Abstract
Olfactory bioassays were performed to investigate the specific odors utilized as host location cues by the beetle parasitoid, Scleroderma guani (Hymenoptera: Bethylidae), a primary biological control agent against Monochamus alternates (Coleoptera: Cerambycidae), the most important vector beetle of the pinewood nematode, Bursaphelenchus xylophilus (Nematoda: Aphelenchoididae), the causal agent for pine wilt disease. Female parasitoids were tested with volatiles and extracts derived from their host beetle. Behavioral assays (Y-olfactometer bioassay and circle arena) demonstrated the response of female parasitoids to odors from host plants damaged by beetle larvae and from their excreta. When available contact cues were additionally provided, the parasitoids showed particularly strong preferences for samples of fresh brown frass of larval beetles. To confirm the electroantennograms (EAG) activity of identified compounds, analyses were repeated with a synthetic blend composed predominantly of compounds in the crude extracts that had revealed apparent electrophysiological activity. Antennal responses to four monoterpenes and three oxygenated monoterpenes were among the strongest, which indicated their potential for use in development of semichemial-based management of the beetle.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Host finding by parasitoids is a complex process, in which host habitat location is the first critical step. Many parasitoids masterly forage and locate their host by virtue of chemical cues originated from the host itself, its products, host plants, or from infestation-associated yeasts and fungi (Sullivan and Berisford 2004; Hart 2013). Infochemicals are important cues for host location in tritrophic systems of parasitoids and beetles (Pettersson et al. 2000; Pettersson 2001a, b).
Typical behaviors of parasitoids before acceptance or rejection of a host are antennating, palpating, test biting, and test feeding (Harrison 1987). Both individual test biting experiences as well as exposure to single host-specific compounds can be sufficient to stimulate feeding in several specialist parasitoids. However, for polyphagous parasitoids, a natural habitat represents a highly diverse mosaic of plants in which they must recognize cues that are emitted by their potential hosts (Randlkofer et al. 2010; Wäschke et al. 2013). To find and identify their host and the plants of their hosts, parasitoids could use various sensory systems, including sight, olfaction, taste and reception of contact cues. A combination of such cues can lead the parasitoid to its food source or oviposition sites. Nevertheless, all these cues vary in both their cost of assessment and accuracy, with some cues being more reliable than others (Fawcett and Johnstone 2003). For example, olfactory cues are likely to be signals that are more reliable. They may allow for host plant location even in a complex environment, if the insect central nervous system receives the volatile information at a fine-scale spatio-temporal resolution (Held et al. 2003; Bruce et al. 2005). However, for the most parasitoids, contact chemoreception indicates more accurate and reliable information about host suitability. Cuticle chemicals of host larvae or pupae can give important information leading to host acceptance or rejection (Pettersson et al. 2000; Williams et al. 2008).
The hosts of the ectoparasitoid Scleroderma guani (Hymenoptera: Bethylidae) tend to be solitary wood-boring insects and are thus concealed in their habitat (trunk, wood or seed) (Evans, 1964; Gauld and Hanson 1995). Therefore, effective searching tactics are required by the parasitoid for finding its hosts. Once S. guani found and invaded chamber structures, they encountered a similar environment with its host larvae or pupae (Evans 1964; Gauld and Hanson 1995). The dispersal of S. guani mainly depends on female adults while they move mainly by walking rather than flying through their complex natural environment. Once detected, female wasps paralyze the larvae or pupae by injecting venom and then lay eggs on the host. Larvae of the wasps are encased in the host and complete their development until adult emergence (Lauzière et al. 2001; Li et al. 2010). Recent studies identified two putative odorant-binding proteins (OBPs) and one putative chemosensory protein (CSP) from S. guani females (Lu et al. 2007). The ultrastructure of the antennal sensilla types in S. guani are morphologically characterized and compared to that of 19 species of parasitic Hymenoptera (Lu et al. 2007). In comparison to monophagous specialists, the highly polyphagous parasitoids such as S. guani possess diverse sensilla types which are likely related to their broad host ranges and complex life styles. Therefore, current hypotheses propose that olfactory and available contact cues may be advantageous in adult host selection behavior through improved recognition, accuracy or selectivity in foraging.
