Abstract
A series of environmentally friendly and sustainable polyurethanes using epoxy soybean oil as feedstock were synthesized with the introduction of double-decker silsesquioxane. Feature is that through the two-step polymerization, double-decker octaphenylsilsesquioxanetetraol was added to partially replace 1,4-butanediol acting as chain extender, and petroleum-based polyol was effectively replaced for polyurethane synthesis. On top of that, POSS tetraol was prepared and characterized by 1H NMR and MALDI-TOF MS. As for the organic–inorganic hybrid nanocomposites, their structures and properties were investigated by FTIR, DSC, TGA, SEM, tensile test techniques, and static contact angle. DSC analysis showed that covalent incorporation of POSS into the PU network would increase the glass transition temperature (T g) of the systems. TG analysis demonstrated that the hybrid nanocomposites were indeed more oxidative thermal stable, compared to virgin polyurethane especially at high temperature. SEM revealed that both nano- and micro-sized POSS aggregates were shown to be dispersed heterogeneously in the polyurethane matrix, despite the expectation to be dispersed or corporate into molecular chains by chemical bonding between OH and NCO. According to the tensile test results, POSS-containing nanocomposites exhibited an increased modulus with an increasing POSS concentration at low POSS content, and with high loading, these values would decline. The results of the static contact angles revealed that the hydrophobicity of the hybrid material was significantly improved with the inclusion of POSS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polyhedral oligomeric silsesquioxanes (POSSs), having the empirical formula (RSiO1.5)n, represent a class of rather versatile building blocks with the three-dimensional structure, which are characterized by the inner inorganic cage-like Si–O framework and external organic substituents that can feature a range of polar or nonpolar functional groups [1]. Generally, the synthetic strategies of POSS are based on the hydrolysis and condensation of trifunctional silsesquioxanes, substitution reactions with retention of the siloxane cage, corner-capping reactions, and the functionalization of the performed POSS cage [2]. In addition, attributing to the readily functionalized approach to POSS [3–7], derivatives possessing various actively functional groups and unique performance can be prepared with one or more reactive functional groups. The enhancement at molecular level through maximizing the surface area and chemical interaction with polymer matrix make POSS widely applied in the design and synthesis of novel organic–inorganic hybrid nanomaterials exhibiting enhanced thermal [8, 9], mechanical properties (e.g., strength, modulus, rigidity), nonflammability [10–12], oxidative resistance [13], and excellent dielectric properties [14–16] via the synergism of organic and inorganic components. For such nanocomposites, currently, synthetic procedures are mainly based on simple physical blending or copolymerization of POSS into polymer matrix. For the latter, it is widespread to introduce POSS into a polymer matrix by covalently bonding as a pendent group [17–20] or a nanocross-linker [21–23]. As a result, a series of POSS-containing nanocomposites were achieved like polystyrene–POSS copolymers [24], polymethacrylate–POSS copolymers [21, 25], norbornyl–POSS copolymers [26], polyethylene–POSS copolymers [27], polypropylene–POSS copolymers [28, 29], poly(ethylene oxide)–POSS copolymers [30], epoxy resin–POSS copolymers [31, 32], polyamide–POSS copolymers [33, 34], polyurethane–POSS copolymers [11, 35, 36], polyimide–POSS copolymers [37, 38], and phenolic–POSS copolymers [2, 39].
Due to their versatility, polyurethanes have been tailored to meet significant applications in coatings, adhesives, sealants, elastomers as well as other fields. However, to endow these materials with excellent and special performance for extraordinary application, a growing number of work have been conducted such as modification with silicon-containing compounds including polysiloxanes and POSS. Raftopoulos and coauthors [36] prepared PU/POSS nanocomposites with 1,2-propanediol isobutyl POSS (PHIPOSS) and aminoethylaminopropyl isobutyl POSS (DIAPOSS) tethered by urethane or urea linkages on the hard segments of polyurethane chains to achieve T g increasing materials. Markovic et al. [40] synthesized a series of polyurethane hybrids octakis (m-isoprenyl-a,a´-dimethylbenzylisocyanato dimethylsiloxy) octasilsesquioxane (Q8M8) as a crosslinking agent. Mather and coauthors [41] controlled the molar ratio of PCL:POSS to obtain poly(ɛ-caprolactone)-POSS multiblock thermoplastic polyurethanes. To my best knowledge, other POSSs with active groups such as amino-group and hydroxyl-group were also successfully introduced to polyurethane chains to acquire high-performance materials [5, 42–44].
