Abstract
The purpose of this study was to analyze the effects of exogenous sodium acetate on astaxanthin accumulation and photoprotection in Haematococcus pluvialis at the non-motile stage. Five or 10 mM sodium acetate increased astaxanthin contents more than two-fold as compared with that in cells without sodium acetate after 6 days of incubation, indicating that exogenous sodium acetate accelerated astaxanthin accumulation at the non-motile stage significantly. Addition of sodium acetate inhibited the chlorophyll fluorescence parameters (ΦPSII, Fv′/Fm′, and qL) as well as photosynthetic rates, indicating that exogenous sodium acetate suppressed photosynthetic activity. However, additional sodium acetate increased respiratory rates. It can be speculated that the enhanced respiration plays an important role in the acceleration of astaxanthin accumulation in the presence of sodium acetate, because acetate can be utilized by the respiratory tricarboxylic acid cycle to generate the carbon skeletons and NAD(P)H for astaxanthin synthesis. Moreover, the level of photoinhibition decreased after adding sodium acetate, which is indicated by the fact that the decrease of the Fv/Fm value from predawn to midday declined on day 4 and day 6. NPQ increased significantly with additional sodium acetate on day 4 and day 6, indicating that additional sodium acetate induced a mechanism to protect algal cells against photoinhibition. Taken together, exogenous sodium acetate enhances astaxanthin accumulation and the photoprotection capacity of H. pluvialis at the non-motile stage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Astaxanthin is an oxygenated carotenoid with high antioxidant capability (Kobayashi and Sakamoto 1999). It is commonly applied in the cosmetic, nutraceutical, and pharmaceutical industries (Hussein et al. 2006). Haematococcus pluvialis, a unicellular green alga, known as the principal source of natural astaxanthin, is mass-cultivated in industrial-scale production (Liu et al. 2014). The cell cycles and proliferation patterns in H. pluvialis are complicated (Zhang et al. 2017a), and a two-stage (motile stage and non-motile stage) culture protocol is widely applied in mass-scale cultivation for astaxanthin production (Borowitzka et al. 1991; Hagen et al. 2001; Wang et al. 2018).
The microalga H. pluvialis has the ability to utilize exogenous organic carbon substrates (Kobayashi et al. 1992), and many previous studies have shown that sodium acetate accelerates cell growth or astaxanthin accumulation (Kobayashi et al. 1993; Orosa et al. 2001). However, the effect of sodium acetate on astaxanthin accumulation is always integrated with increased biomass production (Göksan et al. 2010). Because increasing biomass leads to enhanced astaxanthin as well, it is difficult to distinguish the effect of sodium acetate on the astaxanthin biosynthesis specifically. Furthermore, there is little information about changes of photosynthetic behaviors by adding exogenous sodium acetate at the physiological level at the non-motile stage.
Astaxanthin accumulation in H. pluvialis is commonly induced by high light intensity, and is subject to photosynthetic redox control (Kobayashi et al. 1992; Steinbrenner and Linden 2000, 2003). But it is known that excess light may result in photoinhibition of plant cells (Zhang et al. 2011). Recently, a study showed that the level of photoinhibition in H. pluvialis cultured outdoors decreased as astaxanthin accumulation increased (Zhang et al. 2017b). However, little attention has been given to the effects of additional sodium acetate on the photoinhibition level and photoprotection capacity during astaxanthin accumulation.
This study aims to investigate the effects of exogenous sodium acetate on astaxanthin accumulation and photoprotection capacity in H. pluvialis at the non-motile stage. Algal cells at stationary phase were induced to accumulate astaxanthin without adding new nutrients under outdoor high light illumination, and physiological changes, including photoprotective capacity of cells were analyzed. Determining the photosynthetic behaviors and photoprotection with addition of exogenous sodium acetate would be instructive for optimizing astaxanthin production.