We report on both olfactory and contact cues of S. guani parasitoid. Our aim was to evaluate the host-selection adaptabilities of S. guani with respect to how to find suitable hosts inside trees or logs. First we hypothesize that S. guani can mainly integrate olfactory cues and available contact cues depending on the chemical information from hosts and their micro-environment. Under laboratory conditions, we tested the electrophysiological active responses of S. guani females to different odors.
Materials and methods
Experimental insects and plants
Scleroderma guani was reared on the larvae of Monochamus alternatus (Coleoptera: Cerambycidae) in the laboratory for 15 successive generations prior to use in the experiments. S. guani were reared individually in a vial (7.5 cm in height × 1.2 cm in diameter), blocked with a cotton plug on the port, kept at 25 ± 5 °C, 70 % RH, under a LD 14:10 h. The 3rd instar larvae of M. alternatus were collected from trees within the pine wilt nematode-affected zone in Zhejiang province, China. All 3rd instar larvae of hosts were stored at 8–10 °C prior to use in rearing of S. guani.
The bolts used in the study were cut from logs of two- to three-year old Masson pines, Pinus massoniana (Pinales: Pinaceae), damaged by larvae of M. alternatus and collected from within the pine wilt nematode-affected zone in Yuhang district of Hangzhou city, Zhejiang province, China (N 30°09′–30°34′, E 119°40′–120°23′).
Bolts from undamaged trees were collected from healthy trees without larvae of M. alternates in the pine wilt nematode-affected in Anhui province, China. All bolts were cut to the same dimensions (20.0 cm in length × 8.0 cm in diameter) and their ends wax-sealed in order to avoid desiccation.
Sampling of volatiles and extracts from host and host plants
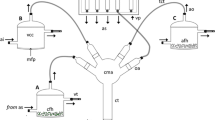
The host tree treatments used for volatile collection experiments were done as in Pettersson et al. (2001a, b) and Fan et al. (2007). The different treatments of host insects and host plant bolts were placed in a plastic oven bag (40 × 44 cm, Reynolds, USA, with an approximate 7,500 ml in volume). Sampling was performed by using dynamic headspace aeration on a Super Q adsorbent. Using two freshly activated charcoal traps, a stream of filtered and moisturized air was pumped into the bag. The air with emitted plant volatiles was withdrawn through a glass collector by a membrane pump (Beijing Institute of Labor Instruments, China) at a rate of 200 ml min-1. The absorbing glass collector contained 75 mg of Super Q (80–100 mesh size, Alltech Association Inc. Deerfield, IL, USA) and headspace was collected for 24 h.
Sixteen collections of volatile and non-volatile compounds were performed simultaneously (Table 1): (1) HP, healthy, undamaged bolts by larvae of M. alternatus; (2) MP, mechanically damaged bolts created by cutting three wounds on the stem in different directions with an axe, folded carefully just as the former logs; (3) L + DP, damaged bolts by 6–8 beetle larvae, combined with their fresh frass; (4) L-DP, damaged bolts without larvae and frass of larval M. alternates; (5) LA/LE, volatiles/extracts from ten beetle larvae; (6) LFA/LFE, volatiles/extracts from larvae combined with its fresh frass; (7) BFA/BFE, volatiles/extracts from fresh brown frass created from the phloem fed by beetle larvae; (8) WFA/WFE, volatiles/extracts from fresh white frass created from the xylem fed by beetle larvae; (9) CA/CE, volatiles/extracts from pupal chambers without beetle larvae; (10) UBFA/UBFE, volatiles/extracts from frass of other woodborers under thin layers of the bark. In treatments (5), extracts were extracted for 5 min in order to avoid internal chemical compounds of larval body penetrating in the solvent. All of the other extracts were extracted for 30 min.
The absorbed volatile compounds from the Super Q collectors were then extracted with 2 ml of HPLC-grade hexane (Sigma-Aldrich Co. LLC). All headspace extracts were stored at −20 °C until used in chemical analyses or behavioral experiments. Biologically active compounds were isolated by coupled EAG.