In recent years, few investigations have been done on the polyurethane/POSS hybrids with respect to sustainable resources. In order to better solve global emergency problems and the shortcoming of pure nature-based polyurethane, further studies are necessary to conducted. Therefore, the purpose of this paper is to propose a synthetic method of novel sustainable polyurethanes modified by POSS. Moreover, we also discussed the thermal and mechanical properties as well as the morphology and surface hydrophobicity of these hybrids after modification. The strategy may hold great promise for future high-end uses of soybean oil-based polyurethane.
Materials and methods
Materials
Phenyltrimethoxysilane (98 %) was purchased from Xiya Reagent Co. (Chengdu China) and used without further purification. Epoxy soybean oil (ESO) was obtained from Hairma Chemical (GZ) Ltd., China. Isophorone diisocyanate (IPDI; Wuxi East Grace Electronic Material Technology Co., Ltd.), stannous octoate (Shanghai Reagent Co.), and 1,4-butanediol (BDO; Aldrich) were purchased to prepare polyurethanes. Prior to use, reagents such as ethyl acetate (EtAc), tetrahydrofuran (THF), and 1,4-butanediol (BDO) need to be dehydrated and stored in the presence of 4A molecular sieves. Unless specially indicated, other reagents in this article were purchased from Shanghai Reagent Co., China and used as received.
Synthesis
Synthesis of double-decker octaphenylsilsesquioxanetetraol (DDT8OH)
The synthesis of octaphenylsilsesquioxane underwent a two-step procedure. Firstly, Octaphenyldicycloocatasiloxane tetrasodium silanolate (Na4O14Si8(C6H5)8) was prepared according to the method reported by Kakimoto and coauthors [45].To prepare DDT8OH, the hydrolysis reaction of Na4O14Si8(C6H5)8 was carried out by following the literature reported by Kazuhiro Yoshida et al. with slight modification. Typically, Na4O14Si8(C6H5)8 (3 g, 2.6 mmol) and tetrahydrofuran (30 mL) were charged into a flask equipped with magnetic stirrer and a nitrogen bubbler. Thereafter, acetic acid (2.4 g) was quickly added to maintain a small nitrogen flow and temperature below 30 °C. After stirring for 3.5 h, deionized water (20 g) was added and the stirring was kept for another 1 h. Remove the solvent with the rotary evaporation and then add 20 mL chloroform. Then the solution was transferred into a separating funnel and washed with the saturated sodium hydrogen carbonate solution and deionized water to obtain a neutral solution. After all the programs, the organic layer was dried on anhydrous magnesium sulfate and condensed under reduced pressure. The crude resultant was purified by passing through a silica gel column to afford white solids.
Prepare and synthesis of soybean oil-based polyurethane
To synthesized sustainable polyurethane, ESO as feedstock needs to be converted into polyol firstly. In our method, epoxy groups were opened with methanol using tetrafluoroboric aid as catalyst according to the literature reported by Petrovic [46]. Finally, a light-yellow viscous SBO polyol with a OH number 177 mg KOH/g was obtained.
Polyurethanes were synthesized by a standard two-step prepolymer method and a typically synthetic procedure as follows: SBO polyol (4 g, 12.62 mmol OH), EtAc (20 mL), stannous octoate (100 μL), and IPDI (1.98 mL) were charged into a flask equipped with a magnetic stirrer, a condenser, and a nitrogen bubbler. Under a high pure nitrogen atmosphere, the pre-polymerization was performed at 80 °C for 2 h with vigorous stirring. After determining the isocyanate (NCO) content according to ASTM D5155-96, stoichiometric amount of chain extender 1,4-butanediol (BDO) was added and the reaction mixture was kept stirring for another 2 h at 80 °C. Finally, the resultant mixture was poured into a Teflon plate, allowed to evaporate the solvent, and then cured at 50 °C for 12 h.
