Abstract
Dryocosmus kuriphilus is one of the most damaging pests of Castanea spp. Behavioral, chemical, and electrophysiological investigations were employed to examine the role of plant volatiles for host location by this thelytokuos cynipid. Y-tube olfactometer bioassays showed that adult wasps are significantly attracted by C. sativa twigs with at least 1-hr-old mechanical damage. Odors of undamaged host seedlings, intact twigs, and twigs with a fresh mechanical damage were not attractive. Wasps were repelled by plant materials of the non-host Prunus laurocerasus. Fourteen compounds, mainly general green leaf volatiles, were identified in the head-space of attractive host plant twigs by gas chromatography coupled to mass spectrometry. All compounds elicited dose-dependent antennal responses in adult wasps. A synthetic blend comprising all identified compounds in the same ratio as in the attractive host source induced significant positive responses in Y-tube olfactometer bioassays. The study gives a basis for future identification of host plant attractants that could contribute to semiochemical-based monitoring and management practices of this pest.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The chestnut gall wasp Dryocosmus kuriphilus Yasumatsu (Hymenoptera: Cynipidae) is one of the most damaging pests of Castanea spp. (Abe et al., 2007). The species is univoltine and thelytokous (Zhu et al., 2007). Adults lay eggs in chestnut buds in early summer, and larvae remain latent until buds expand the following spring. Larvae induce the formation of leaf and twig galls that disrupt shoot elongation, reduce fruit yield by 50%–75%, and even cause the death of young trees as a result of severe consecutive attacks (Payne et al., 1983; Kato and Hijii, 1997). The effectiveness of chemical control is limited by the cryptic nature of the insect, which is in dormant buds for the majority of its life cycle, and by the difficulty of precisely detecting adult emergence. Moreover, chemical treatments often are impracticable due to the natural distribution of chestnut trees in steep terrain.
Gall-inducing cynipids are host-plant specific (Ronquist and Liljeblad, 2001). The oviposition of cynipids and subsequent gall development can be sensitive to host attributes including genotype, age, size, phenology, and nutritional status (Stone et al., 2002). The compositions of cynipid gall-wasp communities correlate with host plant chemistry (Abrahamson et al., 2003), and there is evidence that galls are manipulated by galling cynipids that enhance their food and protective value (Allison and Schultz, 2005).
Given the specificity of the host plant-cypnid interactions, plant volatiles are expected to play a crucial role in host recognition. Changes in host plant chemistry associated with galls provide cues for mate location by adult males in Antistrophus rufus Gillette (Hymenoptera: Cynipidae) (Tooker et al., 2002; Tooker and Hanks, 2004). Females of the same species respond positively to a four-component blend of plant monoterpenes in Y-tube olfactometer bioassays, thus suggesting that plant volatiles probably serve as an olfactory cue for host plant location (Tooker et al., 2005).
Identification of semiochemicals involved in host location by D. kuriphilus is of interest not only from an ecological perspective but also for development of novel pest control strategies. Host plant cues are particularly important in light of the thelytokous parthenogenesis characteristic of this species and the consequent lack of a sex pheromone system. Attractive semiochemicals could be incorporated into traps to detect adult wasps in new areas and to improve the timing of control measures.
In a preliminary study that tested the hypothesis that chestnut leaf volatiles are perceived by antennae of D. kuriphilus adults, electroantennographic (EAG) recordings showed that these insects can detect a wide range of plant volatiles. In addition, some EAG-active compounds were individually attractive to adult wasps in a Y-tube olfactometer bioassay (Germinara et al., 2009a). To better understand the chemical basis of host plant location by D. kuriphilus and in an attempt to find an attractive blend of host-related volatiles, comprehensive behavioral, chemical, and electrophysiological investigations were carried out.