Materials and methods
Strains and culture conditions
The alga Haematococcus pluvialis (strain H6) was obtained from the Institute of Oceanology, Chinese Academy of Sciences. The algal cells were first pre-cultured in modified MCM medium (Sun et al. 2008) at 25 ± 1 °C indoors. Light at an intensity of 200 μmol photon m−2 s−1 was provided from the top by red fluorescent lamps. Aeration with CO2 was adjusted according to pH (7.5–8.5). During the pre-cultivation, the algal cultures were manually shaken three times daily to avoid sticking. Then algal cells (grown phototrophically indoors without additional carbon source) at stationary phase were divided into several 300 mL algal cultures in Erlenmeyer flasks (500 mL). To confirm the effect of sodium acetate on astaxanthin accumulation and photoprotection capacity in H. pluvialis, 0, 5, and 10 mM sodium acetate (Ac) was added to the algal cultures, without adding fresh nutrients. Each set of experiments was done in triplicate. Then the algal cultures were transferred from indoor conditions to outdoor conditions at day 0. During the experiments, the flasks of algal cultures outdoors were manually shaken 5 times a day to avoid sticking, without aeration. Samples of H. pluvialis cells were used for analyses of pigments and physiological changes. The maximal photochemical efficiency of PSII (Fv/Fm) was determined at predawn (6:00) and at midday (12:00), NPQ (non-photochemical quenching) was determined at midday (12:00).
All experiments were done outdoors at the Yunnan Alphy Biotech Co., Ltd. (Yunnan, China). The changes of temperature and solar radiation during the astaxanthin accumulation period are shown in Table 1, as measured by an automatic weather station at Yunnan Alphy Biotech Co., Ltd.
Analytical procedures
Chlorophyll (chlorophyll a and b) and astaxanthin were extracted by methods described in Liu et al. (2002) and were assayed using a UV-visible spectrophotometer according to Lichtenthaler (1987).
The chlorophyll fluorescence parameters, actual photochemical efficiency of PSII (ΦPSII), conversion efficiency of light energy (Fv′/Fm′), the fraction of open PSII centers (qL), maximal photochemical efficiency of PSII (Fv/Fm) and non-photochemical quenching (NPQ) were measured using an FMS-2 pulse modulated fluorometer (Hansatech Instruments, UK) (Jiang et al. 2004; Zhang et al. 2015). NPQ was calculated as Fm/Fm′ − 1, where Fm is the maximal fluorescence yield after dark adaptation, and Fm′ is the maximal yield in a saturation pulse during actinic illumination (Bilger and Björkman 1990), the fast component (qE) and slow component (qI) of NPQ were determined following the protocol of Johnson et al. (1993).
A Clark-type O2 electrode (Hansatech Instruments) was used to measure respiration rates under totally dark conditions and the net photosynthetic O2 evolution rates under saturation actinic light (800 μmol photons m−2 s−1) at room temperature (Zhang et al. 2016b). The total photosynthetic O2 evolution rates are the sum of respiration rates and the net photosynthetic O2 evolution rates.
Chlorophyll a fluorescence (OJIP) transients (Zhang et al. 2016b) of cells were measured with a Handy PEA fluorometer (Hansatech InstrumentsK) and double normalized. All measurements were performed with algal cultures that had been dark-adapted for 10 min at room temperature.
Data in the figures represent the mean ± SD (n = 3) and were subjected to one-way ANOVA and Tukey tests.
Results
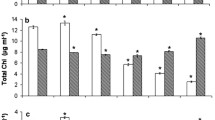
Chlorophyll content increased in the initial 2 days of incubation, and decreased over time after day 2, except in the case of 10 mM Ac which decreased after day 1 (Fig. 1a). Addition of Ac decreased chlorophyll content significantly after 2 days of incubation (P < 0.05). It has been reported that under astaxanthin accumulation conditions, the expression of chlorophyll biosynthesis related genes significantly decreased (Kim et al. 2011). The maximum chlorophyll content (4.00 mg L−1) occurred without Ac addition (0 mM Ac) on day 2. The chlorophyll a/b ratio declined at 1 day of incubation, and basically maintained at relatively high levels between 2.61 and 2.63 in algal cultures without Ac after 2 days (Fig. 1b). Addition of Ac decreased the chlorophyll a/b ratio during the incubation (P < 0.05).