Synthesis of hybrid polyurethane with DDT8OH as a chain extender
The synthesis of hybrid polyurethane was similar to pure polyurethane at the stage of prepolymer. However, different contents of DDT8OH (See Table 1) dissolved in ethyl acetate were added into the prepolymer solution in the chain extending stage, maintaining stirring and ultrasonic dispersion for 30 min. Thereafter, stoichiometric amount of chain extender 1,4-butanediol (BDO) was added to control the overall molar ratio of hydroxyl groups to isocyanate functional groups to be 1:1.05, followed by determining the isocyanate (NCO) content of the above mixture. The chain extension reaction was also performed at 80 °C for 2 h, and the products were poured into a Teflon plate, allowed to evaporate the solvent, and then cured at 50 °C for 12 h. Consequently, a series of sustainable hybrid polyurethanes were successfully synthesized.
Measurements
1H nuclear magnetic resonance (NMR) spectra were recorded on a 400 MHz Bruker Instruments (model Avance 400, Germany) using Acetone-d6 as solvent and tetramethylsilane (TMS) as internal reference. Matrix-assisted ultraviolet laser desorption/ionization time-of-flight mass spectroscopy (MALDI-TOF MS) experiment was carried out on an ultrafleXtreme MALDI-TOF/TOF mass spectrometer. For analysis, the matrix 2,5-dihydroxybenzoic acid dissolved in THF (50 mg/mL) was mixed with double-decker POSS solution (0.1 mg/mL in 1:1 v/v ratio). The ATR-FTIR spectra were obtained with a Nicolet 6700 infrared spectrometer using a DLaTGS detector. All spectra were carried out between 4000 and 500 cm−1 with averaging 32 scans at a resolution of 4 cm−1. The morphology of the samples was observed by Scanning Electron Microscopy (S-4800, Hitachi), and the samples were fractured with liquid nitrogen and coated with gold prior to use for observation, and to avoid carbonizing organic molecular, the accelerating voltage should be chosen as 1 kV. Thermogravimetric analysis (TGA) and Differential Scanning Calorimetry (DSC) of the hybrids were performed using TA Instruments (TGA/1100SF) and (TA-DSC822e), respectively. The thermogravimetric analysis (TGA) was ramped at 20 °C/min under a nitrogen flow rate of 50 mL/min from 25 to 600 °C. While for DSC analysis, samples (5–10 mg) were loaded in alumina pans and cycled twice through a temperature range of 0–150 °C at a nitrogen flow rate of 50 mL/min. The tensile properties were measured according to ASTM D 882-97 on a tensile tester model and the gage length was 4 mm. The extension rate was 10 mm/min, and five specimens were used for each sample.
Results and discussion
Synthesis
Synthesis of double-decker octaphenylsilsesquioxanetetraol (DDT8OH)
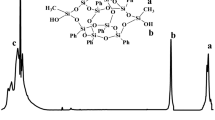
Toward our final research, DDT8OH was synthesized firstly as shown in Scheme 1. According to the literature reported by Kakimoto and coauthors [45], octaphenyldicycloocatasiloxane tetrasodium silanolate [Na4O14Si8(C6H5)8] was synthesized. Thereafter, the hydrolysis reaction between [Na4O14Si8(C6H5)8] and acetic acid was carried out to afford DDT8OH. To verify the successful synthesis of DDT8OH, 1H NMR and MALDI-TOF MS were taken into analysis. In the 1H NMR spectra shown in Fig. 1, the resonance at 2.90 ppm was attributed to the protons of hydroxyl groups, whereas the signals of resonance in the range of 6.60–7.90 ppm were ascribed to the protons of benzene, and the ratio of integral intensity for these protons was measured to be 1:10, which were successfully consistent with the value calculated according to the structure formula. The molecular weight of DDT8OH was measured by MALDI-TOF MS (shown in Fig. 2). It was seen that DDT8OH possessed the molecular weight of 1068 [1091.08–23], which fully agrees with the value calculated according to the structure formula. In short, the results of 1H NMR and MALDI-TOF MS indicate the successful synthesis of DDT8OH.
