Abstract
Soy-polyol has been synthesized via a low energy two-step continuous route thus avoiding intermediate steps and chemicals. The functional groups of soy-polyol thus produced were identified by Fourier transform infrared (FTIR) spectroscopy which confirmed the cleavage of the double bonds, the formation of new epoxy linkages and the presence of hydroxyl groups. The change in chemical structure and physical properties of the soy polyol was further characterized and the results indicated a successful conversion with reduced unsaturation, increased hydroxyl number and increased viscosity. Polyurethane foam was prepared from soy-polyol using isocyanate and thermogravimetric analysis was used to study its thermal decomposition behaviour. Multiple transitions were identified in relation to depolymerization and bond dissociation. Density and compressive strength of the soy-foam were found to be satisfactory. An investigation of microstructure of soy foam by scanning electron microscope and X-ray computed tomography revealed the internal cell morphology and cell structure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Renewable, biodegradable, agricultural resources are gaining increasing attention of many researchers because of growing environmental awareness and their potential to replace petrochemical derivatives. Soybean oil is an inexpensive, readily available, renewable resource with multiple sites of reactivity including ester and olefinic sites which provides an excellent platform for polymeric materials particularly as a potential alternative to petroleum-based monomers. Soybean oil is mainly composed of tri-glyceride molecules derived from unsaturated fatty acids such as oleic acid (22%), linoleic acid (55%), and linolenic acid (7%). In addition to being a renewable resource, there are several other compelling reasons to use soybean oil as starting materials: The production of polyols from petrochemicals requires a great deal of energy. Furthermore, the process itself is costly and adversely affects the environment. Thus, it is desirable to replace petroleum-based polyols with the more versatile, renewable, less costly, and more environmentally friendly biobased polyols. Several strategies have been employed to prepare polyols from vegetable oils [1–9]. Some of the early methods of preparing polyols from various vegetable oils suffer from certain drawbacks. Premature degradation occurs by these processes as a result of high temperatures and a relatively long reaction time. Besides, these reactions of preparing polyols from vegetable oils are not very selective. By-products in addition to alcohol groups are created during transformation. Greases or waxes often result as a consequence of such chemical transformation. Many available methods of preparing polyols from vegetable oils do not produce products with desirable viscosity and often have strong odour. Polyols are generally used to produce polyurethane foams which are continuing to grow at a rapid rate throughout the world. This growth can be attributed to their light weight, excellent strength to weight ratio, energy absorbing performance and comfort features. Most of the commercially available rigid polyurethane (PU) foams are derived from polypropylene oxide (PPO) triols and polymeric methylene diphenyl diisocyanate (polymeric MDI). However, foams based on PPO polyols have rather low oxidative stability, are sensitive to hydrolysis and have moderate water absorption [10]. An unresolved need therefore exists to devise a more efficient method to prepare soy oil based polyol of improved and selectable functionality and development of PU foam of satisfactory performance.
In this work, the interest lies in preparing bio-polyol from soy-bean oil in a two-step continuous synthesis so that unnecessary intermediate steps are avoided and the synthesized polyol has low odour. More specifically the process includes adding performic acid to the soybean oil in a controlled temperature environment wherein the said performic acid reacts to form epoxidized soybean oil. This is followed by high temperature ring opening and hydroxylation of the epoxidized soybean oil to form soy oil based polyol. These are consecutive non-stop steps. The reaction is not stopped after the formation of epoxidized soy oil or to purify the intermediate products. The polyol was characterized for the presence of functional groups and their transformation during synthesis. The level of unsaturation, hydroxyl numbers and the viscosities were also measured to assess the chemical transformation. Polyurethane (PU) foam was prepared from this soy-polyol using isocyanate, surfactants and water as blowing agent and was characterized for thermal, mechanical and morphological characteristics.
Experimental Procedures
Polyol Preparation
Materials
Formic acid (85%) and hydrogen peroxide (30%) was supplied by Sigma-Aldrich. 1% sodium hydroxide solution was prepared from laboratory grade sodium hydroxide pellets supplied by Fisher Scientific. Sodium chloride, dichloro methane and anhydrous sodium sulphite were supplied by Fisher. Commercially available purified soybean oil was used as the precursor.