Effects of different Ac concentrations (0, 5, 10 mM) on pigment parameters in H. pluvialis at the non-motile stage. a Chlorophyll content. b Chlorophyll a/b ratio. c Astaxanthin content. d Astaxanthin/chlorophyll ratio. e The algal color of H. pluvialis on day 6 of incubation. Mean ± SE of three replicates
Astaxanthin content increased during the whole incubation (Fig. 1c). Astaxanthin content of Ac-supplemented cells increased significantly after 2 days (P < 0.05), compared with cells without adding Ac. There was no significant difference in the astaxanthin content when the Ac concentration was varied between 5 and 10 mM (P > 0.05). The astaxanthin content in H. pluvialis cells with 5 or 10 mM Ac increased more than two-fold as compared with that in cells without Ac after 6 days, indicating exogenous Ac significantly accelerated astaxanthin accumulation at the non-motile stage. Meanwhile the astaxanthin/chlorophyll ratio increased over time during the incubation (Fig. 1d). The astaxanthin/chlorophyll ratio in presence of Ac was significantly higher than that in absence of Ac after 2 days (P < 0.05). After 6 days of incubation, both 10 and 5 mM Ac increased the astaxanthin/chlorophyll ratio, with values up to 5.5 times and 3.78 times higher than that without Ac, respectively.
In Erlenmeyer flasks, the color of the Ac-treated algal culture was much redder than that without Ac on day 6 (Fig. 1e). On day 6, astaxanthin had already spread gradually throughout the cell in presence of Ac, while only a small amount of astaxanthin was present in absence of Ac (microscopic observation of cell morphology, not shown). The algal color and microscopic observation of cell morphology revealed that the addition of Ac actually accelerated astaxanthin accumulation, which was closely associated with pigmental changes. Therefore, addition of Ac facilitated astaxanthin accumulation and inhibited chlorophyll biosynthesis at the non-motile stage.
The actual photochemical efficiency of photosystem II (PSII) (ΦPSII), an indication of energy in photochemistry (Zhang et al. 2015), decreased sharply in the initial 2 days of incubation, and then increased during the incubation (Fig. 2a). Addition of 10 mM Ac decreased ΦPSII and electron transport rate (ETR) (data not shown) significantly during the incubation (P < 0.05). Conversion efficiency of light energy (Fv′/Fm′) represents energy conversion efficiency of antenna pigments (Maxwell and Johnson 2000). The changes of Fv′/Fm′ was similar with changes of ΦPSII, decreased significantly after 1 day in presence of Ac (Fig. 2b). Additional Ac decreased the fraction of open PSII centers (qL) (Kramer et al. 2004) significantly after day 3 (P < 0.05) (Fig. 2c).
Changes in the respiratory O2 consumption capacity and total photosynthetic O2 evolution capacity in the absence or presence of Ac were detected (Fig. 3). The respiratory O2 consumption capacity without Ac basically maintained constant between 2.83 and 3.44 μmol O2 mg−1Chl min−1 (Fig. 3a). Addition of Ac significantly enhanced respiratory O2 consumption capacity (P < 0.05) after 2 days of incubation. The respiratory rate increased to 4.16 μmol O2 mg−1Chl min−1 after 6 days when 10 mM Ac was supplied (Fig. 3a). The total O2 evolution capacity steeply decreased in the initial 2 days, and basically maintained constant thereafter (Fig. 3b). Addition of Ac decreased the total photosynthetic O2 evolution capacity significantly after day 4 (P < 0.05).
The effects of Ac on chlorophyll a fluorescence (OJIP) transients during the incubation are double normalized in Fig. 4. The addition of Ac changed the OJIP transients after 3 days, indicating that the addition of Ac affected the photosynthetic electron transport chain.