Synthesis of sustainable polyurethane and hybrid polyurethanes
Hybrid polyurethanes with double-decker POSS in the main chains were synthesized through the reaction between soybean oil polyol and IPDI using DDT8OH and BDO as chain extenders (See Scheme 2). A series of ATR-FTIR were taken to identify the chemical structures of the polyurethanes as shown in Fig. 3. All PUs exhibit absorption peaks around 3340 cm−1 ascribed to the stretching vibration of NH. For Si–O–Si groups in the silsesquioxane cages, the stretching vibration occurred around 1100 cm−1. However, for the PU–POSS hybrids, perhaps the unfortunate overlap between Si–O–Si groups and that of the aliphatic ether lead to small difference among the IR spectra [47].
DSC analysis
DSC analyses of all samples were conducted to evaluate the effect of POSS on the motion of the macromolecular chains, and all T g (the midpoint of the stepwise decrease of the heat flow trace observed during heating) values are reported in Table 2. With the increasing of POSS fraction in the polymer, the T g was slightly shifted to higher temperatures from a T g = 38 °C (pure PU) to a T g = 65 °C (PU/POSS with 12.59 wt% POSS). It was convinced that the relatively rigid structure of the bulky POSS cages would restrict the chain motion, thereby decreasing the free volume, and consequently, higher temperatures were required to provide the requisite thermal energy to cause a glass transition in the hybrids [48]. Besides, according to other reports, with high POSS content, a serious aggregation occurred, which would increase the free volume since the aggregation particles acted as solid lubricant and resulted in the decrease of the T g. Both the two opposite factors contributed to the change of T g, but in the current system, perhaps the former played a leading role (Fig. 4).
Thermal decomposition behavior
Thermal decomposition behaviors of the hybrids were examined with TGA (see Fig. 5) at a heating rate of 20 °C/min in a nitrogen atmosphere. For analysis, we defined T d5 as the onset of decomposition temperature, where the samples showed 5 % weight loss. As it can be seen, the thermal decomposition of PU hybrid was a two-step process. The initial thermal decomposition (5 wt% weight loss) of PU hybrid due to the decomposition of urethane bonds took place above 200 °C, while the second decomposition process attributed to soybean oil chain scission occured later on at 340 °C [49–51]. It can be noted that all polyurethanes show a similar TGA decomposition trend, which demonstrated that the degradation mechanism does not significantly alter despite the introduction of DDT8OH. Clearly, the 5 % weight loss temperatures (T d5) for hybrids were significantly higher than pure polyurethane, especially with 4.48 % POSS content, whose T d5 can be as high as 250 °C, almost 37 °C higher than pure polyurethane. However, beyond 4.48 %, with the increasing concentration of DDT8OH, the onset of decomposition temperature gradually decreased. In this context, two possible facts could be envisaged about this thermal phenomenon. Firstly, the incorporation of DDT8OH, which displayed excellent thermal stability, into polyurethane chains through chemical bonds could significantly retard the movement and scission of molecular chains, consequently improving T d5. Secondly, when POSS content was beyond 4.48 wt%, aggregation between POSS became serious, which would reduce the overall effectiveness in inhibiting chain scission. In fact, both the two opposite effects determined the change of T d5 together. In addition, with high POSS content, TGA curves above 350 °C became flatter which indicated that the decomposition rates during the second decomposition process of the hybrids were slower. It was convinced that the silicon dioxide from the oxidation of POSS at high temperature would be coated on the surface and suppress the release of gaseous products from segmental decomposition, which made the material heat insulating and flame resistance [52, 53]. The fact that superior thermal stability was in accordance with higher char residual (see Table 2) was also proved above conclusion.
