Abstract
Polyol derived from soybean oil was made from crude soybean oil by epoxidization and hydroxylation. Soy-based polyurethane (PU) foams were prepared by the in-situ reaction of methylene diphenyl diisocyanate (MDI) polyurea prepolymer and soy-based polyol. A free-rise method was developed to prepare the sustainable PU foams for use in automotive and bedding cushions. In this study, three petroleum-based PU foams were compared with two soy-based PU foams in terms of their foam characterizations and properties. Soy-based PU foams were made with soy-based polyols with different hydroxyl values. Soy-based PU foams had higher T g (glass transition temperature) and worse cryogenic properties than petroleum-based PU foams. Bio-foams had lower thermal degradation temperatures in the urethane degradation due to natural molecular chains with lower thermal stability than petroleum skeletons. However, these foams had good thermal degradation at a high temperature stage because of MDI polyurea prepolymer, which had superior thermal stability than toluene diisocyanate adducts in petroleum-based PU foams. In addition, soy-based polyol, with high hydroxyl value, contributed PU foam with superior tensile and higher elongation, but lower compressive strength and modulus. Nonetheless, bio-foam made with high hydroxyl valued soy-based polyol had smaller and better distributed cell size than that using low hydroxyl soy-based polyol. Soy-based polyol with high hydroxyl value also contributed the bio-foam with thinner cell walls compared to that with low hydroxyl value, whereas, petroleum-based PU foams had no variations in cell thickness and cell distributions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The global economy hastens the development of world PU production and consumption [1]. Although global PU demand is growing rapidly [2], there are environmental concerns associated with the use of petroleum-based PU foams. While there are several ways to degrade PU foams [3], landfills have been the most common form of waste disposal. However, a lack of degradability and the closing of landfill sites have led to concerns about PU foams. Petroleum-based PU foams do not biodegrade for several hundred years under anaerobic conditions found in landfills, because PU cannot degrade to provide carbon and nitrogen sources for microbial growth [4]. Even exposed to extreme degradation conditions, such as thermal aging at 90 °C for 60-day period, mechanical deformation at 8 % over 1,000 h, dry and wet freeze–thaw cycling, and weather aging under 1,000-year alpha and gamma dose irradiation, petroleum-based PU foams have little or virtually no significant degradation [4]. As a result, petroleum-based PU foams can remain in landfills for a very long time [5], whereas, materials having a bio-content allow fungal attacks on bio-based PU foams by breaking down their labile chemical moieties [6] such as ester groups [7–9]. Therefore, increasing the bio-renewable content helps PU foams to biodegrade.
In addition, natural plants or vegetables can reduce carbon dioxide (CO2) footprints by absorbing CO2 gas during growth [10]. The substitution of petroleum-based polyols with vegetable/plant oil-based polyols is expected to reduce carbon footprints and promote the management of natural sources, especially crop planting. Polyols from sustainable sources are renewable and have a superior economic allocation compared with petrochemical ingredients [10]. Current commercial bio-polyols are most produced from palm, soya, corn and castor [11, 12]. Petrochemicals are facing upward price pressure from feedstocks, higher crude oil values and uncertainty [13]. The natural materials already have economical advantages in price [14] with petroleum-based competitors. Furthermore, it has been reported that bio-based PU foam achieves higher thermal and dimensional stability than traditional polypropylene oxide-based foams due to its abundant triglycerides [15].
A number of researchers have studied vegetable oil-based water-blown PU foams [11, 16–18]. The literature on foam characterization and properties of soy-based rigid PU foams blown by physical blowing agents are also available [19] as well as for soy-based PU resins [20]. However, no research has directly addressed the foam characterization and properties of flexible water-blown PU foams or their hydroxyl values, where vegetable oil-based PU foams are compared with petroleum-based PU foams. This paper studies three petroleum-based foams and two soy-based PU foams to show their differences in cell morphologies, foam mechanical and thermal properties. This work also predicts the potential commercialization of high hydroxyl valued soy-based polyol made from crude soybean oil by epoxidization and hydroxylation.