Methods and Materials
Insects
Chestnut galls containing immature stages of the chestnut gall wasp were collected in an infested orchard near Avellino (southern Italy) during June and July and kept in glass containers at 25 ± 2°C, 55 ± 5% relative humidity, and under natural light conditions. Newly emerged adults were collected daily and kept individually in glass vials (i.d. 2 × 8 cm) covered with a fine mesh net and starved for at least 12 hr prior to behavioral and electrophysiological tests.
Plants
We used the host plant wild Castanea sativa Mill. and the non-host plant Prunus laurocerasus L. (Rosales). One meter long branches were cut from plants grown in a field near the University of Molise in Campobasso, Italy and placed individually into 2000-ml glass jars containing water. Branches were used for the experiments within 24 hr of cutting the branch from the tree. For some experiments, C. sativa seedlings about 1-yr-old (8–10 leaves, length 20–30 cm) were supplied by the Fruit Tree Unit of the Agricultural Research Council (Caserta, Italy). All plants used were in the flowering stage (June–July) when infestation by D. kuriphilus occurs in the field.
Chemicals
(E)-2-hexenal, hexyl acetate, (Z)-3-hexenyl acetate, (E)-2-hexenyl acetate, hexanol, (E)-3-hexenol, (Z)-3-hexenol, (E)-2-hexenol, (Z)-3-hexenyl butyrate, (E)-2-hexenyl butyrate, (E)-caryophyllene, (Z)-3-hexenyl hexanoate, methyl salicylate, geranyl acetate were purchased from Sigma-Aldrich (Milan, Italy), and were 97–99% pure. (E)-caryophyllene was an enantiomeric mixture.
Olfactometer Bioassays
A glass Y-tube olfactometer (each arm 23 cm long at 75°C angle, stem 30 cm long, 3.0 cm i.d.) was used to examine the behavioral responses of D. kuriphilus adults to plant volatiles. Each arm of the Y-tube was connected to a glass cylinder (9 cm long and 3.0 cm i.d.) as an odor source container. The apparatus was put into an observation chamber (90 × 75 × 40 cm) and illuminated from above by two 36-W cool white fluorescent lamps providing uniform lighting (2500 lux) inside the tube. A purified (activated charcoal) and humidified airflow maintained at 60 ml/min by a flowmeter was pumped through each arm.
In order to determine an attractive source of host plant volatiles, four dual-choice experiments were performed with host plant materials: (1) host plant seedlings vs. clean air; (2) intact host plant twigs with two leaves and three buds vs. clean air; (3) host plant twigs 0–60 min after mechanical damage (fresh damage) vs. clean air; (4) host plant twigs 60–120 min after mechanical damage (old damage) vs. clean air.
To prevent contamination of volatiles emitted by host plant seedlings, the surface of the soil around the stem of the plant was covered with pieces of aluminum foil. Individual seedlings were equilibrated for 1 hr at room temperature in a two-part glass vessel 60 cm high and with a diameter of 32 cm. The upper part of the vessel was provided with a Drechsel-bottle head with the inlet connected to the flowmeter and the outlet to an arm of the olfactometer. An empty glass vessel was used as a control.
To test the specificity of the host plant signal, three additional tests were carried out: (1) intact non-host plant twigs vs. clean air; (2) non-host plant twigs with old mechanical damage vs. clean air; (3) host plant twigs with old mechanical damage vs. non-host plant twigs with old mechanical damage. Twigs used for the experiments were taken from the cut branches just before the start of the experiment, and their cut ends were placed in glass tubes containing water to maintain the physiological water content (Tasin et al., 2005). Each twig (ca. 2 g) included at least two leaves and three buds. Mechanical damage was achieved by cutting twigs into pieces (0.5 cm wide) (van Tol et al., 2002).
We determined the response of adult wasps to a synthetic blend comprising 14 identified compounds mixed in the same ratio as in the head-space sample of host plant twigs with old mechanical damage. In this case, the odor chamber contained a filter paper disk (0.5 cm2) loaded with 5 μl of a 0.01, 0.1 or 1 M synthetic volatile blend dissolved in mineral oil (Sigma-Aldrich, Milan, Italy), while the control chamber contained a filter paper disk loaded with 5 μl of mineral oil. Both disks were suspended in the center of the cross section.