Morphology
Scanning electron microscopy (SEM) was performed to identify changes in surface morphology related to the POSS contents. Figure 6 displays a group of SEM images of the samples with 4.48, 7.20, 9.75, and 12.59 wt% POSS, and it could be seen that the images of the composites displayed numerous micron-sized particles on the fracture surfaces. The number as well as the size of particles increased as POSS loading increased. At low POSS content, the morphology of the PU hybrid still displayed relatively smooth structure, except for numerous micron-sized particles about 200 nm dispersing on. However, as POSS loading increased, the dispersed particles gradually seeped into the organic matrix and small particles gradually aggregated into larger ones with a size of 650 nm. The phenomenon occurred because POSS had a strong aggregation effect through physical interactions similar to the reports by A. Strachota et al. [54, 55]. For these strong physical interactions, it might be interpreted that due to benzene ring being hydrophobic and inert to PU chains, the only direction of the nano-cages interacting with the matrix would be the hydroxyls hung on the open face of the POSS cage. In addition, the incorporation of POSS could also change the material morphology as numerous detachment regions and holes appeared (Fig. 6d). On increasing POSS content, particles with relatively large size fell into corresponding holes, which might be derived from the removal of the solvent EtAc enveloped to the space between particles and polymers.
Mechanical properties of PU–POSS hybrids
Tensile tests were carried out at room temperature to evaluate the static mechanical properties of PU/POSS hybrids, and the results are shown in Fig. 7. With low POSS content (not above 7.20 wt%), hybrid polyurethanes displayed brittle fracture, which was attributed to the changes of macromolecular bonds and angles. However, in the case of hybrid PU containing 12.59 wt% of POSS, there existed an unexpected yield point and ductile fracture manifested in the movement and rearrangement of polymer chain segment started. The tensile tests indicated that all of the POSS-containing hybrid polyurethanes behaved like glassy polymer. Compared to pure PU, POSS-containing nanocomposites exhibited an increased modulus with an increasing POSS concentration at low content, but with high loading, this value would drastically decline. This trend could be interpreted on the basis of two factors: 1. The nano-reinforcement of POSS cages on the polymeric matrices and the increase in cross-link density of the networks by chemical introduction of POSS led to mechanical enhancement. 2. At higher POSS content, there was a possibility of over-crosslinking and serious aggregation, which resulted in mechanical impairment.
Surface hydrophobicity
The surface hydrophobicity of the organic–inorganic hybrid polyurethanes was investigated, since the DDT8OH cages in the hybrid polyurethanes were the derivatives of organosilicon compound which was famous for low free energy. In order to certify the effect of DDT8OH on surface wettability, all hybrid polyurethanes were studied by means of static contact angle measurements. Water and ethylene glycol were used as probe liquids to obtain the static contact angles, respectively. The static contact angles of pure PU and the hybrid polyurethanes are shown in Table 3. From Table 3, a conclusion that both the water and ethylene glycol contact angles of the hybrid polyurethanes were mildly heightened with the increasing concentration of DDT8OH in the copolymers (see Fig. 8) in comparison with the pure PU could be drawn. For hybrid 5, the water contact angle reached 103.5°, illustrating that the hydrophobicity of the hybrid material was significantly improved.
Conclusions
In this work, a series hybrid polyurethanes based on sustainable epoxy soybean oil were synthesized using double-decker octaphenylsilsesquioxanetetraol (DDT8OH) to replace 1,4-butanediol (BDO) as chain extender. According to the analysis of 1H NMR and IR, the DDT8OH was successfully synthesized and introduced into the molecular chains. By controlling the adding amounts of inorganic constitutes, the mass percentage of DDT8OH could reach up to 12.59 wt%. Thereafter, a series of characterizations were conducted to evaluate the quality of the material. TGA revealed that the thermal stabilities of the nanocomposites were enhanced, especially for the thermal decomposition at high temperature. The DSC results suggested that the nanocomposites displayed increased glass transition temperatures (T g), making molecular chains more rigid. SEM indicated that POSS was homogeneously dispersed in the polymer matrix at low DDT8OH concentration; however, serious aggregation would occur with high loading. In terms of tensile tests, the nanocomposites showed enhanced mechanical performance to some extent. The hydrophobicity of the hybrid material was significantly enhanced as the static contact angle displayed. Overall, the results of the present work confirm that the incorporation of double-decker silsesquioxane into sustainable polyurethane molecular chains can make it possible to prepare hybrids with relatively perfect physical properties and will open up new pathways for the development of environmentally friendly polymer finally.