Experimental
Preparation of Soy-Based Polyol with a High Hydroxyl Value
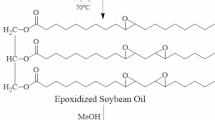
Soy-based polyol was prepared from soybean oil by introducing hydroxyl functionality to the unsaturated sites of its triglycerides structures following the procedure developed by Ghosh-Roy [21]. Crude soybean oil (Energrow Inc., Newton, Canada) was treated with a mixture of sodium tungstate and tungstic acid in the presence of hydrogen peroxide for epoxidation of the unsaturated sites. The epoxidized soybean oil was then subjected to controlled acid hydrolysis at 70 °C for 4 h to obtain soybean oil-based polyol. Finally, the hydroxylation solution was extracted with organic solvent and washed to neutrality. Soy-based polyol was obtained after removal of the solvent.
Preparation of PU Foams
Materials
Low odor soy-based polyol (Soyol® 2999) was donated by Urethane Soy Systems (Volga, South Dakota, USA). Its bio-renewable content was 98 %, determined by ASTM D6866. Its hydroxyl value was 67 mg KOH/g according to ASTM D4274 with a viscosity of 2,456 cps at 25 °C. Its acid value was <1.1 mg KOH/g. Its moisture content was <0.02 % according to the data provided by the supplier.
Rubinate® 9480 MDI (15.2 % NCO), a member of the polyurea prepolymer isocyanate family, was donated by Huntsman (West Point, GA, USA) and used to produce durable foams. Its functionality was 2.0, as provided by the supplier. This prepolymer also contained 7–13 % propylene carbonate as solvent.
Dibutyltin dilaurate and tertiary amine were generously donated by Air Products and Chemicals Inc. (Allentown, PA, USA). Dibutyltin dilaurate was used as a foaming catalyst. Tertiary amine was used as a gelling catalyst. A copolymer of silicone and furandione was used as a surfactant to achieve superior cell structures, which were also donated by Air Products and Chemicals Inc. All of the catalysts and surfactants were used directly. Distilled water was used as a blowing agent to generate foams.
Foam Preparation
PU foams were prepared by a free-rise method according to the formulation listed in Table 1, which was in terms of 100 parts of soy-based polyol. Soy-based polyol was mixed with the additives (catalysts, surfactant and blowing agent) using a spatula at ambient temperature for 5 min, and then isocyanate was added and mixed for another 20 s. Afterwards, the resultant mixture was quickly transferred into a deep foil pan (13 × 10 × 2 cubic inch) for foaming to obtain the PU foam. Finally, the resulting soy-based PU foam was kept at room temperature for several days to cure.
Petroleum-based PU cushions were obtained from Schukra of North America Ltd (Windsor, Ontario, Canada). These water-blown foams were made with polyether polyols and toluene diisocyanate. The ILD (Indentation Load Deflection) tests were performed by the supplier and the values were recorded at 25 % compression according to ASTM D3574. The ILD values of petroleum-based PU foams were 264, 271 and 331 N, respectively, according to the data provided by the foregoing supplier. The ILD values were only used as a reference to assess the mechanical material behavior. It was not used in our analyses as it does not provide explicit information on the material characteristics. All the foams were coded as shown in Table 2.
Foam Characterizations
Fourier Transform Infrared Analysis (FTIR)
Foam fragments preparation: Soy-based PU foams fragments were prepared by crushing in liquid nitrogen, while petroleum-based PU foams fragments were obtained by sanding. These Foam fragments were used for Fourier Transform Infrared Analysis (FTIR) and Differential Scanning Calorimetry (DSC) measurements.
Sample preparation: a small amount of soybean oil, soy-based polyol or foam fragments was ground with 100 mg of dry KBr. A KBr pellet was then prepared in a standard device with 10 MPa pressure for 1 min. The thickness of the pellet was 300 μm.
The spectra were obtained against pure KBr on a Bruker Tensor 27 spectrophotometer at a resolution of 4 cm−1 with a coaddition of 60 scans. All the data analyses were performed with the OPUS v5.5 software from Bruker.
Scanning Electron Microscope (SEM) Investigation
A sample stub with thin foam slab was gold-coated by a sputter-coating (BOT 341F) with evaporated gold (4 nm thickness), and then the morphology was examined using a SEM (Hitachi S-2500, Hitachi High Technologies Inc., Tokyo, Japan) at an acceleration voltage of 10 kV.
The cell morphologies including Feret diameter (the longest distance between any two points along the selection boundary, which was used to describe the cell size), Feret distribution (cell size distribution), and the thickness of cell wall were statistically analyzed by ImageJ.