Individual chestnut wasps were released at the open end of the stem. Each experiment lasted 10 min. A choice was recorded when the insect moved 3 cm up an arm of the Y-tube crossing the decision line (marked on both arms) and remained beyond that line for more then 30 sec. The time spent by test insects in each arm also was recorded.
After 5 individuals were tested, the olfactometer was cleaned with acetone and dried (200°C for 30 min). Treatments between arms were switched to avoid position bias. For each test stimulus at least 30 wasps were tested.
Sampling of Volatiles
Volatiles emitted by C. sativa twigs were collected by Solid Phase Microextraction (SPME) using a manual SPME holder with a polydimethylsiloxane coated fiber (100 μm) (Supelco, Bellefonte, PA). A chestnut twig similar to those used for behavioral tests was cut into pieces (0.5 cm) on aluminum foil and was used immediately for the extraction, or was kept exposed to air at room temperature (23 ± 1°C) for 60 min. Plant material then was enclosed in a 25 ml screw top mini flask with a PTFE thread Mininert™ Valve (Sigma-Aldrich, Milan, Italy) and a SPME was exposed to the head-space of the sample. Individual analyses were standardized following the method for volatile organic compounds described in Bech et al. (2008). The method parameters were P=permeation time = 1 min, E=exposure time = 60 min, S=storage time = 30 sec, and T=thermal desorption of SPME fiber time = 15 min. Before each extraction, the fiber was conditioned for 30 min in a GC injection port at 250°C. For both fresh and old mechanical damage, samples from five different plants were analyzed.
Coupled Gas Chromatography-Mass Spectrometry (GC-MS)
SPME extracts were analyzed in a Fisons 8000 series gas chromatograph coupled with a Fisons MD 800 quadrupole mass spectrometer using a CPWAX 52 CB capillary column (30 m × 0.32 mm i.d., 0.25 μm film thickness) (Ruther, 2000). The oven temperature was kept at 40°C for 3 min, programmed to rise 3°C min−1 to 230°C and hold at 230°C for 30 min. Injection temperature was kept at 240°C. Helium was used as carrier gas at a flow rate of 2 ml min−1. Mass spectra were recorded in the electron impact mode (EI) at 70 eV and at a source temperature of 200°C. Mass range was from 25 to 450 m/z at a scan time of 0.9 sec and an interscan delay of 0.1 sec. Compounds were identified tentatively by comparison of mass spectra with those of authentic samples in the NIST database (2005) and confirmed by comparison of mass spectra and retention times with those of authentic reference compounds. In order to determine the relative composition of plant extracts, for each analysis the sum of peak areas was set to 100% and the mean contribution of each individual compound was calculated.
Electroantennography (EAG)
Electrophysiological activity of identified compounds to adult wasps was determined by EAG (Germinara et al., 2009a). The head of a 1-d-old specimen was cut off and a glass pipette filled with 0.1 M KCl solution, which served as the neutral electrode, was inserted into its base. The distal end of the last antennal segment was put in contact with the end of a similar pipette (0.2–0.3 mm i.d.) serving as the recording electrode. AgCl-coated silver wires were used to maintain the electrical continuity between the antennal preparation and an AC/DC UN-6 amplifier in DC mode connected to a PC equipped with the EAG 2.0 program (Syntech Laboratories, Hilversum, The Netherlands). A stream of charcoal-filtered humidified air (500 ml/min) was directed constantly onto the antenna through a stainless steel delivery tube (1 cm i. d.) with the outlet positioned at approximately 1 cm from the antenna.