Chemical identification and quantification of collected volatiles
The collected volatile compounds were identified using an Agilent gas chromatographer (GC) (6890 N) coupled with a mass spectrometry (MS) system (5973 MSD, Agilent Technologies, Inc. USA). The system was equipped with a DB-WAX polyethylene glycol 20000 column (60 m × 0.25 mm ID, 0.15-µm film thickness, J&W Scientific, USA). For analyses using the DB-WAX column, the initial oven temperature was kept at 40 °C for 2 min and then increased to 220 °C at a programmed rate of 5 °C min−1. The inlet was operated under the splitless injection mode, and the injector temperature was maintained at 250 °C with a constant flow rate at 1.0 ml min−1. Volatile compounds were identified by comparing their retention times and spectra with those of synthetic standards. Referenced mass spectra from the NIST02 library (Scientific Instrument Services, Inc., USA) were also used.
Behavioral bioassay
Because S. guani moves through a very complex habitat mainly by walking, visual cues, which may under these circumstances be of little accuracy, were neglected in the first instance. Consequently, this study focused on the attraction behavior of S. guani to olfactory and contact cues of its host M. alternatus. Olfactory cues were analyzed in two different laboratory bioassays: (1) a Y-olfactometer without contact, and (2) a contact test in a circle arena. These two types of assays were chosen as they present olfactory and contact cues in different distances and modalities, and allow analyzing for different behavioral contexts (cf. “Materials and methods”).
Y-tube olfactometer behavioral experiments
A Y-tube olfactometer (stem, 20 cm; arms, 15 cm at 75° angle; internal diameter, 1.5 cm) was used to determine the behavioural responses of female S. guani to sample compounds of the aforementioned volatiles and extracts from the hosts and host plants (Wei et al. 2007; Pettersson et al. 2000; Pettersson 2001a). In the dual-choice tests, the sample compounds were compared respectively versus the control and conducted as follows: (1) HP, MP, L-DP, L + DP; (2) CA, UBFA, WFA, BFA, LA, LFA; and (3) CE, UBFE, WFE, BFE, LE, LFE. A pump (Beijing Institute of Labour Instruments, China) was used to draw air through activated charcoal and a water jar so that it was purified of any contaminating odors and humidified before passing over the target and entering the Y-tube. Airflow through each of the olfactometer arms was maintained at 200 ml min−1 by a flowmeter. Headspace compounds (Table 1) were applied to 1 × 1 cm pieces of filter paper at a volume of 5 μl and placed in the airstream entering one of the olfactometer arms and with a control (equal volume of HPLC-grade hexane) in the other. The solvent evaporated prior testing behavioral responses. Each individual parasitoid was introduced into the Y-tube at the entrance of the stem and thus had a choice between the test odor and the control. New filter papers with the extracts and dichloromethane were used for every parasitoid. Female parasitoids were tested individually between 9h00 and 18h00. The position of the arms containing treatment and control odors was reversed to avoid position bias after every five individuals had been tested. Each female parasitic wasp was placed in the olfactometer for 5 min. A ‘‘no choice’’ was recorded when the wasp remained inactive for the duration of the testing period. The samples of different treatments were applied to a piece of filter paper (1 × 1 cm) at a volume of 5 μl and placed inside one arm of the Y-tube olfactometer. A same-sized filter paper impregnated with equal volume of HPLC-grade hexane was set in the other arm as the control. Per treatment, each parasitoid was allowed to acclimatize on the sphere for 2 min, and then one of 16 different odor sources was applied for 4 min.
Contact behavioral experiments
To investigate the parasitoids’ reaction to contact cues, contact tests were conducted in a circle arena where different odor samples were offered. The circle arenas were prepared and modified as described by Pettersson (2001a). The following choices were offered simultaneously in a glass Petri dish of 12 cm in diameter. In the multiple choice assay, the different sample compounds were compared with the control and conducted as follows: (1) HP, MP, L-DP, L + DP; (2) CA, UBFA, WFA, BEA, LA, LFA; and (3) CE, UBFE, WFE, BEE, LE, LFE. Twenty mated females (5–6 days) per dish were tested separately. In the three treatments, each compound was separately applied to 1 × 1 cm pieces of filter paper, at a volume of 5 μl, and placed in the glass Petri dish. A same-sized filter paper impregnated with equal volume of hexane was set in the glass Petri dish as the control. The solvent evaporated prior testing behavioral responses. Choice preference was expressed as arena residence time (min) of female S. guani on different samples within 20 min. In a bioassay with successful selection, female S. guani walk, search and probe throughout the arena, and generally do not change positions within 20 min after making their selection. The arena was sealed with a thin film with air vents for ventilating and avoiding parasitoids escape. Per treatment, each parasitoid was allowed to acclimatize on the arena for 2 min. All odor sources were renewed after two test runs. A ‘‘no choice’’ was stated once the wasp remained inactive for the duration of the testing period. The tests were conducted at 25–26 °C under a lamp (100 Lx) hanging approximately 0.5 m above the roof of the experimental arena. The parasitoids were starved 3–4 h prior to testing.