Mechanical Tests
All the foams were conditioned at 23 °C and 45 % relative humidity. The foam slabs were then cut with a saw followed by polishing with a belt sander (Model 31–710, Rockwell International, Pittsburgh, USA). The size of the grit was 120. The length, width, and thickness were measured after polishing. The thickness was parallel to the foam rise direction.
Five slabs (Type C, 5 cm × 5 cm × 3 cm) were used for the tensile strength test using a Zwick universal testing machine (Zwick/Z100, Zwick GmbH & Co. KG, Germany) according to ASTM D1623 under 23 °C and 45 % relative humidity. The rate of crosshead movement was 1.3 mm/min.
Eight slabs (5 cm × 5 cm × 3 cm) were tested for compressive strength using an Instron universal testing machine (Model 3367, Instron) following ASTM D3574 at 23 °C and with 45 % relative humidity. The rate of crosshead movement was 50 mm/min. The value at 50 % deformation was recorded as the compressive strength.
Thermal Analyses
Thermal analyses for PU foams were performed with a thermogravimetric analyzer (TGA Q50, TA Instruments). Runs of TGA were conducted in the ramp mode from room temperature to 800 °C under nitrogen at a flow rate of 60 mL/min. The heating rate was 10 °C/min. Sample weights of TGA were approximately 3 mg in their original state.
Runs of DSC (Q2000, TA Instruments) were performed in the temperature range of −85 to 150 °C under nitrogen at a flow rate of 50 mL/min. The heating rate was 10 °C/min in the ramp mode. Samples weights of DSC runs were approximately 5 mg in oven-dried foam fragments.
Results and Discussion
Characterization of Synthesized Soy-Based Polyol
The hydroxyl value of the final synthesized soy-based polyol was 164 mg KOH/g, which was determined according to ASTM D4274. Its acid value was 3.2 mg KOH/g measured in accordance with ASTM D974. Its viscosity was 4830 cps at 25 °C, determined by a Brookfield Synchro-Electric Viscometer (Model RVT). The changes in the chemical structures of soy-based polyol and soybean oil were evidenced through FTIR spectra as shown in Fig. 1, where the unsaturated bonds in soybean oil disappeared, and the hydroxyl groups were inserted into the final product. However, a trace amount of unsaturated bonds remained in the soy-based polyol and upon autoxidation it yielded a number of odoriferous low molecular weight (Mw) compounds such as aldehydes, ketones, and carboxylic acids [22].
Cryogenic Observations
Soy-based PU foams were broken easily into pieces in liquid nitrogen. However, petroleum-based PU foams could not be crushed at cryogenic temperatures. It was concluded that petroleum-based PU foams had good low-temperature resistance compared to the bio-based PU foams.
Characterization of PU Foams
The FTIR spectra of both petroleum-based and bio-based PU foams are shown in Fig. 2, and the assignments of major bands are presented in Table 3. Both spectra of bio-foams exhibited typical spectroscopic fingerprints of triglycerides in the region of 2,700–3,000 cm−1 [23, 24], whereas, petroleum-based PU foams showed typical fingerprinting of polyether polyol [25]. All spectra exhibited a broad strong peak due to the N–H stretching vibration in the region around 3,336 cm−1. The peak owing to the carbonyl stretching bands appeared at 1,731 cm−1 [26, 27].
Large amounts of isocyanates are used to keep the same NCO index when high hydroxyl valued soy-based polyol is employed. Some isocyanates appeared to undergo dimerization reactions to form uretdione structures. As a result, a characteristic carbonyl stretching band in uretdione structures was found at 1,799 cm−1 in B2 [27–29]. It can be seen from Fig. 3 that the uretdione appeared to be in the inactive state along with extension of storing time. In addition, B2 showed a weak intensity absorption band at 2,273 cm−1 due to the existence of small number of residual NCO groups [30, 31], which is associated with the uretoneimine structure (see Fig. 2) [32]. Meanwhile, it showed a detectable loss even after 8 weeks of storage as shown in Fig. 3.
Water-blown PU foams were expanded by the CO2 gas released from the reaction between water and NCO groups. The influence of gas expansion should be neglected, because the foam comprised of mostly open cells as observed in Fig. 4. Petroleum-based foams appeared to have porous structures exhibiting high open cell content. In addition, the effect of the moisture content of polyol could also be neglected due to its very low amount as reported by its supplier (max 0.02 %).