Just before the experiment, solutions of authentic standards of identified compounds were applied to filter paper (Whatman No. 1) strips (1 cm2) inserted in a Pasteur pipette (15 cm long) and used as an odor cartridge. For each compound, seven doses ranging from 10−3 to 40 μM in 10 μl mineral oil were tested. Over 1 sec, 2.5 cm3 of vapor from an odor cartridge were blown by a disposable syringe into the air stream flowing over the antennal preparation. Stimuli were applied in ascending doses. Control (10 μl of mineral oil) and standard stimuli (10 μl of 1 M Z3-hexen-1-ol solution) were applied at the beginning of the experiment, and after each group of 3 randomly selected odors. Intervals between stimuli were 30 sec. For each compound, EAG responses were recorded from 5 antennae of different insects.
Data Analysis
The amplitude (mV) of the EAG response to each test stimulus was adjusted to compensate for solvent and/or mechanosensory artefacts by subtracting the mean EAG response of the two nearest mineral oil controls (Raguso and Light, 1998). To compensate for the decrease of the antennal responsiveness during the experiment, the resulting EAG amplitude was corrected according to the EAG response to the standard stimulus (Den Otter et al., 1991).
In dose-response curves, the activation threshold was considered to be the lowest dose at which the lower limit of the standard error of the mean response was greater than the upper limit of the standard error for the lowest dilution tested (Sant’ana and Dickens, 1998). Saturation level was taken as the lowest dose at which the mean response was equal to or less than the previous dose (Germinara et al., 2009b).
A χ2 test was employed to determine the significance of differences between the number of wasps choosing the treatment or control arm of the olfactometer. A paired-sample t-test was used to analyze the differences between the time spent by wasps in each arm.
Results
Behavioral Response to Plant Materials
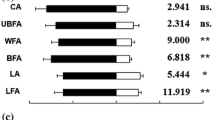
In behavioral tests with plant materials, the percentage of adult wasps responding was generally high. Odors from undamaged host seedlings, intact twigs, and twigs with fresh mechanical damage did not elicit a significant response from adult wasps measured either as first choice or as time spent in the treatment arm when clean air was the alternative (Table 1). Insects presented with host twigs with old mechanical damage vs. clean-air control exhibited a significant preference for the damaged twigs (χ2 = 10.80, d.f. = 1, P = 0.001) and spent significantly more time in the treatment arm (Table 1).
When insects could choose between non-host intact twigs and clean air, there was no preference but they spent significantly more time in the control arm (Table 1). Moreover, insects presented with non-host plant twigs with old mechanical damage vs. clean air control significantly preferred and spent more time in the control arm (Table 1). A significant positive response to host twigs with old mechanical damage was confirmed also in the presence of non-host twigs with a similar damage (Table 1).
Chemical Analyses
GC-MS analysis revealed 15 volatile compounds in the SPME extracts from damaged chestnut twigs (Table 2). Fourteen of them were confirmed by comparison of retention times and mass spectra to those of authentic samples, while one compound remained unidentified. Chemical analysis showed differences between the volatiles collected from chestnut twigs with fresh or old mechanical damage. The volatile blend collected from freshly damaged twigs lacked the green leaf aldehyde (E)-2-hexenal. One hour after mechanical damage, there was a significant reduction in the relative amount of (Z)-3-hexenyl acetate and a significant increase in that of hexanol and (E)-2-hexenol (Table 2).
EAG Dose-Response Characterization
The sensitivity of adult D. kuriphilus antennae toward increasing concentrations of authentic standards of identified compounds are reported in Fig. 1. For all compounds, the amplitude of the EAG response increased with dose. The lowest activation thresholds were recorded for (E)-2-hexenal and (E)-2-hexenyl acetate at the 0.01 μM dose. The activation threshold for the remaining compounds was 0.1 μM for hexanol, (Z)-3-hexenol, (E)-caryophyllene, and geranyl acetate, 1 μM for hexyl acetate, (Z)-3-hexenyl acetate, (E)-3-hexenol, (Z)-3-hexenyl butyrate, (E)-2-hexenyl butyrate, and methyl salicylate, and 10 and 20 μM for (E)-2-hexenol and (Z)-3-hexenyl hexanoate, respectively.