The maximal photochemical efficiency of PSII (Fv/Fm) is an indication of photoinhibition in plants (Takahashi et al. 2009; Zhang et al. 2015). To investigate the effect of additional Ac on the photoprotection capacity at the non-motile stage, the changes of Fv/Fm in the absence or presence of Ac were measured at predawn (6:00) and at midday (12:00), respectively (Fig. 5). With increased time, the Fv/Fm at predawn increased in the initial 4 days and then decreased after 4 days in the absence of Ac. In Ac-treated cells, Fv/Fm at predawn maintained constant during the initial 4 days and then decreased over time after day 4. Compared with the Fv/Fm measured at predawn, the Fv/Fm at midday declined significantly in the absence or presence of Ac during the incubation, suggesting that the high light at midday led to photoinhibition. Compared with the Fv/Fm measured at predawn, the Fv/Fm value at midday in the absence or presence of Ac decreased 74% and 57%, respectively, after 1 day of incubation, suggesting that the photoinhibition in cells without Ac was more severe than that in Ac-treated cells.
Non-photochemical quenching (NPQ) is used to indicate the protective efficiency of the photosynthetic mechanism (Masojídek et al. 2000); qE and qI are the fast and slow component of NPQ, respectively (Fig. 6). Values of NPQ and qE decreased during the incubation (Fig. 6a, b). In contrast, the qI value increased sharply over 4 days, then decreased slightly after day 4 (Fig. 6c). Addition of Ac significantly increased NPQ and qE during the incubation (P < 0.05). There was no significant difference in qI values between 0 and 10 mM Ac-treated cells (P > 0.05).
Discussion
Chlorophyll fluorescence parameters, ΦPSII, ETR, Fv′/Fm′, and qL in Ac-treated cells significantly decreased compared with that in cells without Ac (Fig. 2a), suggesting that photosynthetic efficiency was restricted in presence of Ac. The presence of acetate was reported to strongly influence the photosynthetic fluorescence parameters as well, due to generation of reducing power in the chloroplast causing a dark reduction of plastoquinones in Chlamydomonas reinhardtii (Johnson and Alric 2012). The changes in OJIP transients (Fig. 4) after 3 days showed that addition of Ac had a direct effect on the photosynthetic electron transport chain. Moreover, additional Ac significantly inhibited the total photosynthetic O2 evolution capacity (Fig. 3b). In contrast, during astaxanthin accumulation, the respiratory O2 consumption capacity was significantly enhanced by adding Ac (Fig. 3a) and thus it can be proposed that Ac was absorbed and utilized by H. pluvialis as substrate for respiratory metabolism. The substrates for astaxanthin synthesis (glycerate-3-phosphate, glyceraldehyde-3-phosphate and pyruvate) are derived mainly from photosynthetic carbon fixation, glycolysis, or from gluconeogenesis (Wingler et al. 2000; Zhang et al. 2016a). Therefore, it is proposed that the enhanced respiration plays an important role in the acceleration of astaxanthin accumulation in presence of Ac.
Exogenous Ac is transported across the cell membrane by the proton-linked monocarboxylate transporter protein (Becker et al. 2005), and then is assimilated to form acetyl coenzyme A (acetyl-CoA) through the condensation reaction with coenzyme A, catalyzed by acetyl-CoA synthetase (Ke et al. 2000; Lin and Oliver 2008; Boyle and Morgan 2009). After adding Ac, the metabolite flux rate in the glyoxylate cycle, TCA cycle, and pentose phosphate pathway increases (Yang et al. 2000, 2002; Hong and Lee 2007; Boyle and Morgan 2009). These metabolic pathways provide carbon skeletons and NAD(P)H, which are indispensable for astaxanthin biosynthesis (Boyle and Morgan 2009; Perez-Garcia et al. 2011). Exogenous Ac enhances the metabolite flux rate in fatty acid synthesis in C. reinhardtii as well (Boyle and Morgan 2009). Accumulation of fatty acids is linearity correlated with astaxanthin content (Zhekisheva et al. 2002). Hence, it is postulated that Ac enhanced astaxanthin accumulation in the following ways: (i) increased acetyl-CoA directly enhanced respiratory rates to provide carbon skeletons and NAD(P)H for astaxanthin biosynthesis; (ii) increased acetyl-CoA indirectly enhanced fatty acid synthesis to accelerate astaxanthin accumulation. Moreover, it was reported that oxidative stress in Ac-treated H. pluvialis cells played a part in the posttranslational activation of carotenoid biosynthesis (Kobayashi et al. 1993). Further studies are needed to address these possibilities.