In this study, petroleum-based PU foams were found to have insignificantly decreasing foam densities depending on their increased ILD values. This could be due to additional water introduced into the reaction which resulted in more CO2 generation and consequently a reduction in density (see Table 4). But the variations are acceptable for cushion application. The selected formulation yielded bio-foams with an affordable foam density around 55–60 kg/m3, which were found to be a bit higher than petroleum-based foams. It is worth noting that soy-based polyol with high hydroxyl value provided a small increase of foam density compared to low hydroxyl valued soy-based polyol. This increased value was 5 kg/m³ under the same level of NCO index.
Figure 5 shows the cell size distributions of PU foams. Although the cell size distributions of petroleum-based foams were similar, the same conclusion cannot be drawn for soy-based PU foams, which were found to be down-shifted. It can be observed from Fig. 5 that PU foam made with low hydroxyl valued soy-based polyol (B1) had wider cell size range (Feret diameter) and lower cell distribution frequency (Feret diameter distribution) compared to the foam made with high hydroxyl valued soy-based polyol (B2). The mean cell size of B1 (448 μm) was higher than that of B2 (377 μm) after profiling hundreds of cells.
Thermal Properties of PU Foams
DSC analyses of foam samples were conducted to observe subtle phase changes and to evaluate the effect of natural polyols on foam characteristics. The DSC results clearly showed that soy-based and petroleum-based PU foams have different thermal behaviors (Fig. 6). Most petrochemical polyols had terminal primary hydroxyl groups, unlike soy-based polyols which possessed most middle hydroxyl groups in triglycerides structures [15]. As a result, the effective crosslinking density of soy-based region was higher than that of petroleum-based domain when the polyol was crosslinked with isocyanate. Alternatively, soy-based polyols reduced the hard domain size and the soft domain fraction in soy-based foams [33], and led to a flatter and higher T g of the networks than petrochemical polyol as indicated in Fig. 6. Similar results were also reported by Javni et al. [15] and Zhang et al. [33]. This finding also demonstrates that petrochemical polyol is a fairly crystalline material, while soy-based polyol has a broad distribution of interdomain spacings due to abundant triglyceride structures of amorphous type.
The three petroleum-based PU foams had very close T g values which were around 56 °C due to the presence of the same polyether polyol used. However, the two soy-based polyols had different hydroxyl values. Soy-based polyol with high hydroxyl values required more isocyanate polymers in the reactions in order to keep the same level of NCO index. Unquestionably, more isocyanate polymers facilitated secondary reactions, and produced more uretdione and biuret structures. Therefore, high hydroxyl valued soy-based polyol contributed B2 a wider peak and a higher T g value compared to B1 with low hydroxyl value. Moreover, high hydroxyl valued soy-based polyol having high viscosity might consist of a higher percentage of longer chains than commercial soy-based polyol (Soyol® 2999, low hydroxyl value) with the exception of some low molecular weight odoriferous chemicals. It has been reported that longer chains lead to higher crystallinity temperatures [34] by exhibiting higher toughness and flexibility [35]. Higher flexibility led to lower compressive strength as well as compressive modulus, which is in line with the results in Table 4. For the cryogenic performance of PU foams, both the hydroxyl value and the amount of isocyanate prepolymer used played a significant role in foam low temperature resistance. Petroleum-based foams were found to have superior cryogenic properties compared to bio-based foams when they were observed in liquid nitrogen under compression.
The thermal properties of the foam cores were studied by discarding foam skins. A typical decomposition curve of PU foam with two distinct steps is shown in Fig. 7. The decomposition temperatures (T d) of petroleum-based foams are correlated with their ILD. It is known that more water generates more urea structures. As a result, high ILD foam has a bit lower thermal stability than low ILD foam. The thermal degradation temperatures observed from TGA are reported in Table 5. Urea formation was found to be a dominant factor in the water-blown PU foam, especially during the initial stages of foam rise, according to Li et al. [36] and Grünbauer et al. [37]. Urethane structures were broken down at lower temperatures (first stage) with the evolution of isocyanate and alcohol, and resulted in the collapse of the cellular structure. Polyurea and polyol structures might have been cleaved at high temperature (second stage) by forming complex degradation products. The T d at 5 % weight loss (T d5) was closer to the decomposition of urethane bonds, whereas, the T d at 50 % weight loss (T d50) was mostly correlated with the degradation of polyurea and polyol structures. Both cell structure and foam ingredients had an important effect on foam thermal behavior. It appears from Table 5 that both T d5 and T d50 decreased with the increase in their respective petroleum-based foam ILDs, since the increase in ILD was attributed to the decrease in the amount of water-related adducts.