The amplitude of the EAG response to hexyl acetate, (E)-2-hexenyl acetate, hexanol, (E)-3-hexenol, (Z)-3-hexenol, (E)-2-hexenol, (Z)-3-hexenyl butyrate, (E)-2-hexenyl butyrate, and methyl salicylate decreased from 20 to 40 μM doses indicating saturation of receptors at the lowest dose.
Behavioral Response to a Synthetic Blend
The behavioral responses of adult wasps to different doses of a multicomponent blend comprising the 14 commercially available authentic standards in the same ratio as in the head-space sample of host plant twigs with old mechanical damage are reported in Table 3.
At the 0.05 and 0.5 μM doses, a significant attraction of adult wasps, either as first choice or time spent in the treatment arm of the olfactometer was recorded. At the 5 μM dose, adult wasps did not show any significant preference for either the treatment or the control arm.
Discussion
In Y-tube olfactometer bioassays, adults of the chestnut gall wasp were attracted by odors of leaf- and bud-bearing C. sativa twigs with at least one-h-old mechanical damage. Odors of undamaged host seedlings, intact twigs, and twigs with fresh mechanical damage were unattractive. Moreover, insects were able to discriminate between host and non-host plant materials, suggesting specificity of host-plant chemical signals. Chemical analysis showed that the blend of volatiles emitted by artificially damaged C. sativa twigs changed significantly over time. From the first to the second hour after mechanical damage, the additional presence of (E)-2-hexenal was detected. In addition, there was a significant decrease in the relative proportion of (Z)-3-hexenyl acetate and a significant increase in that of hexanol and of (E)-2-hexenol. These changes might account for the attractiveness of old mechanically damaged twigs compared to the neutral effect of the freshly damaged ones.
The majority of compounds identified in the head-space of attractive host-plant samples were C6 alcohols, esters, and the aldehyde (E)-2-hexenal. These so-called green leaf volatiles (GLVs) contribute to the “green odor” of the leaves of numerous plant species. GLVs may be produced constitutively or induced by leaf damage caused abiotically or by herbivory (Visser, 1986; Loughrin et al., 1996; Turlings et al., 1998; Ruther et al., 2002) and by light-dark transitions (Graus et al., 2004; Chamberlain et al., 2006). Additional components were the terpenoids (E)-caryophyllene and geranyl acetate and the aromatic methyl salicylate. These compounds are released in response to plant damage (Takabayashi et al., 1994; Bolter et al., 1997; Hoballah and Turlings, 2005).
Volatile compounds emitted upon mechanical and/or herbivore damage are important in the host selection process for several phytophagous insects (Loughrin et al., 1996; Dickens, 2000; Kalberer et al., 2001; van Tol et al., 2002). In some cases, a neutral effect of undamaged host plant material also has been recorded (Bolter et al., 1997; Ruther et al., 2000; van Tol et al., 2004). The advantages of adult D. kuriphilus being attracted to a damaged plant remain unclear.
All identified compounds elicited EAG dose-dependent responses with different activation thresholds. At doses from 0.05 to 0.5 μM, a synthetic blend comprised of all 14 EAG-active volatiles in the same ratio as in the head-space of the attractive host plant materials elicited a significant attraction in adult wasps, suggesting that these compounds are involved in locating host plants. One compound could not be identified and therefore it was not included in the EAG and behavioral tests. However, it seems unlikely that this compound may play a role in host plant location since its relative proportion in the natural blend did not change significantly over time.
In a previous study, (E)-2-hexenal did not elicit a positive response in the adult wasps (Germinara et al., 2009a), therefore, the additional presence of this compound in the odor blend of old mechanically damaged twigs could not explain its attractiveness as compared to the blend of freshly damaged twigs. This, along with the ubiquitous nature of all identified chestnut volatiles, suggests that host location by the chestnut wasp is more likely mediated by a host-specific ratio of ubiquitous volatiles rather than by a single compound. The exploitation of ubiquitous compounds even by the monophagous D. kuriphilus supports the hypothesis of ratio-specific odor recognition as the most prevalent mechanism mediating host plant location by phytophagous insects (Visser, 1986; Bruce et al., 2005).