Method
Performic acid was pre-formed by low temperature (10–15 °C) reaction of formic acid and hydrogen peroxide. The performic acid mixture was slowly added with soybean oil placed in water bath at 15 °C with constant stirring. Temperature of the reaction mixture was controlled at 15–20 °C to prevent premature dissociation of performic acid and thus to regulate the reaction in a controlled way. Temperature of the reaction was then increased to 65–70 °C and the reaction was allowed to continue for 2 h at this temperature with constant stirring. 1% sodium hydroxide and dichloro methane was added to the mixture and left overnight for separation. After the separation of aqueous layer the pH was checked for neutral followed by washing with saturated sodium chlorite, shaking and separation. Anhydrous sodium sulphite was then added to the mixture with constant stirring and left for 1 h. The synthesized liquid (polyol) was then vacuum-distillated in a rotary evaporator.
Polyurethane Foam Formation
Materials
Soy polyol prepared from the soybean oil was used as a starting material for polyurethane foam formation. Aromatic diisocyanate, polymeric diphenylmethane diisocyanate was supplied by Woodbridge Foam Corporation, Ontario, Canada. Silicone surfactant, and the catalysts dibutyltin dilaurate and tertiary amine, respectively, were obtained from Air Products Inc., Allentown, PA. Distilled water was used as the blowing agent. The silicone surfactant aids in the dispersion of hydrophilic water into hydrophobic soy polyol. The concentrations of all the ingredients have been expressed in parts per hundred parts of polyol, php. A typical formulation used in the present work is presented in Table 1.
Methods of Foaming
Soy polyol and all other ingredients were mixed in the required proportions in ambient conditions for approximately 5 min and then isocyanate was added and mixed well for another minute. Characteristic fibre formation time (time taken for gel formation) was 1 min which represents the reactivity of the components in the polyurethane foam system. After fibre formation, the mixture was poured into an open mold and allowed to expand freely and was left for 1 h at room temperature. It was then cured for 1 h at 75 °C followed by room temperature curing for another 24 h before cutting into the test specimens.
Characterization
Soy Polyol
The soy polyol was characterized to determine its physical properties and the nature of chemical transformation. Viscosity was measured using Brookfield viscometer at 30.5 rpm at room temperature. Iodine number was measured according to ASTM test method D1959 [11] to determine the level of unsaturation and is expressed in terms of the number of centigrams of iodine absorbed per gram of the sample (% iodine absorbed). The hydroxyl content of the soy polyol was determined using the procedure outlined in American Oil Chemists’ Society (AOCS) Tx 1a-66 [12]. Hydroxyl number is defined as the milligrams of potassium hydroxide equivalent to the hydroxyl groups of one gram of sample. The procedure includes the use of pyridine and n-butyl alcohol as solvents and reaction of acetic anhydride with the hydroxyl groups of the soy polyol. Excess acetic anhydride was quenched with water and back titrated with 1.0 N KOH in ethanol with phenolphthalein as the indicator. The acid number was determined using the acid value procedure according to AOCS Cd 3d-63. Isopropyl alcohol and toluene were used to dissolve the soy polyol, and the acid was immediately titrated with 0.1 N KOH in ethanol using phenolphthalein to indicate the endpoint. Functional groups were identified using Bruker FT-IR model TENSOR 27 using at least 64 scan in ATR mode. Before recording a spectrum, the sample holder was flushed with N2 to remove moisture and CO2 and the background was recorded. The sample cell was constantly purged with dry nitrogen while the signal was acquired.
Soy Foam
Soy foam was characterized by several techniques. Thermogravimetric analysis (TGA) was carried out by TA instrument’s thermogravimetric analyzer (TA Q-500), at a heating rate of 10 °C/min in a nitrogen atmosphere and the reproducibility of the results was checked by several runs. The polyurethane (PU) foam density was measured using water immersion method and the compressive strength was measured by Zwick tensile tester (Z100), according to ASTM 1622-93 method at a speed of 1.5 mm/min with a preload of 2 N. The microstructure of PU foam was observed by scanning electron microscope (Hitachi S-2500) at an accelerating voltage of 15 kV. The samples were fractured in liquid nitrogen and then coated with gold using a sputter coater. The foam samples were also examined using the high-resolution X-ray micro-computed tomography system SkyScan 1172 (SkyScan, Belgium). The X-ray tube was operated at 40 kV and 150 μA. Scanning of the specimens was done with 180 degree rotation around the vertical axis and a single rotation step of 0.4. The cross-sectional pixel size was 4.01 μm. After a half circle (180°) was completed, the entire set of radiographs was synthesized by computer software NRecon.