Soy-based PU foams might be very similar to petroleum-based foam as far as the hydrolysis is concerned [38, 39]. It can be observed from Table 5 that soy-based PU foams had lower T d5 than petroleum-based PU foams at the first stage (urethane degradation). This was probably due to the weak urethane linkages between -NCO groups in isocyanates and hydroxyl groups in natural triglycerides structures. Unlike petrochemical polyols that are susceptible to oxidation to ketones, soy-based polyols consist of more stable paraffinic chains and ester groups [15]. The higher thermal stability of soy-based polyols contributes to bio-foams having a higher T d50 than petroleum-based foams. Considering their degradable proportions, bio-foams had higher thermal stability than petroleum-based foams. This result was in line with the finding of Javni et al. [15].
Though B2 had more isocyanate content than B1 due to its higher hydroxyl value, both its T d5 and T d50 were much lower than those of B1. This could be due to a combination of cell structures and polyol chain length. B2 was predicted to contain a trace amount of low molecular weight odoriferous chemicals by exhibiting soya odor. Moreover, the thinner cells in B2 might have caused its cellular structures to collapse more easily compared to the thicker cells in B1, which is indicated in Table 4. The cell wall thickness of petroleum-based foams is expected not to have an effect on their thermal behaviors due to their much closer values.
Mechanical Properties of PU Foams
Foam cell morphology has a direct influence on the foam mechanical properties, such as compressive strength and tensile strength. Both petroleum-based and soy-based PU foams have very close compression behaviors to each other. Their typical compression behaviors are described in Fig. 8. The cell walls were bent at the beginning of the compression (<10 %), which indicated foam elasticity and showed compressive modulus. As the compression deformation was increased, cell walls buckled and yielded a plateau collapse around 10–25 % deformation. Finally, as the deformation reached the densification strain (over 30 %), the cell walls were found to be crushed together and the material itself was compressed.
For petroleum-based PU foams, the variability of compressive strength depends on the foam morphologies, including cell size, cell wall and foam density. It is understood that petroleum-based foam has an increased compression at 50 % deformation with the increase in their ILD due to their similar cell size and closed cell wall thickness. Good cellular structure helps to disperse tension under compression and the applied stress. Soy-based PU foams had fine cell structures, where each polyhedral cell was covered by a thin membranous window especially of B1 (see Fig. 4; B1, B2). Natural polyol might have contributed to giving the foam a three-dimensional cell wall structure, because of triglyceride molecules [11], which helped the cell structure to stand the compressive strength in particular for thicker cell walls. Although B2 had a slightly higher foam density and smaller cells, its cell walls were found to be much thinner than B1. The thicker cell walls and struts provided B1 with superior support and thereby exhibited higher compressive strength (Table 4) and higher compressive modulus (Table 4). The results of foam compressive strengths were well in agreement with their compression modulus and their cell wall thickness.
Cells are stretched under applied tensile forces. The tensile behaviors of petroleum-based PU foams (A1, A2 and A3) are very near to each other. Meanwhile, soy-based PU foams (B1 and B2) also have very close tensile curves except at the end of fracture, which is shown in Fig. 9. The tensile strength of petroleum-based foams dropped suddenly over their yield point, but soy-based foams exhibited fracture inhibitions due to their three-dimensional networks from their abundant triglycerides structures. This inhibition also displayed much more energy absorbance or toughness during the stretch stage.
The tensile strength and elongation of PU foams under maximum load are shown in Table 4. The results showed that selected petroleum-based foams had similar tensile strengths due to their similar cell ingredients and cell characteristics when the testing variations are considered (Table 4). The existence of a small amount of residual NCO in B2 implied that most of the NCO groups could react with urethane and urea to form allophanate and biuret with the exception of inactive uretdione [40]. These adducts, which proceeded in secondary reactions, provided stronger linkages than urethane bonds and resulted in great tensile properties. So, the superiority in the tensile strength of B2 came from its high secondary reactivity. It can be seen from Table 4 that petroleum-based PU foams exhibited a decrease in elongation and showed a relationship with the increase in the ILDs. Due to the testing variations, there were almost no differences in the compressive strengths and moduli of A1 and A2 due to their close ILDs, however, A3 with much higher ILD had a higher compressive strength and modulus. Like petroleum-based foams, the elongation of soy-based foams was consistent with their compressive modulus. In addition to a superior tensile strength, B2 also had higher flexibility, which could be best used for cushion material.