EAG active responses
The antennae of parasitoids were prepared as described by Wei and Kang (2006) and Williams et al. (2008) with a few modifications. The head of the insect was removed, and the tips of the antennae were cut off. The reference electrode (Ringer’s filled glass capillary Ag–AgCl electrode) was inserted in the hemocoel of the cranial cavity, while the recording electrode was connected to the two antennae in one individual. Signals from the antennae were passed through a high-impedance DC amplifier (CS-55, Syntech, Hilversum, The Netherlands) in a signal connection interface box (Auto Spike, IDAC 2/3, Syntech). EAG responses of antennae from S. guani adult females were detected to sixteen synthetic compounds. Standards of 1R-(+)-α-pinene, 1S-(-)-α-pinene, (-)-camphene, 1S-(+)-3-carene, R-(-)-α-phellandrene, R-(+)-limonene, terpinolene, myrcene, cumene, (+)-longifolene, (-)-trans-caryophylene, (-)-camphor, D(+)-camphor, (+)-fenchone and L(-)fenchone were obtained from Sigma, Aldrich, Acros Organics or Fluka Chemical Co., and were ≥95–98 % pure. The selected single compounds were diluted with paraffin oil in a grade of 1, 10, 100, and 1,000 μg per 10 μl. Each tested compound at the aforementioned dosages was applied to a piece of filter paper (0.5 × 5.0 cm2) at a volume of 10 μl and was placed inside a pipette testing. Filter papers with the chemical compounds or hexane control were refreshed after each test. Two controls were used: a pipette containing 10 μl paraffin oil only on filter paper, and a pipette containing 10 μl 100 μg hexane in paraffin oil only on filter paper (hexane standard). EAG recording began 5 min after the antennal preparation was mounted. At this time, the following test protocol was used for each recording trial. The tests were in the following order: paraffin oil, hexane, samples (from low concentration to high concentration), paraffin oil, hexane. For each chemical, order of delivery of the four concentrations was random. The controls throughout the recording session permitted standardization of antennal responses. Test compounds and controls were applied (0.1 s pulse) at 60 s intervals for antennal recover. EAGs were measured as maximum amplitude of depolarization (mV). Each chemical was tested on 10–25 individuals of females.
Data analysis and statistics
Data were analyzed using the SPSS statistical program (version 13.0; SPSS Inc., USA). Each experiment was replicated 4–6 times. A χ2 test was used to determine the significance of the differences between the numbers of parasitoids choosing each arm of the olfactometer (Wei et al. 2007). Parasitoids that did not make a choice were excluded from statistical analyses. Unsuccessful parasitic wasp response rates in choice experiments ranged from 8 to 20 %. One-way analysis of variance (ANOVA) and least significant difference (LSD) multiple comparisons were performed to assess the differences in the amount of time spent exploring each filter paper of different odor treatments. EAG responses were control-adjusted with the paraffin oil and expressed as proportional responses relative to the hexane standard (Williams et al. 2008).