Astaxanthin accumulation is usually conducted by high light induction (Scibilia et al. 2015); however, excess light energy may accelerate the generation of reactive oxygen species (ROS), resulting in photoinhibition (Zhang et al. 2011). The decrease of Fv/Fm from predawn to midday in absence of Ac was significantly greater than that in presence of Ac (Fig. 5), indicating the level of photoinhibition decreased after adding Ac during astaxanthin accumulation. Concurrently, NPQ can protect photosystems against photoinhibition by preventing the generation of ROS caused by excess light (Niyogi 2000). qE is the main component of NPQ, controlled by the xanthophyll cycle, dissipating excess absorbed light energy in PSII; qI is to quantify energy trapped in closed reaction center of PSII during thermal dissipation processes (Masojídek et al. 2000; Zhang et al. 2017b). During astaxanthin accumulation, NPQ and qE were significantly enhanced by addition of Ac (Fig. 6a, b), suggesting that additional Ac induced a protective mechanism to protect cells against photoinhibition. However, the precise mechanisms in enhancement of astaxanthin accumulation and photoprotection capacity, and the interrelation between increased astaxanthin accumulation and enhanced photoprotection capacity after adding Ac at the non-motile stage have not been clarified. Further studies are needed to address these questions.
Conclusion
Exogenous sodium acetate (Ac) significantly accelerated astaxanthin accumulation in H. pluvialis at the non-motile stage. Exogenous Ac suppressed photosynthetic activity and facilitated respiratory activity. It can be speculated that the enhanced respiration plays an important role in acceleration of astaxanthin accumulation after adding Ac. Moreover, the level of photoinhibition decreased after adding Ac during astaxanthin accumulation. Concurrently, NPQ increased significantly with additional Ac, indicating additional Ac induced a mechanism to protect H. pluvialis cells against photoinhibition. Taken together, exogenous Ac enhances astaxanthin accumulation and photoprotection capacity in H. pluvialis at the non-motile stage.
References
Becker HM, Hirnet D, Fecher-Trost C, Sultemeyer D, Deitmer JW (2005) Transport activity of MCT1 expressed in Xenopus oocytes is increased by interaction with carbonic anhydrase. J Biol Chem 280:39882–39889
Bilger W, Björkman O (1990) Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynth Res 25:173–185
Borowitzka MA, Huisman JM, Osborn A (1991) Culture of the astaxanthin-producing green alga Haematococcus pluvialis, 1. Effects of nutrients on growth and cell type. J Appl Phycol 3:295–304
Boyle NR, Morgan JA (2009) Flux balance analysis of primary metabolism in Chlamydomonas reinhardtii. BMC Systems Biol 3:1–14
Göksan T, Ak İ, Şevket G (2010) An alternative approach to the traditional mixotrophic cultures of Haematococcus pluvialis Flotow (Chlorophyceae). J Microbiol Biotechnol 20:1276–1282
Hagen C, Grünewald K, Xyländer M, Rothe E (2001) Effect of cultivation parameters on growth and pigment biosynthesis in flagellated cells of Haematococcus pluvialis. J Appl Phycol 13:79–87
Hong SJ, Lee CG (2007) Evaluation of central metabolism based on a genomic database of Synechocystis PCC6803. Biotechnol Bioprocess Eng 12:165–173
Hussein G, Sankawa U, Goto H, Matsumoto K, Watanabe H (2006) Astaxanthin, a carotenoid with potential in human health and nutrition. J Nat Prod 69:443–449