Conclusions
Petroleum-based PU foams could be replaced by bio-based PU foams without compromising the integrity except cryogenic properties. The hydroxyl value of soy-based polyol exerted important effects on cell morphologies and foam properties. Soy-based polyols with high hydroxyl values made by our group exhibited superior tensile strength, high tensile elongation, comparatively higher degradability and high T g compared to low hydroxyl valued soy-based polyol. However, the synthesized soy-based polyol still needed further purification to eliminate soya odor.
References
Anon. (2011) Markets and Markets market report #CH 1596: MDI, TDI and polyurethane market by type, applications, prices, regulations trends and global forecasts 2011–2016. Publishing Date: July 2011
Global Industry Analysis, Inc. (2011) Foamed plastics (polyurethane)—a global strategic business report. #MCP-2069. Released in February 2011
Howard GT (2002) Biodegradation of polyurethane: a review. Int Biodeterior Biodegradation 49:245–252
Urgun-Demirtas M, Singh D, Pagilla K (2007) Laboratory investigation of biodegradability of a polyurethane foam under anaerobic conditions. Polym Degrad Stab 92:1599–1610
Lichtenberg F (2002) The environmental fate of TDI/MDI-based polyurethane foams, Alliance for the polyurethanes industry, Proceedings of the technical program of the polyurethane foam association meeting, Arlington, May 9, 2002
Oprea S, Doroftei F (2011) Biodegradation of polyurethane acrylate with acrylated epoxidized soybean oil blend elastomers by Chaetomium globosum. Int Biodeterior Biodegradation 65:533–538
Raquez J-M, Deléglise M, Lacrampe M-F, Krawczak P (2010) Thermosetting (bio)materials derived from renewable resources: a critical review. Prog Polym Sci 35:487–509
Uyama H, Kuwabara M, Tsujimoto T, Kobayashi S (2003) Enzymatic synthesis and curing of biodegradable epoxide-containing polyesters from renewable resources. Biomacromolecules 4:211–215
Shogren RL, Petrovic Z, Liu Z, Erhan SZ (2004) Biodegradation behavior of some vegetable oil-based polymers. J Polym Environ 12:173–178
Anon (2010) Life cycle impact of soybean production and soy industrial products. Omni Tech International prepared for the United Soybean Board, USA
Petrović ZS (2008) Polyurethanes from vegetable oils. Polym Rev 48:109–155
Meyer H-P (2011) Sustainability and biotechnology. Org Process Res Dev 15:180–188
ICIS Pricing. www.icispricing.com
Ash M (2011) Oil crops outlook: foreign competition may diminish the export potential for US soybeans next fall. US Department of Agriculture. June 10, 2011. P. 16
Javni I, Zhang W, Petrović ZS (2004) Soybean-oil-based polyisocyanurate rigid foams. J Polym Environ 12:123–129
Banik I, Sain MM (2008) Water blown soy polyol-based polyurethane foams of different rigidities. J Reinf Plast Compos 27:357–373
Khazabi M, Gu R, Sain M (2011) Fiber reinforced soy-based polyurethane spray foam insulation. Part 1: cell morphologies. BioResources 6:3757–3774
Gu R, Khazabi M, Sain M (2011) Fiber reinforced soy-based polyurethane spray foam insulation. Part 2: thermal and Mechanical Properties. BioResources 6:3775–3790
Guo A, Javni I, Petrovic Z (2000) Rigid polyurethane foams based on soybean oil. J Appl Polym Sci 77:467–473
Petrovic ZS, Yang L, Zlatanic A, Zhang W, Javni I (2007) Network structure and properties of polyurethanes from soybean oil. Appl Polym Sci 105:467–473
Ghosh-Roy S (2010) Master thesis. Novel approaches for synthesis of polyols from soy oils. Faculty of Forestry, University of Toronto. Issued on January 19, 2010
Frankel EN (1980) Lipid oxidation. Prog Lipid Res 19:1–22