References
Shouming W, Hayakawa T, Kakimoto M-a, Oikawa H (2008) Synthesis and characterization of organosoluble aromatic polyimides containing POSS in main chain derived from double-decker-shaped silsesquioxane. Macromolecules 41:3481–3487
Kuoa SW, Changb FC (2011) POSS related polymer nanocomposites. Prog Polym Sci 36:1649–1696
Tamaki R, Tanaka Y, Asuncion MZ, Choi J, Laine RM (2001) Octa(aminophenyl)silsesquioxane as a nanoconstruction Site. J Am Chem Soc 123:12416–12417
Ak M, Gacal B, Kiskan B, Yagci Y, Toppare L (2008) Enhancing electrochromic properties of polypyrrole by silsesquioxane nanocages. Polymer 49:2202–2210
Neumann D, Fisher M, Tran L, Matisons JG (2002) Synthesis and characterization of an isocyanate functionalized polyhedra oligosilsesquioxane and the subsequent formation of an organic-inorganic hybrid polyurethane. J Am Chem Soc 124:13998–13999
Laine RM, Roll MF (2011) Polyhedral phenylsilsesquioxanes. Macromolecules 44:1073–1109
Cai H, Kai X, Liu X, Fua Z, Chen M (2012) A facile synthesis of octa(carboxyphenyl)silsesquioxane. Dalton Trans 41:6919–6921
Hongyao X, Yang B, Wang J, Guang S, Li C (2007) Preparation, Tg improvement, and thermal stability enhancement mechanism of soluble poly(methyl methacrylate) nanocomposites by incorporating octavinyl polyhedral oligomeric silsesquioxanes. J Polym Sci Pol Chem 45:5308–5317
Gnanasekaran D, Ajit Walter P, Reddy BSR (2013) Influence of moieties on morphology, thermal, and dielectric properties in polyamide-polyhedral oligomeric silsequioxanes nanocomposites. Polym Eng Sci 53:1637–1644
Kun W, Kandola BK, Kandare E, Yuan H (2011) Flame retardant effect of polyhedral oligomeric silsesquioxane and triglycidyl isocyanurate on glass fibre-reinforced epoxy composites. Polym Compos 32:378–389
Wei K, Wang L, Zheng S (2013) Organic–inorganic polyurethanes with 3, 13-dihydroxypropyloctaphenyl double-decker silsesquioxane chain extender. Polym Chem 4:1491–1501
Bourbigot S, Turf T, Bellayer S, Duquesne S (2009) Polyhedral oligomeric silsesquioxane as flame retardant for thermoplastic polyurethane. Polym Degrad Stab 94:1230–1237
Musto P, Abbate M, Pannico M, Scarinzi G, Ragosta G (2012) Improving the photo-oxidative stability of epoxy resins by use of functional POSS additives: a spectroscopic, mechanical and morphological study. Polymer 53:5016–5036
Leu CM, Te Chang Y, Wei KH (2003) Polyimide-side-chain tethered polyhedral oligomeric silsesquioxane nanocomposites for low-dielectric film applications. Chem Mater 15:3721–3727
Wahab MA, Mya KY, He C (2008) Synthesis, morphology, and properties of hydroxyl terminated-POSS/Polyimide low-k nanocomposite films. J Polym Sci Pol Chem 46:5887–5896
Ye Y, Yen Y, Chen W, Cheng C, Chang F (2008) A simple approach toward low-dielectric polyimide nanocomposites: blending the polyimide precursor with a fluorinated polyhedral oligomeric silsesquioxane. J Polym Sci Pol Chem 46:6296–6304
Wang J, Ye Z, Joly H (2007) Synthesis and characterization of hyperbranched polyethylenes tethered with polyhedral oligomeric silsesquioxane (POSS) nanoparticles by chain walking ethylene copolymerization with acryloisobutyl-POSS. Macromolecules 40:6150–6163
Lee KM, Knight PT, Chung T, Mather PT (2008) Polycaprolactone-POSS chemical/physical double networks. Macromolecules 41:4730–4738
Frank KL, Exley SE, Thornell TL, Morgan SE, Wiggins JS (2012) Investigation of pre-reaction and cure temperature on multiscale dispersion in POSS/epoxy nanocomposites. Polymer 53:4643–4651
