Woodcock CM (1998) Insect supersense: mate and host location by insect model systems for exploiting olfactory interactions. Biochemist 20:8–13
Pollini A (1998) Manuale di entomologia applicata. Edagricole-Edizioni Agricole, Bologna
Raguso RA, Light DM (1998) Electroantennogram responses of male Sphinx perelegans hawkmoths to floral and “green-leaf volatiles”. Entomol Exp App 86(3):287–293. https://doi.org/10.1046/j.1570-7458.1998.00291.x
Rao S, Cossé AA, Bartelt RJ, Zilkowski BW (2002) Field responses of cereal leaf beetle, Oulema melanopus (Coleoptera: Chrysomelidae) to its aggregation pheromone. In: Entomological Society of America annual meeting, Fort Lauderdale, 17-20 November 2002
Reddy GV, Guerrero A (2004) Interactions of insect pheromones and plant semiochemicals. Trends Plant Sci 9(5):253–261. https://doi.org/10.1016/j.tplants.2004.03.009
Ruther J, Reinecke A, Hilker M (2002) Plant volatiles in the sexual communication of Melolontha hippocastani: response towards time-dependent bouquets and novel function of (Z)-3-hexen-1-ol as a sexual kairomone. Ecol Entomol 27(1):76–83. https://doi.org/10.1046/j.1365-2311.2002.0373a.x
Sun R, Wang Y, Chin CK, Garrison SA (2001) Volatile compounds in Asparagus officinalis L. in: X international Asparagus symposium, A. Uragami, Niigata, 589 pp 257–266. https://doi.org/10.17660/ActaHortic.2002.589.35
Tasin M, Anfora G, Ioriatti C, Carlin S, De Cristofaro A, Schmidt S, Bengtsson M, Versini G, Witzgall P (2005) Antennal and behavioral responses of grapevine moth Lobesia botrana females to volatiles from grapevine. J Chem Ecol 31(1):77–87. https://doi.org/10.1007/s10886-005-0975-3
Thibout E, Pierre D, Mondy N, Lecomte C, Biemont JC, Auger J (2005) Host-plant finding by the asparagus fly, Plioreocepta poeciloptera (Diptera: Tephritidae), a monophagous, monovoltine tephritid. B Entomol Res 95:393–399. https://doi.org/10.1079/BER2005370
Thorpe WH, Crombie AC, Hill R, Darrah JH (1947) The behaviour of wireworms in response to chemical stimulation. J Exp Biol 23(3-4):234–266
Turlings TC, Lengwiler UB, Bernasconi ML, Wechsler D (1998) Timing of induced volatile emissions in maize seedlings. Planta 207(1):146–152. https://doi.org/10.1007/s004250050466
Van den Berg J, Torto B, Pickett JA, Smart LE, Wadhams LJ, Woodcock CM (2008) Influence of visual and olfactory cues on field trapping of the pollen beetle, Astylus atromaculatus (Coleoptera: Melyridae). J Appl Entomol 132(6):490–496. https://doi.org/10.1111/j.1439-0418.2007.01259.x
Visser JH (1979) Electroantennogram responses of the Colorado beetle, Leptinotarsa decemlineata, to plant volatiles. Entomol Exp App 25(1):86–97. https://doi.org/10.1111/j.1570-7458.1979.tb02851.x
Visser JH (1986) Host odour reception in phytophagous insects. Annu Rev Entomol 31:121–144. https://doi.org/10.1146/annurev.en.31.010186.001005
Wei J, Kang L (2011) Roles of (Z)-3-hexenol in plant-insect interactions. Plant Signal Behav 6(3):369–371. https://doi.org/10.4161/psb.6.3.14452
Weissbecker B, Schütz S, Klein A, Hummel HE (1997) Analysis of volatiles emitted by potato plants by means of a Colorado beetle electroantennographic detector. Talanta 44(12):2217–2224. https://doi.org/10.1016/S0039-9140(97)00037-4
Acknowledgments
The skilful cooperation of Adriano Cotugno for assistance in insect rearing and Y-tube olfactometer bioassays is gratefully acknowledged.
Funding
This research was financially supported by the Apulia Region (PSR program 2014-2020 - Sottomisura 16.2, DDS n.167, 28 July 2020. Title: “Innovazioni e soluzioni sostenibili per l’asparago pugliese (AS_PARA)”.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
All authors approve of the submission of the manuscript.
Conflict of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Rights and permissions
About this article
Cite this article
Pistillo, O.M., D’Isita, I. & Germinara, G.S. Olfactory Response of the Spotted Asparagus Beetle, Crioceris duodecimpunctata (L.) to Host Plant Volatiles. J Chem Ecol 48, 41–50 (2022). https://doi.org/10.1007/s10886-021-01323-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-021-01323-5