Results and Discussion
Conversion of Soybean Oil to Soy Polyol
The structure and composition of soybean oil varies with region, soil and weather conditions. The soybean oil triglycerides contain both saturated and unsaturated fatty acids. The unsaturated fatty acids are susceptible to oxidation and hydroxyl groups can be introduced to the oil chain after oxidation. In the present study, the process of making soy polyol involved epoxidation and subsequent hydroxylation of soybean oil in a continuous two step process and thus avoiding unnecessary intermediate steps and chemicals. The soy polyol produced was odorless, of light-yellow colour suitable for production of polyurethanes without further purification. Furthermore, the obtained product was found to completely dissolve in ethanol. The results indicate that soy oil was converted into a highly functionalized hydroxylated product. Figure 1 shows the soy polyol produced by the above mentioned procedure.
Characterization
Table 2 shows the density, viscosity, iodine value and hydroxyl number of soybean oil as well as of soy polyol. The density of soy polyol is 1.02 gm/cc which is higher than the density of soybean oil (0.902 gm/cc). Iodine number obtained from the synthesized polyol was found to be 32 which is much lower than the iodine number obtained from soybean oil that is 134. This signifies that the number of unsaturations present in polyol is much lower than that of soy oil as hydroxyl groups were introduced. Viscosity is quite sensitive to the higher molar mass fractions of polymers and oligomers [13]. Increase in viscosity was expected in synthesized soy-polyol as the presence of polar groups increases interaction between the molecules. The viscosity of polyol had increased to 12,000 cps from 72 cps for soy oil. Others have also reported an increase in viscosity [14, 15]. The hydroxyl number is a measure of the degree of dehydration of soy oil. The hydroxyl number for the synthesized polyol was found to be 169. This value is important in establishing reactivity with acids and isocyanates.
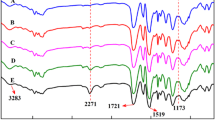
Figure 2a and b show the FTIR spectra of soybean oil and soy polyol. The soy polyol was characterized by a broad peak around 3500 cm−1, assigned to hydroxyl stretching which is not present in the spectra of soy oil. The FTIR of polyols also showed a peak near 1098 cm−1, which is characteristic to the presence of secondary hydroxyl group. The soy polyol mixture was also characterized by the complete disappearance of the C–H stretching band from C=C–H group at 3005 cm−1, and the C=C stretch at 1650 cm−1 (Fig. 2b), both these bands were present in the soy oil spectra (Fig. 2a). Besides, two small bands at 860 cm−1 and 1165 cm−1, assigned to the epoxy groups can also be observed. This set of FTIR spectra further indicates disappearance of the aliphatic ester carbonyl peak (1750 cm−1) in soy polyol.
Preparation of Soy Based PU Foam
Polyurethanes are produced by the polyaddition reaction of the isocyanate group with the polyol in the presence of a catalyst and other additives. The reaction product is a polymer containing the urethane linkage, –RNHCOOR–. When water is used as a blowing agent, isocyanate group reacts with water to form a urea linkage and carbon dioxide gas in the form of bubbles. As more carbon dioxide is generated, the bubbles expand and the foam begins to rise. Alongside the expansion of bubbles, a polymerization reaction takes place in the liquid phase leading to an increase in viscosity. A typical PU foam produced in this study from the soy polyol is shown in Fig. 3. The foam was odorless and nearly white in colour as shown in the figure. The compressive strength is 40.8 kPa and showed complete recovery after compression.
Characterization
The PU foam is a complex mixture of unreacted starting materials and chemical functional groups associated with the polymerization reaction. The FTIR spectrum of the PU foam prepared from soy oil-based polyol is shown in Fig. 4. The spectrum exhibits several characteristic peaks of urethane bonds at 3385 cm−1 (–NH stretching), 1741 cm−1 (–CO stretching), 1514 cm−1 (–NH bending) and 1382 cm−1 (–OCONH asymmetric stretching). The FTIR spectrum also shows characteristic peaks of unreacted NCO groups at 2348 cm−1, emerging carbodiimide at 2276 cm−1 and uretoneimine at 1379 cm−1. A decrease in the absorption intensity of the carbonyl group and –OH stretching can also be observed because of the result of reactions of these groups in soy polyol with isocyanate.