Wabel C (1998) Doctoral dissertation. Influence of Lecithin on Structure and Stability of Parenteral Fat Emulsions. Chapter 4. Department of Chemistry and Pharmacy, Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU). Erlangen, Germany. Issued on July 30, 1998
Çaylı G, Küsefoğlu S (2008) Biobased polyisocyanates from plant oil triglycerides Synthesis, polymerization, and characterization. J Appl Polym Sci 109:2948–2955
Molero C, de Lucas A, Rodríguez JF (2006) Recovery of polyols from flexible polyurethane foam by “split-phase” glycolysis with new catalysts. Polym Degrad Stab 91:894–901
David D, Silverstein MS (2009) Porous polyurethanes synthesized within high internal phase emulsions. J Polym Sci Part A: Polym Chem 47:5806–5814
Meyer-Stork LS, Höcker H, Berndt H (1992) Syntheses and reactions of urethanes of cellobiose and cellulose-containing uretdione groups. J Appl Polym Sci 44:1043–1049
Halpaad R, Kocher J, Laas H-J, Richter F (2006) Isocyanates containing uretdione groups. US Patent # 7098289. Issued on August 29, 2006
Grogler G, Kopp R, Hess H (1988) Process for the preparation of complexes of one mol of dimerized or low-oligodimerized 4,4′-diisocyanatodiphenylmethane and two to three mol of 4,4′-diisocyanatodiphenylmethane, corresponding complexes, and their use for polyurethane production. US Patent #4720545 Issued on January 19, 1988
Chuayjuljit S, Sangpakdee T, Saravari O (2007) Processing and properties of palm oil-based rigid polyurethane foam. J Metals Mater Miner 17:17–23
Blagbrough IS, Mackenzie NE, Ortiz C, Scott AI (1986) The condensation reaction between isocyanates and carboxylic acids. A practical synthesis of substituted amides and anilides. Tetrahedron Lett 27:1251–1254
Hatchett DW, Kodippili G, Kinyanjui JM, Benincasa F, Sapochak L (2005) FTIR analysis of thermally processed PU foam. Polym Degrad Stab 87:555–561
Zhang L, Jeon HK, Malsam J, Herrington R, Macosko CW (2007) Substituting soybean oil-based polyol into polyurethane flexible foams. Polymer 48:6656–6667
Bunjes H, Westesen K, Koch MHJ (1996) Crystallization tendency and polymorphic transitions in triglyceride nanoparticles. Int J Pharm 129:159–173
Güner FS, Yağcı Y, Erciyes AT (2006) Polymers from triglyceride oils. Prog Polym Sci 31:633–670
Li X, Cao H, Zhang Y (2006) Structures and physical properties of rigid polyurethane foams with water as the sole blowing agent. Sci China, Ser B: Chem 49:363–370
Grünbauer HJM, Thoen JA, Folmer JCW, Lieshout HCV (1992) Polymer morphology of water-blown rigid polyurethane foam: development of new polyols. J Cell Plast 28:36–47
Dai Z, Hatano B, Kadokawa J, Tagaya H (2002) Effect of diaminotoluene on the decomposition of polyurethane foam waste in superheated water. Polym Degrad Stab 76:179–184
Gerlock JL, Braslaw J, Mahoney LR, Ferris FC (1980) Reaction of polyurethane foam with dry steam: kinetics and mechanism of reactions. Journal of Polymer Science: Polymer Chemistry Edition 18:541–557
Rogers ME, Long TE (eds) (2003) Synthetic methods in step-growth polymers. In: Wiley, Hoboken. ISBN 0-471-38769-X
Acknowledgments
The authors are grateful to the Natural Sciences and Engineering Research Council of Canada (NSERC)-Collaborative Research and Development (CRD) and Ontario BioCar Initiative for their financial support. The authors thank Dr. William Altenhof for providing commercial petroleum-based PU foams and Mr. Nolan Rempe for his work through Ontario BioaCar Youth Outreach by BioCar. The authors also thank Huntsman, Urethane Soy Systems, and Air Products for their generous donations.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Gu, R., Konar, S. & Sain, M. Preparation and Characterization of Sustainable Polyurethane Foams from Soybean Oils. J Am Oil Chem Soc 89, 2103–2111 (2012). https://doi.org/10.1007/s11746-012-2109-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-012-2109-8