Jiang CD, Gao HY, Zou Q, Jiang GM, Li LH (2004) Leaf orientation, photorespiration and xanthophyll cycle protect young soybean leaves against high irradiance in field. Env Exp Bot 55:87–96
Johnson GN, Young AJ, Scholes JD, Horton P (1993) The dissipation of excess excitation energy in British plant species. Plant Cell Environ 16:673–679
Johnson X, Alric J (2012) Interaction between starch breakdown, acetate assimilation, and photosynthetic cyclic electron flow in Chlamydomonas reinhardtii. J Biol Chem 287:26445–26452
Ke J, Behal RH, Back SL, Nikolau BJ (2000) The role of pyruvate dehydrogenase and acetyl-coenzyme A synthetase in fatty acid synthesis in developing Arabidopsis seeds. Plant Physiol 123:497–508
Kim DK, Hong SJ, Bae JH, Yim N, Jin E, Lee C-G (2011) Transcriptomic analysis of Haematococcus lacustris, during astaxanthin accumulation under high irradiance and nutrient starvation. Biotechnol Bioprocess Eng 16:698–705
Kobayashi M, Kakizono T, Nagai S (1993) Enhanced carotenoid biosynthesis by oxidative stress in acetate-induced cyst cells of a green unicellular alga, Haematococcus pluvialis. Appl Environ Microbiol 59:867–873
Kobayashi M, Kakizono T, Yamaguchi K, Nishio N, Nagai S (1992) Growth and astaxanthin formation of Haematococcus pluvialis in heterotrophic and mixotrophic conditions. J Ferment Bioeng 74:17–20
Kobayashi M, Sakamoto Y (1999) Singlet oxygen quenching ability of astaxanthin esters from the green alga Haematococcus pluvialis. Biotechnol Lett 21:265–269
Kramer DM, Johnson G, Kiirats O, Edwards GE (2004) New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosynth Res 79:209–218
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Meth Enzymol 148:350–382
Lin M, Oliver DJ (2008) The role of acetyl-coenzyme A synthetase in Arabidopsis. Plant Physiol 147:1822–1829
Liu JG, Yin MY, Zhang JP, Liu W, Meng ZC (2002) Dynamic changes of inorganic nitrogen and astaxanthin accumulation in Haematococcus pluvialis. Chin J Oceanol Limnol 20:358–364
Liu JG, Li QQ, Liu Q, He M, Zhang L, Liu YD, Ding Y, Zhang Z, Lin W, Song P, Li L, Huang Y, Han C (2014) Screening of unicellular microalgae for biofuels and bioactive products and development of a pilot platform. Algol Stud 145:99–117
Masojídek J, Torzillo G, Kopecký J, Koblížek M, Nidiaci L, Komenda A, Lukavska A, Sacchi A (2000) Changes in chlorophyll fluorescence quenching and pigment composition in the green alga Chlorococcum sp. grown under nitrogen deficiency and salinity stress. J Appl Phycol 12:417–426
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence—a practical guide. J Exp Bot 51:659–668
Niyogi KK (2000) Safety valves for photosynthesis. Curr Opin Plant Biol 3:455–460
Orosa M, Franqueira D, Cid A, Albade J (2001) Carotenoid accumulation in Haematococcus pluvialis in mixotrophic growth. Biotechnol Lett 23:373–378
Perez-Garcia O, Escalante FM, De-Bashan LE, Bashan Y (2011) Heterotrophic cultures of microalgae: metabolism and potential products. Water Res 45:11–36
Scibilia L, Girolomoni L, Berteotti S, Alboresi A, Ballottari M (2015) Photosynthetic response to nitrogen starvation and high light in Haematococcus pluvialis. Algal Res 12:170–181
Steinbrenner J, Linden H (2000) Regulation of two carotenoid biosynthesis genes coding for phytoene synthase and carotenoid hydroxylase during stress-induced astaxanthin biosynthesis in the green alga Haematococcus pluvialis. Plant Physiol 125:810–817