Results
Response to volatiles in the olfactometer
In the Y-olfactometer, the mated female S. guani showed significant preference for volatiles collected from bolts damaged by the beetle larvae with frass compared to the solvent control (percent responses >70 % in all cases, P < 0.01; Fig. 1a). Odor collected from healthy plants (HP) was also attractive to parasitoids (60.98 %). However, volatiles originated from mechanically damaged plants (MP) and damaged plant logs without larvae and frass of the beetle (L-DP) were unattractive to parasitoids (48.78 % and 53.66 %, respectively). The adult females showed clear preference for the complex of host-frass volatiles and extract to the solvent control (LFA, 70.732 %; LFE, 73.171 %, P < 0.01, Fig. 1b, c). The volatiles and extracts collected from fresh larval frass were attractive to the female parasitoids (BFA, 58.537 %, P < 0.01, Fig. 1a; BFE, 63.415 %, P < 0.05, Fig. 1c; WFA, 65.854 %; WFE, 63.415 %, P < 0.01, Fig. 1b, c). However, there were no significant differences between volatiles collected from the pupal chambers and the frass of other woodborers under thin layers of the bark and the solvent control (CA, UBFA, percent responses <54 % in all cases, P > 0.05; Fig. 1b). Moreover, the female parasitoids significantly preferred the olfactometer arm containing volatiles from the beetle larvae without any feces to the solvent control (LA, 60.976 %, P < 0.05, Fig. 1b), but there was no clear difference between the olfactometer arm containing extracts from the beetle larvae without any feces to the solvent control (LE, percent responses <57 % in all cases, P > 0.05, Fig. 1c).
Responses of Scleroderma guani adult females in a Y-olfactometer to compounds from headspace volatiles and extracts of host plant logs versus a solvent control (hexane). For abbreviations of different treatments: HP, healthy bolts; MP, mechanically damaged bolts; L + DP, damaged bolts; L-DP, damaged bolts without any larvae and frass; LA/LE, volatiles/extracts from larvae; LFA/LFE, volatiles/extracts from larvae combined with its frass; BFA/BFE, volatiles/extracts from brown frass; WFA/WFE, volatiles/extracts from white frass; CA/CE, volatiles/extracts from pupal chambers; UBFA/UBFE, volatiles/extracts from frass under thin bark layers. a Responses of parasitoids to headspace volatiles from host plant logs b responses of parasitoids to headspace volatiles from hosts with their frass or pupal chambers c responses of parasitoids to extracts from hosts with their frass or pupal chambers. χ2 test for differences between numbers of parasitoids in each arm (mean + SE, n = 41 in each treatment; df = 1; *P < 0.05; **P < 0.01; ns not significant)
Response to extracts in a contact bioassay
When a parasitoid could choose between volatiles from its hosts and host plants and the solvent control, female adults of S. guani spent significantly more time on the filter paper containing volatiles from damaged host plants by 3–4 instars larvae of M. alternates (Fig. 2a; ANOVA; F 4,95 = 15.156, P < 0.001). However, there were no clear differences in the amount of time spent exploring each filter paper of HP, MP and L-DP (arena residence time <3.3 min, Fig. 2a). When a parasitoid could choose between extracts from hosts and its frass in the micro-habitat versus the solvent control, female parasitoids spent significantly more time on the filter paper containing extracts from LFA and LA (Fig. 2b; ANOVA; F 6,133 = 12.636, P < 0.001). When a parasitoid could choose between extracts of hosts and its frass in the micro-habitat versus the solvent control, female parasitoids spent significantly more time on the filter paper containing extracts from LFE, LE and BFE (Fig. 2c; ANOVA; F 6,133 = 12.636, P < 0.001). In addition, female parasitoids spent more than 6 min on exploring on the filter paper containing extracts from BFE (Fig. 2c).
Responses of Scleroderma guani adult females to different odor cues in the arena: compounds from different headspace volatiles and extracts of host and host plants versus a solvent control (hexane). For abbreviations of different treatments, please see Fig. 1. a Residence time of adult females spent on headspace volatiles collected from host bolts b residence time of adult females spent on headspace volatiles collected from hosts with their frass or pupal chambers c residence time of adult females spent on extracts collected from hosts with their frass or pupal chambers. Same letters on bars indicate no significant differences (P < 0.05). For abbreviations of different treatments, please see “Materials and Methods” for explanation (mean + SE, n = 20 in each treatment)
EAG responses to volatiles from hosts and host plants
EAG analyses were repeated with 16 synthetic compounds according to the behavior response of the crude extracts. The chemicals were selected on the basis of their wide distribution in Masson pines, P. massoniana (Fan et al. 2007). S. guani female parasitoids showed characteristic EAG response profiles to 16 volatile compounds (Fig. 3). Four general masson pine Pinus massoniana monoterpenes (1S-(-)-α-pinene, R-(-)-α-phellandrene, (+)-longifolene, R-(+)-limonene), and three oxygenated monoterpenes (D(+)-camphor, (+)-fenchone, L(-)-fenchone), elicited the higher EAG responses in female wasps (Fig. 3). When sixteen compounds that showed relatively large EAG responses were tested for their EAG dose-responses, most of these compounds elicited significant EAG responses at <10 µg in the odor cartridge (Fig. 3). A oxygenated monoterpene, (+)-fenchone, evoked the highest increases in EAG response at a cartridge dose of between 1 and 10 µg, and 100 and 1,000 µg. (+)-Fenchone evoked higher EAG responses than its optical isomer L(-)-fenchone. Other highly EAG active compounds, 1S-(-)-α-pinene, 1S-(+)-3-carene and (+)-longifolene, evoked the highest increases between 100 and 1,000 µg. The less EAG-active compounds reached maximum EAG responses at 1,000 µg.