Tan BH, Hussain H, Leong YW, Lin TT, Tjiua WW, He C (2013) Tuning self-assembly of hybrid PLA-P(MA-POSS) block copolymers in solution via stereocomplexation. Polym Chem 4:1250–1259
Wang Z, Leng S, Wang Z, Li G, Hao Yu (2011) Nanostructured organic-inorganic copolymer networks based on polymethacrylate-functionalized octaphenylsilsesquioxane and methyl methacrylate: synthesis and characterization. Macromolecules 44:566–574
Huang J, Xiao Y, My KY, Liu X, He C, Dai J, Siow YP (2004) Thermomechanical properties of polyimide-epoxy nanocomposites from cubic silsesquioxane epoxides. J Mater Chem 14:2858–2863
Markovic E, Clarke S, Matisons J, Simon GP (2008) Synthesis of POSS-methyl methacrylate-based cross-linked hybrid materials. Macromolecules 41:1685–1692
Cardoen G, Coughlin EB (2004) Hemi-telechelic polystyrene-POSS copolymers as model systems for the study of well-defined inorganic/organic hybrid materials. Macromolecules 37:5123–5126
Kotal A, Si S, Paira TK, Mandal TK (2008) Synthesis of semitelechelic POSS-Polymethacrylate hybrids by thiol-mediated controlled radical polymerization with unusual thermal behaviors. J Polym Sci Pol Chem 46:1111–1123
Mather PT, Jeon HG, Romo-Uribe A (1999) Mechanical relaxation and microstructure of poly(norbornyl-POSS) copolymers. Macromolecules 32:1194–1203
Waddon AJ, Zheng L, Farris RJ, Bryan Coughlin E (2002) Nanostructured polyethylene-POSS copolymers: control of crystallization and aggregation. Nano Lett 10:1149–1155
Fina A, Tabuani D, Frache A, Camino G (2005) Polypropylene–polyhedral oligomeric silsesquioxanes (POSS) nanocomposites. Polymer 46:7855–7866
Li X, Xiaolong L (2007) Effect of annealing on the structure and properties of polyvinylidene fluoride hollow fiber by melt-spinning. J Appl Polym Sci 103:935–941
Goseki R, Hirai T, Ishida Y, Kakimoto M-a, Hayakawa T (2012) Rapid and reversible morphology control in thin films of poly(ethylene oxide)-block-POSS-containing poly(methacrylate). Polym J 44:658–664
Ni C, Ni G, Zhang L, Mi J, Yao B, Zhu C (2011) Syntheses of silsesquioxane (POSS)-based inorganic/organic hybrid and the application in reinforcement for an epoxy resin. J Colloid Interface Sci 362:94–99
Ni Y, Zheng S (2007) Nanostructured thermosets from epoxy resin and an organic-inorganic amphiphile. Macromolecules 40:7009–7018
Baldi F, Bignotti F, Ricco L, Monticelli O, Ricco T (2006) Mechanical and structural characterization of POSS modified polyamide. J Appl Polym Sci 100:3409–3414
Ding Y, Chen G, Song J, Gou Y, Shi J, Jin R, Li Q (2012) Properties and morphology of supertoughened polyamide 6 hybrid composites. J Appl Polym Sci 126:194–204
Madbouly SA, Otaigbe JU, Nanda AK, Wicks DA (2007) Rheological behavior of POSS/polyurethane-urea nanocomposite films prepared by homogeneous solution polymerization in aqueous dispersions. Macromolecules 40:4982–4991
Raftopoulos KN, Janowski B, Apekis L, Pissis P, Pielichowski K (2013) Direct and indirect effects of POSS on the molecular mobility of polyurethanes with varying segment Mw. Polymer 54:2745–2754
Chen Y, Kang E-T (2004) New approach to nanocomposites of polyimides containing polyhedral oligomeric silsesquioxane for dielectric applications. Mater Lett 58:3716–3719
Wright ME, Petteys BJ, Guenthner AJ, Fallis S, Yandek GR, Tomczak SJ, Minton TK, Brunsvold A (2006) Chemical modification of fluorinated polyimides: new thermally curing hybrid polymers with POSS. Macromolecules 39:4710–4718