Steinbrenner J, Linden H (2003) Light induction of carotenoid biosynthesis genes in the green alga Haematococcus pluvialis: regulation by photosynthetic redox control. Plant Molec Biol 52:343–356
Sun YH, Liu JG, Zhang XL, Lin W (2008) Strain H 2-419-4 of Haematococcus pluvialis induced by ethyl methanesulphonate and ultraviolet radiation. Chin J Oceanol Limnol 26:152–156
Takahashi S, Milward SE, Fan DY, Chow WS, Badger MR (2009) How does cyclic electron flow alleviate photoinhibition in Arabidopsis? Plant Physiol 149:1560–1567
Wang N, Guan B, Kong Q, Duan L (2018) A semi-continuous cultivation method for Haematococcus pluvialis from non-motile cells to motile cells. J Appl Phycol 30:773–781
Wingler A, Lea PJ, Quick WP, Leegood RC (2000) Photorespiration: metabolic pathways and their role in stress protection. Phil Trans Roy Soc B 355:1517–1529
Yang C, Hua Q, Shimizu K (2000) Energetics and carbon metabolism during growth of microalgal cells under photoautotrophic, mixotrophic and cyclic light-autotrophic/dark-heterotrophic conditions. Biochem Eng J 6:87–102
Yang C, Hua Q, Shimizu K (2002) Integration of the information from gene expression and metabolic fluxes for the analysis of the regulatory mechanisms in Synechocystis. Appl Microbiol Biotechnol 58:813–822
Zhang CH, Liu JG, Zhang LT (2017a) Cell cycles and proliferation patterns in Haematococcus pluvialis. Chin J Oceanol Limnol 35:1205–1211
Zhang CH, Zhang LT, Liu JG (2016b) The role of photorespiration during astaxanthin accumulation in Haematococcus pluvialis (Chlorophyceae). Plant Physiol Biochem 107:75–81
Zhang LT, He ML, Liu JG, Li L (2015) Role of the mitochondrial alternative oxidase pathway in hydrogen photoproduction in Chlorella protothecoides. Planta 241:1005–1014
Zhang LT, Li L, He ML, Liu J (2016a) The role of photorespiration during H2 photoproduction in Chlorella protothecoides under nitrogen limitation. Plant Cell Rep 35:1–4
Zhang LT, Su F, Zhang CH, Gong F, Liu J (2017b) Changes of photosynthetic behaviors and photoprotection during cell transformation and astaxanthin accumulation in Haematococcus pluvialis grown outdoors in tubular photobioreactors. Int J Molec Sci 18:33
Zhang LT, Zhang ZS, Gao HY, Xue ZC, Yang C, Meng XL, Meng QW (2011) Mitochondrial alternative oxdiase pathway protects plants against photoinhibition by alleviating inhibition of the repair of photodamaged PSII through preventing formation of reactive oxygen species in Rumex K-1 leaves. Physiol Plant 143:396–407
Zhekisheva M, Boussiba S, Khozin-Goldberg I, Zarka A, Cohen Z (2002) Accumulation of oleic acid in Haematococcus pluvialis (Chlorophyceae) under nitrogen starvation or high light is correlated with that of astaxanthin eaters. J Phycol 38:325–331
Acknowledgements
We thank Dr. John van der Meer (Pan-American Marine Biotechnology Association) for his English editing.
Contributions
Chunhui Zhang and Jianguo Liu designed the study and wrote the manuscript; Chunhui Zhang and Litao Zhang performed the experiments and analyzed the data. All authors read and approved the manuscript.
Funding
This work was financial supported by National Natural Science Foundation of China (Nos. 31572639, U1706209).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, C., Zhang, L. & Liu, J. Exogenous sodium acetate enhances astaxanthin accumulation and photoprotection in Haematococcus pluvialis at the non-motile stage. J Appl Phycol 31, 1001–1008 (2019). https://doi.org/10.1007/s10811-018-1622-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-018-1622-z