EAG responses of Scleroderma guani adult females to different synthetic compounds (mean + SE, n = 15, in each treatment). Abbreviations used: 1 (-)-camphene, 2 1S-(+)-α-pinene, 3 1R-(+)-α-pinene, 4 1S-(-)-α-pinene,5 (-)-camphor, 6 D(+)-camphor, 7 1S-(+)-3-carene, 8 R-(-)-α-phellandrene, 9 (-)-trans-caryophylene, 10 cumene, 11 (+)-fenchone, 12 L(-)fenchone, 13 terpinolene, 14 myrcene, 15 (+)-longifolene, 16 R-(+)-limonene
Discussion
In the host-parasite system of M. alternatus and S. guani, we investigated available chemical cues from hosts and host plant when the parasitoid S. guani was searching for concealed hosts. Female S. guani prefer the headspace volatiles from host plants damaged by larval M. alternatus, and stay for a longer time on a contact arena. S. guani also showed significant attraction to odors from volatiles emitted from frass of beetle larvae. However, the wasps showed only a very weak attraction to odors from volatiles and extracts from frass of other woodborers under thin layers of the bark. In contrast, when contact cues were provided, the wasps strongly preferred extracts of fresh brown frass created from the phloem to fresh white frass created from the xylem fed by beetle larvae or to the control. The olfactometer behavioral bioassay and EAG responses of female S. guani revealed that olfactory cues are sufficient for host plant identification at a long distance, while available contact cues can provide more accurate and reliable infochemicals about host suitability at a short distance. In the common habitat of parasitoids and their hosts, information chemicals from host larva and its frass can already provide important cues for host habitat.
S. guani females do not immediately perceive host quality, but need subsequent feeding experience on the hosts to adjust their last preference and acceptance of hosts. A previous study of Li et al. (2009) confirmed that the pre-imaginal and adult experience of S. guani induced the variation in adult behavioral preference through improved recognition, accuracy or selectivity in foraging. The current results show that S. guani can rely on volatiles and extracts from the host and the host plant, including those released from plants damaged by host larvae, healthy plants, the complex of host larvae and its frass, and host larvae.
When S. guani had direct access to the volatiles and extracts of L + DP, BFA/E, and LFA/E in the circle arena, they were able to discriminate their host chemical information mainly based on contact cues. They clearly preferred the host-infested plant logs and the host frass, especially the fresh brown frass created from the phloem fed by beetle larvae. This implies that volatiles and extracts from frass of beetle larvae play a role in host location for S. guani. Monoterpene hydrocarbons are predominant chemical components of the constitutive volatiles of stressed pines P. massoniana (Fan et al. 2007), which commonly are colonized by the Japanese pine sawyer, M. alternatus. The monoterpene hydrocarbons from stressed trees were more attractive to the ovipositing female of the sawyer beetles than from healthy trees.
In EAG recordings, parasitoids S. guani antennae appear to display certain sensitivity to the variety of these odors with higher dose (>100 µg). At first glance, S. guani antennae appear to display similar sensitivity to a variety of odorants as reflected in their EAG responses. This finding is perhaps not surprising due to the parasitoid’s wide coleopterous, even lepidopterous, larval host range. The amplitudes of response to at least four monoterpenes: 1S-(-)-α-pinene, R-(-)-α-phellandrene, (+)-longifolene, R-(+)-limonene were equivalently high. In addition, three oxygenated monoterpenes: D(+)-camphor, (+)-fenchone, and L(-)-fenchone appear to be involved in habitat location by S. guani. This implies that oxygenated monoterpenes can act synergistically with the monoterpenes in attracting S. guani.