Lee Y-J, Kuo S-W, Huang W-J, Lee H-Y, Chang F-C (2004) Miscibility, specific interactions, and self-assembly behavior of phenolic/polyhedral oligomeric silsesquioxane hybrids. J Polym Sci Polym Phys 42:1127–1136
Markovic E, Nguyen K, Clarke S, Constantopoulos K, Matisons J, Simon GP (2013) Synthesis of POSS-Polyurethane hybrids using octakis(m-isoprenyl-α,α’dimethylbenzylisocyanato dimethylsiloxy) octasilsesquioxane (Q8M8™I) as a crosslinking agent. J Polym Sci Pol Chem 51:5038–5045
Huitron-Rattinger E, Ishida K, Romo-Uribe A, Mather PT (2013) Thermally modulated nanostructure of poly(ε-caprolactone)-POSS multiblock thermoplastic polyurethanes. Polymer 54:3350–3362
Turri S, Levi M (2005) Structure, dynamic properties, and surface behavior of nanostructured ionomeric polyurethanes from reactive polyhedral oligomeric silsesquioxanes. Macromolecules 38:5569–5574
Knight PT, Lee KM, Qin H, Mather PT (2008) Biodegradable thermoplastic polyurethanes incorporating polyhedral oligosilsesquioxane. Biomacromolecules 9:2458–2467
Janowski B, Pielichowski K (2008) Thermo(oxidative) stability of novel polyurethane/POSS nanohybrid elastomers. Thermochim Acta 478:51–53
Seino M, Hayakawa T, Ishida Y, Kakimoto M-a (2006) Hydrosilylation polymerization of double-decker-shaped silsesquioxane having hydrosilane with diynes. Macromolecules 39:3473–3475
Petrović ZS (2008) Polyurethanes from vegetable oils. Polym Rev 48:109–155
Liu H, Zheng S (2005) Polyurethane networks nanoreinforced by polyhedral oligomeric silsesquioxane. Macromol Rapid Commun 26:196–200
Wang W, Guo Y-l, Otaigbe JU (2009) The synthesis, characterization and biocompatibility of poly(ester urethane)/polyhedral oligomeric silesquioxane nanocomposites. Polym 50:5749–5757
Morent R, De Geyter N, Van Vlierberghe S, Beaurain A, Dubruel P, Payen E (2011) Influence of operating parameters on plasma polymerization of acrylic acid in a mesh-to-plate dielectric barrier discharge. Prog Org Coat 70:336–341
Ruijun G, Konar S, Sain M (2012) Preparation and characterization of sustainable polyurethane foams from soybean oils. J Am Oil Chem Soc 89:2103–2111
Bandyopadhyay-Ghosh S, Ghosh SB, Sain M (2010) Synthesis of soy-polyol by two step continuous route and development of soy-based polyurethane foam. J Polym Environ 18:437–442
Vaia RA, Maguire JF (2007) Polymer Nanocomposites with prescribed morphology: going beyond nanoparticle-filled polymers. Chem Mater 19:2736–2751
Sanchez C, Julián B, Belleville P, Popall M (2005) Applications of hybrid organic-inorganic nanocomposites. J Mater Chem 15:3559–3592
Strachota A, Kroutilová I, Kovárová J, Matějka L (2004) Epoxy networks reinforced with polyhedral oligomeric silsesquioxanes (POSS). Thermomechanical properties. Macromolecules 37:9457–9464
Liu Y, Ni Y, Zheng S (2006) Polyurethane networks modified with octa(propylglycidyl ether) polyhedral oligomeric silsesquioxane. Macromol Chem Phys 207:1842–1851
Acknowledgements
This work was supported by research Grants from the National Key Technology Research and Development Program (2012BAD32B03-4) and the Cooperative Innovation Foundation of Industry, Academy and Research Institutes (BY2013015-10) in Jiangsu Province of China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, J., Jiang, P., Li, X. et al. Synthesis and characterization of sustainable polyurethane based on epoxy soybean oil and modified by double-decker silsesquioxane. J Mater Sci 51, 2443–2452 (2016). https://doi.org/10.1007/s10853-015-9557-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-015-9557-0