Adult wasps were significantly repelled by non-host volatiles, indicating that these compounds may act as antagonists. This evidence is in accordance with previous reports on the tendency of specialists to be deterred (Bernays et al., 2000) or repelled (Linn et al., 2005) by non-host compounds. The capability of the adult wasps to actively avoid non-host plant volatiles and not just to passively ignore them could be important for optimizing host location, and it could have important evolutionary consequences (Forbes et al., 2005; Feder and Forbes, 2007). Moreover, repellent compounds from non-host plants may have potential for the use in the control of insect pests (Ukeh et al., 2010).
Many previous studies have shown that only a small number of host plant volatiles out of all of those electrophysiologically active to an insect are involved in host location (Rojas, 1999; Dickens, 2000; Fraser et al., 2004; Tasin et al., 2006), suggesting that not all 14 components of the synthetic blend are necessary for attraction. Laboratory and field studies examining the behavioral responses of D. kuriphilus with various dosages of individual synthetic compounds and their mixtures are in progress to find out those that are crucial in host plant location. Identification of such compounds may contribute to the development of effective monitoring and management tools for this pest.
References
Abe, Y., Melika, G., and Stone, G. N. 2007. The diversity and phylogeography of cynipid gallwasps (Hymenoptera: Cynipidae) of the Oriental and eastern Palaearctic regions, and their associated communities. Orient. Insects 41:169–212.
Abrahamson, W. G., Hunter, M. D., Melika, G., and Price, P. W. 2003. Cynipid gall-wasp communities correlate with oak chemistry. J. Chem. Ecol. 29:209–223.
Allison, S. D. and Schultz, J. C. 2005. Biochemical responses of chestnut oak to a galling cynipid. J. Chem. Ecol. 31:151–166.
Bech, J. J., Smith, L., and Merrill, G. B. 2008. In situ volatile collection, analysis, and comparison of three Centaurea species and their relationship to biocontrol with herbivory insects. J. Agric. Food Chem. 56:2759–2764.
Bernays, E. A., Oppenheim, S., Chapman, R. F., Kwon, H., and Gould, F. 2000. Taste sensitivity of insect herbivores to deterrents is greater in specialists than in generalists: a behavioural test of the hypothesis with two closely related catarpillars. J. Chem. Ecol. 26:547–563.
Bolter, C. J., Dicke, M., van Loon, J. J. A., Visser, J. H., and Posthumus, M. A. 1997. Attraction of Colorado potato beetle to herbivore-damaged plants during herbivory and after its termination. J. Chem. Ecol. 23:1003–1023.
Bruce, T. J. A., Wadhams, L. J., and Woodcock, C. M. 2005. Insect host location: a volatile situation. Trends Plant Sci. 10:269–274.
Chamberlain, K., Khan, Z. R., Pickett, J. A., Toshova, T., and Wadhams, L. J. 2006. Diel periodicity in the production of green leaf volatiles by wild and cultivated host plants of stemborer moths, Chilo partellus and Busseola fusca. J. Chem. Ecol. 32:565–577.
Den Otter, J. C., Tchicaya, T., and Schutte, A. M. 1991. Effects of age, sex and hunger on the antennal olfactory sensitivity of tsetse flies. Physiol. Entomol. 16:173–182.
Dickens, J. C. 2000. Orientation of Colorado potato beetle to natural and synthetic blends of volatiles emitted by potato plants. Agric. For. Entomol. 2:167–172.
Feder, J. L., and Forbes, A. A. 2007. Habitat avoidance and speciation for phytophagous insect specialists. Funct. Ecol. 21:585–597.