The above-mentioned eight compounds evoked higher increases in EAG response at a cartridge dose of between 100 and 1,000 µg. However, the parasitoid did not respond to β-pinene, camphene and myrcene which were general monoterpene hydrocarbons in the volatiles of pine tree. Similarly, Pettersson et al. (2000) used GC-EAD to demonstrate that several oxygenated monoterpenes, such as fenchone and camphor, evoked responses in the female parasitoid Roptrocerus xylophagorum (Ratzeburg). The monoterpene hydrocarbon α-pinene was reported to elicit EAG responses as those of the oxygenated monoterpenes α-terpineol in a sympatric pteromalid parasitoid, Dinotiscus dendroctoni (Ashmead), which attacks many of the same host species and life stages as R. xylophagorum (Salom et al. 1991). However, the parasitoid did not respond to α-pinene, β-pinene, camphene, limonene, or myrcene (Pettersson et al. 2000). The authors thought the lack of EAD responses by R. xylophagorum to monoterpene hydrocarbons supports the hypothesis that the constitutive odors from the first trophic level may not play an important role in host foraging by this species (Pettersson et al. 2000).
The EAG-active oxygenated monoterpenes associated with the habitat of S. guani’s hosts have a variety of possible biological/biochemical origins. They can be produced through transformations of tree constitutive monoterpenes resulting from spontaneous oxidations (Borden et al. 1986), bioconversions by sawyer beetle-associated microbes (Leufvén 1987), or detoxification processes of feeding beetle larvae or adults (White et al. 1980). S. guani has been widely used in China as a biological control agent the exotic pest M. alternates, a primary vector of the pinewood nematode, Bursaphelenchus xylophilus (Ding et al. 2001). This parasitoid may also prove useful in managing other cerambycid insects through efforts aimed at augmentation and conservation of this species. Increased knowledge about the host location mechanisms and olfactory cues involved in these processes should assist in efforts to maximize the potential of S. guani as a control agent of its beetle hosts.
References
Borden JH, Hunt DWA, Miller DR, Slessor KN (1986) Orientation in forest Coleoptera: an uncertain outcome of responses by individual beetles to variable stimuli. In: Payne TL, Birch MC, Kennedy CE (eds) Mechanisms in insect olfaction. Oxford University Press, Oxford, pp 97–109
Bruce TJA, Wadhams LJ, Woodcock CM (2005) Insect host location: a volatile situation. Trend Plant Sci 10:270–274
Ding Y, Lu C, Han B, Pu H, Wu M (2001) Relationship among growth potential of pine, population density of Monochamus alternatus, and pathogenicity of Bursaphelenchus xylophilus. Chin J Appl Ecol 12:351–354
Evans HE (1964) A synopsis of the American Bethylidae (Hymenoptera: Aculeata). Bull Mus Comp Zool 132:1–222
Fan JT, Sun JH, Shi J (2007) Attraction of the Japanese pine sawyer, Monochamus alternatus, to volatiles from stressed host in China. Ann For Sci 64:67–71
Fawcett TW, Johnstone RA (2003) Mate choice in the face of costly competition. Behav Ecol 14:771–779
Gauld ID, Hanson PE (1995) The chrysidoid families. In: Hanson PE, Gauld ID (eds) The Hymenoptera of Costa Rica. Oxford University Press, Oxford, pp 464–503
Harrison GD (1987) Host-plant discrimination and evolution of feeding preference in the Colorado potato beetle Leptinotarsa decemlineata. Physiol Entomol 12:407–415
Hart SJ (2013) The early stage of wood decay: wood/fungus interaction and its attraction to xylophagous Coleoptera, especially cerambycids and their hymenopteran parasitoids. PhD Dissertation. Imperial College, London
Held DW, Gonsiska P, Potter DA (2003) Evaluating companion planting and non-host masking odors for protecting roses from the Japanese beetle (Coleoptera: Scarabaeidae). J Econ Entomol 96:81–87