As described before PU foams usually contain several structures, mainly related to the monomers (polyether polyol) in addition to groups those are derived from the starting isocyanates, such as urethane, urea, allophanate and others. This thermal degradation range of PU foam is strongly influenced by the physical characteristics of the PU, namely, internal crosslinking, hydrogen bonds and the inner crystalline structure [16]. The thermal degradation of urethane group occurs randomly in the polymer structure and follows two simultaneous mechanisms [17, 18]: depolymerisation (of precursors, polyol, isocyanate and urea groups) and dissociation (of a six-membered ring transition). Figure 5 shows a representative thermogram of soy polyurethane foam produced in this study. The TGA and DTG plot is characterized by a multi-stage decomposition process with the initial decomposition starting around 270 °C corresponding to the decomposition of urethane bond [10], [19] and [20]. Urethanes are known to be relatively thermally unstable materials [20, 21]. Decomposition of the urethane bond starts at about 150–220 °C leading to the formation of primary amine and olefin or to the formation of secondary amine and carbon dioxide. The weight loss with a maximum at 400 °C is associated with other segments of the remaining structure and most likely represent the degradation of the polyol backbone.
Figure 6a and b show the scanning electron micrographs of soy foam at different magnifications. From both these images it is clear that the PU foam has thermodynamically stable polygonal cell structures. The size and shapes of the pores are quite regular and good cell formation is observed. The observation is also confirmed by the X-ray micro-CT investigation (Fig. 7). Reconstruction results are shown as cross sections corresponding to extremely thin slices through the foam (Fig. 7).
Conclusions
A method has been presented to synthesize soy-based polyol from soy oil in a two step continuous process. FTIR spectroscopy revealed the cleavage of double bonds and presence of hydroxyl groups in the synthesised polyol structure. The soy polyol obtained exhibited higher viscosities (12,000 cps) and lower unsaturation (iodine value: 32) compared to soybean oil. Polyurethane foam produced from soy polyol was found to have comparable density and compressive strength and showed multiple decomposition steps under thermal analysis with initial decomposition starting around 270 °C. The cellular microstructure of soy foam shows polygonal cell structure. This article therefore, suggests a successful conversion of soy oil to soy foam with comparable properties.
References
Chuayjuljit S, Sangpakdee T, Saravari O (2007) J Met Mater Miner 17(1):17–23
Guo A, Javni I, Petrovic Z (1999) ACS PMSE Preprints 80:503
Chian KS, Gan LH (1998) J Appl Polym Sci 68:509
Dahlke B, Poltrock R, Larbig H, Scherzer HD (1998) J Cell Plast 34:361
Hoefer R, Daute P, Grutzmacher R, Westfechtel A (1997) J Coat Technol 69:65
Reed D (1997) Urethane Technol 14:20
Baser SA, Khakhar DV (1993) Cell Polym 12:390
Saggese EJ, Bilyk A, Artymyshyn B, Zubillaga M (1980) J Cell Plast 16:102
Lyon CK, Garrett VH, Frankel EN (1974) J Am Oil Chem Soc 51:331
Szycher M (1999) Szycher’s handbook of polyurethanes. CRC Press, Boca Raton
ASTM (1997) In: Annual book of standards. ASTM, West Conshohocken, PA, Method D1959-97
AOCS (1997) Hydroxyl value of epoxidized oils, official methods and recommended practices of the AOCS, 5th edn. AOCS Press, Champaign, Official Method Tx 1a-6
Doi M, Edwards SF (1992) The theory of polymer dynamics, vol 73. Oxford University Press, New York
Guo A, Cho Y, Petrovic ZS (2000) J Polym Sci A Polym Chem 38:3900
John J, Bhattacharya M, Turner RB (2002) J Appl Polym Sci 86:3097–3107
Pielichowski K, Kulesza K, Pearce EM (2003) J Appl Polym Sci 88:2319–2330
Ravey M, Pearce EM (1997) J Appl Polym Sci 63:47–74
Bakirova IN, Vluev VI, Demchenko IG, Zenitova LA (2002) J Polym Sci A 44(6):615–622
Molero C, de Lucas A, Rodríguez JF (2008) Polym Degrad Stab 93:353–361
Banik I, Sain M (2008) J Reinf Plast Comp 27(4):357–373
Banik I, Sain M (2008) J Reinf Plast Comp 27:1515–1524
Acknowledgements
The authors acknowledge Dr. I. Banik, S. Ghosh Roy, and V. Mavisakalyan from the Faculty of Forestry at the University of Toronto for their help. They also acknowledge Arkema Canada Inc., BioCar® and Natural Sciences and Engineering Research Council of Canada for supporting the project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bandyopadhyay-Ghosh, S., Ghosh, S.B. & Sain, M. Synthesis of Soy-Polyol by Two Step Continuous Route and Development of Soy-Based Polyurethane Foam. J Polym Environ 18, 437–442 (2010). https://doi.org/10.1007/s10924-010-0186-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-010-0186-z