Forbes, A. A., Fisher, J., and Feder, J. L. 2005. Habitat avoidance: overlooking an important aspect of host-specific mating and sympatric speciation? Evolution 59:1552–1559.
Fraser, A. M., Mechaber, L. W., and Hildebrand, J. G. 2004. Electroantennographic and behavioural responses of the sphinx moth Manduca sexta to host plant headspace volatiles. J. Chem. Ecol. 29:1813–1833.
Germinara, G. S., De Cristofaro, A., Paparatti, B., Speranza, S., Stacchiotti, M., and Rotundo, G. 2009a. Electroantennographic responses of Dryocosmus kuriphilus to Castanea sativa leaf volatiles. Acta Hort. 844:387–394.
Germinara, G. S., De Cristofaro, A., and Rotundo, G. 2009b. Antennal olfactory responses to individual cereal volatiles in Theocolax elegans (Westwood) (Hymenoptera: Pteromalidae). J. Stored Prod. Res. 45:195–200.
Graus, M., Schnitzler, J.-P., Hansel, A., Cojocariu, C., Rennenberg, H., Wisthaler, A., and Kreuzwieser, J. 2004. Transient release of oxygenated volatile organic compounds during light-dark transitions in grey poplar leaves. Plant Physiol. 135:1967–1975.
Hoballah, M. E. and Turlings, C.J. 2005. The role of fresh versus old leaf damage in the attraction of parasitic wasps to herbivore-induced maize volatiles. J. Chem. Ecol. 31:2003–2018.
Kalberer, N. M., Turlings, T. C. J., and Rahier, M. 2001. Attraction of a leaf beetle (Oreina cacaliae) to damaged host plants. J. Chem. Ecol. 27:647–661.
Kato, K. and Hijii, N. 1997. Effects of gall formation by Dryocosmus kuriphilus Yasumatsu (Hym., Cynipidae) on the growth of chestnut trees. J. Appl. Entomol. 121:9–15.
Linn, C. Jr., Nojima, S., and Roelofs, W. 2005. Antagonistic effects of nonhost fruit volatiles on chemically-mediated discrimination of host fruit by Rhagoletis pomonella flies infesting apple, hawthorn (Crataegus spp.) and flowering dogwood (Cornus florida). Ent. Exp. Appl. 114:97–105.
Loughrin, J. H., Potter, D. A., Hamilton-Kemp, T. R., and Byers, M. E. 1996. Role of feeding-induced plant volatiles in aggregative behaviour of the Japanese beetle (Coleoptera: Scarabaeidae). Environ. Entomol. 25:1188–1191.
Payne, J. A., Jaynes, R. A., and Kays, S. J. 1983. Chinese chestnut production in the United States: practice, problems and possible solutions. Econ. Bot. 37:187–200.
Raguso, R. A. and Light, D. M. 1998. Electroantennogram responses of male Sphinx perelegans hawkmoths to floral and ‘green leaf volatiles’. Ent. Exp. Appl. 86:287–293.
Rojas, C. J. 1999. Electrophysiological and behavioural responses of the cabbage moth to plant volatiles. J. Chem. Ecol. 25:1867–1883.
Ronquist, F. and Liljeblad, J. 2001. Evolution of the gall wasp-host plant association. Evolution 55:2503–2522.
Ruther, J. 2000. Retention index database for identification of general green leaf volatiles in plants by coupled capillary gas chromatography-mass spectrometry. J. Chromatogr. A 890:313–319.
Ruther, J., Reinecke, A., Thiemann, K., Tolasch, T., Francke, W., and Hilker, M. 2000. Mate finding in the forest cockchafer, Melolonta hippocastani, mediated by volatiles from plants and females. Physiol. Entomol. 25:172–179.
Ruther, J., Reinecke, A., and Hilker, M. 2002. Plant volatiles in the sexual communication of Melolontha hippocastani: response towards time-dependent bouquets and novel function of (Z)-3-hexen-1-ol as a sexual kairomones. Ecol. Entomol. 27:76–83.