Lauzière I, Brodeur J, Pérez-Lachaud G (2001) Host stage selection and suitability in Cephalonomia stephanoderis Betrem (Hymenoptera: Bethylidae), a parasitoid of the coffee berry borer. Biol Control 21:128–133
Leufvén A (1987) Chemical interactions between the spruce bark beetle (Ips typographus), its host tree (Picea abies), and associated microorganisms (especially yeasts) during tree colonisation. PhD Dissertation. Department of Chemical Ecology, Göteborg University, Sweden
Li L, Miller DR, Sun JH (2009) The influence of prior experience on preference and performance of a cryptoparasitoid Scleroderma guani (Hymenoptera: Bethylidae) on beetle hosts. Ecol Entomol 34:725–734
Li L, Wei W, Liu ZD, Sun JH (2010) Host adaptation of a gregarious parasitoid Sclerodermus harmandi in artificial rearing. BioControl 55:465–472
Lu D, Li X, Liu X, Zhang Q (2007) Identification and molecular cloning of putative odorant-binding proteins and chemosensory protein from the Bethylid wasp, Scleroderma guani Xiao et Wu. J Chem Ecol 33:1359–1375
Pettersson E (2001a) Volatile attractants for three Pteromalid parasitoids attacking concealed spruce bark beetles. Chemoecology 11:89–95
Pettersson E (2001b) Volatiles from potential hosts of Rhopalicus tutela a bark beetle parasitoid. J Chem Ecol 27:2219–2231
Pettersson E, Sullivan B, Anderson P, Berisford C, Birgersson G (2000) Odor perception in the bark beetle parasitoid Roptrocerus xylophagorum exposed to host associated volatiles. J Chem Ecol 26:2507–2525
Randlkofer B, Obermaier E, Hilker M, Meiners T (2010) Vegetation complexity—the influence of plant species diversity and plant structures on plant chemical complexity and arthropods. Basic Appl Ecol 11:383–395
Salom SM, Birgersson G, Payne TL, Berisford CW (1991) Electroantennogram responses of the southern pine beetle parasitoid Dinotiscus dendroctoni (Ashmead) (Hymenoptera: Pteromalidae) to potential semiochemicals. J Chem Ecol 17:2527–2538
Sullivan BT, Berisford CW (2004) Semiochemicals from fungal associates of bark beetles may mediate host location behavior of parasitoids. J Chem Ecol 30:703–717
Wäschke N, Meiners T, Rostas M (2013) Foraging strategies of insect parasitoids in complex chemical environments. In: Wajnberg E, Colazza S (eds) Recent advances in chemical ecology of insect parasitoids, Wiley-Blackwell, UK, pp 193–224
Wei JN, Kang L (2006) Electrophysiological and behavioral responses of a parasitic wasp to host plant volatiles induced by two leaf miner species. Chem Senses 31:467–477
Wei J, Wang L, Zhu J, Zhang S, Nandi OI, Kang L (2007) Plants attract parasitic wasps to defend themselves against insect pests by releasing hexenol. PLoS ONE 2:e852
White RA, Agosin M, Franklin RT, Webb JW (1980) Bark beetle pheromones: evidence for physiological synthesis mechanisms and their ecological implications. Z Angew Entomol 90:255–274
Williams L III, Rodriguez-Saona C, Castle SC, Zhu S (2008) EAG-active herbivore-induced plant volatiles modify behavioral responses and host attack by an egg parasitoid. J Chem Ecol 34:1190–1201
Acknowledgments
We thank Dr. Daniel R. Miller (USDA Forest Service) for his review of an earlier version of the manuscript. Thanks also extend to two anonymous reviewers for their constructive comments which improved the quality of the manuscript greatly. This work was supported by the National Natural Science Foundation of China (31370650 and 31360519), the CAS Knowledge Innovation Key Research Program (KSCX2-EW-05) and the State Key Laboratory of Integrated Management of Pest Insects and Rodents (ChineseIPM1205).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Torsten Meiners.
Rights and permissions
About this article
Cite this article
Li, L., Liu, Z. & Sun, J. Olfactory cues in host and host-plant recognition of a polyphagous ectoparasitoid Scleroderma guani . BioControl 60, 307–316 (2015). https://doi.org/10.1007/s10526-015-9651-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-015-9651-x