Sant’ana, J. and Dickens, J. C. 1998. Comparative electrophysiological studies of olfaction in predaceous bugs, Podisus maculiventris and P. nigrispinus. J. Chem. Ecol. 24:965–984.
Stone, G. N., Schönrogge, K., Atkinson, R. J., Bellido, D., and Pujade-Villar, J. 2002. The population biology of oak gall wasps (Hymenoptera: Cynipidae). Annu. Rev. Entomol. 47:633–668.
Takabayashi, J., Dicke, M., and Posthumus, M. A. 1994. Volatile herbivore-induced terpenoids in plant-mite interactions: variation caused by biotic and abiotic factors. J. Chem. Ecol. 20:1329–1354.
Tasin, M., Anfora, G., Ioriatti, C., Carlin, S., De Cristofaro, A., Schmidt, S., Bengtsson, M., Versini, G., and Witzgall, P. 2005. Antennal and behavioural responses of grapevine moth Lobesia botrana females to volatiles from grapevine. J. Chem. Ecol. 31:77–87.
Tasin, M., Backman, A. C., Bengtsson, M., Ioriatti, C., and Witzgall, P. 2006. Essential host plant cues in the grapevine moth. Naturwissenschaften 93:141–144.
Tooker, J. F. and Hanks, L. M. 2004. Stereochemistry of host plant monoterpenes as mate location cues for the gall wasp Antistrophus rufus. J. Chem. Ecol. 30:473–477.
Tooker, J. F., König, W. A., and Hanks, L. M. 2002. Altered host plant volatiles are proxies for sex pheromones in the gall wasp Antistrophus rufus. Proc. Natl. Acad. Sci. U.S.A. 99:15486–15491.
Tooker, J. F., Crumrin, A. L., and Hanks, L. M. 2005. Plant volatiles are behavioural cues for adult females of the gall wasp. Chemoecology 15:85–88.
Turlings, T.C.J., Urs, B., Lengwiler, U.B., Bernasconi, M.L., and Wechsler, D. 1998. Timing of induced volatile emissions in maize seedlings. Planta 207:146–152.
Ukeh, D. A., Birkett, M. A., Bruce, T. J. A., Allan, E. J., Pickett, J. A., and Mordue (Luntz) A. J. 2010. Behavioural responses of the maize weevil, Sitophilus zeamais, to host plant (stored-grain) and non-host plant volatiles. Pest Manag. Sci. 66:44–50.
van Tol, R. W. H. M., Visser, J. H., and Sabelis, M. W. 2002. Olfactory responses of the vine weevil, Otiorhynchus sulcatus, to tree odours. Physiol. Entomol. 27:213–222.
van Tol, R. W. H. M., Visser, J. H., and Sabelis, M. W. 2004. Behavioural responses of the vine weevil, Otiorhyncus sulcatus, to semiochemicals from conspecifics, Othiorhyncus salicicola, and host plants. Ent. Exp. Appl. 110:145–150.
Visser, J. H. 1986. Host odor perception in phytophagous insects. Annu. Rev. Entomol. 31:121–144.
Zhu, D-H., He, Y-Y., Fan, Y-S., Ma, M-Y., and Peng, D-L. 2007. Negative evidence of parthenogenesis induction by Wolbachia in a gallwasp species, Dryocosmus kuriphilus. Ent. Exp. Appl. 124:279–284.
Acknowledgments
This research was partly supported by the Campania Region, Contribution number 28/08 of the research program: “Studio per il controllo ecocompatibile del Cinipide del castagno”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Germinara, G.S., De Cristofaro, A. & Rotundo, G. Chemical Cues for Host Location by the Chestnut Gall Wasp, Dryocosmus kuriphilus . J Chem Ecol 37, 49–56 (2011). https://doi.org/10.1007/s10886-010-9893-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10886-010-9893-0