Abstract
Non-motile cells of Haematococcus pluvialis grow slowly, whereas motile cells grow fast and divide frequently. Cultivation from non-motile cells to motile cells of H. pluvialis was implemented to promote semi-continuous production. When old cultures which consist of non-motile cells were inoculated in fresh medium with an inoculation amount less than 15%, zoospores were produced in the non-motile cells and developed into motile cells, as the concentration of astaxanthin inducer in the medium was below the threshold value. This process was accomplished within 3 days after inoculation. Furthermore, enhancing KNO3 content to 1200 mg L−1 or reducing light intensity to 20 μmol photons m−2 s−1 could increase growth during the late culturing period of H. pluvialis and postpone the next round of transformation from motile cells to non-motile cells. A semi-continuous cultivation method for H. pluvialis from non-motile cells to motile cells is proposed in order to regulate the life cycle and promote industrial production. This cultivation mode shortens the inoculum cultivation stage and simplifies the production process of H. pluvialis, showing considerable commercial potential.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Haematococcus pluvialis, a unicellular green alga, is the most promising producer of natural astaxanthin. Haematococcus pluvialis synthetizes astaxanthin to protect the cells from oxidative stress (Li et al. 2008) under high light (Steinbrenner and Linden 2003), high temperature (Tjahjono et al. 1994) or nutrient deprivation (Boussiba et al. 1999). Astaxanthin (3,3′-dihydroxy-β,β-carotene-4,4′-dione) possesses stronger antioxidant activity than many other carotenoids and vitamins (Guerin et al. 2003) and is highly effective in eliminating free radicals, reducing DNA damage and improving the immune system (Bustos-Garza et al. 2013). Due to its health benefits, astaxanthin is widely desired by the food, feed, nutraceutical and pharmaceutical industries (Ahmed et al. 2015).
Culture conditions affect the cell type of H. pluvialis, and the main factor leading to carotenoid accumulation is nitrogen starvation (Borowitzka et al. 1991). Haematococcus pluvialis grows as motile cells in normal conditions and begins to form non-motile cells (aplanospores) under stress conditions (Liu et al. 2000). Non-motile cells of H. pluvialis grow little, while motile cells grow fast and divide frequently (Boussiba 2000). Because of the transition from motile cells to non-motile cells when environmental conditions become unfavorable for normal cell growth, two-stage cultivation of H. pluvialis has been generally adopted (Fabregas et al. 2001). Nitrogen and light are important factors in algae cultivation (Fuentes-Grünewald et al. 2012). In the two stages of H. pluvialis, nitrogen, light and dissolved oxygen affect both cell growth and astaxanthin accumulation significantly (Kobayashi et al. 1992; Lee and Ding 1995; Kim et al. 2006; Kang et al. 2010; Wan et al. 2015). At present, many studies have focused on accumulation of astaxanthin and the promotion of the transformation from motile cells to non-motile cells (Zhang et al. 1999; Hata et al. 2001; Pérez-López et al. 2014). Commercial production of astaxanthin from H. pluvialis is viable in outdoor photobioreactors and open ponds and different systems have been applied in different process steps. For example, the Aquasearch Growth Module technology has been used for large-scale biomass production, and either AGM technology or simpler open pond technology have been used for carotenogenesis and astaxanthin accumulation (Olaizola 2000).
Kobayashi et al. (1997) divided the life cycle of H. pluvialis into four stages: vegetative cell growth stage, encystment stage, maturation stage and germination stage. When mature cysts are centrifuged, washed and transferred into fresh medium, they germinate and produce daughter cells. Wayama et al. (2013) visualized the dynamics of astaxanthin accumulation and subcellular changes during encystment in H. pluvialis with three-dimensional transmission electron microscopy, clearly showing the asexual life cycle of H. pluvialis.
In our previous work, red colonies of H. pluvialis were cultivated into motile cells and proliferated (Wang et al. 2016). After motile cells grew into non-motile cells and accumulated astaxanthin, however, non-motile cells were not transformed to motile cells again in large scale cultivation, due to the activity decrease of the non-motile cells. Integral life cycle and continuous production of H. pluvialis were not achieved, and industrial production mode of H. pluvialis was single-track. Thus, a semi-continuous cultivation method from non-motile cells to motile cells is proposed in this study, which combines regulating the life cycle, semi-continuous cultivation and enhancing cell growth, aiming at promoting industrial production of H. pluvialis. Furthermore, the specific culture environment required in this semi-continuous cultivation is provided.
Materials and methods
Haematococcus pluvialis FACHB-712 was from the Freshwater Algae Culture Collection at the Institute of Hydrobiology, China, and maintained in the School of Food Science and Engineering, Ocean University of China. The composition of revised MCM medium was (in mg L−1): KNO3 600, KH2PO4 20, MgSO4·7H2O 200, CaCl2 20, H3BO3 0.0012, MnSO4·H2O 0.0084, ZnSO4 0.0072, CuSO4·5H2O 0.0062, Na2MoO4·2H2O 0.0007, CoCl2·2H2O 0.0005, EDTA·2Na 0.0004, FeSO4·7H2O 0.0004.
Motile cell cultivation was in moderate conditions: the green cells were cultivated at 22 °C for 10 days, with a day/night cycle of 12 h:12 h and daylight intensity of 34 μmol photons m−2 s−1.
Non-motile cell cultivation was under stress conditions: the red cells were obtained by exposing green cells to continuous light of 101 μmol photons m−2 s−1 at 30 °C for another 10 days.
Semi-continuous cultivation from non-motile cells to motile cells was in moderate conditions: non-motile cells were transferred into fresh medium with an inoculation amount not more than 15% and cultivated at 22 °C for 10 days, with a day/night cycle of 12 h:12 h and daylight intensity of 20 μmol photons m−2 s−1. In particular, KNO3 content in the medium was increased to 1200 mg L−1.
Motile and non-motile cell numbers were determined using a hemocytometer. The dry cell weight (g L−1) was measured by spectrophotometry and calculated using the equation (Katsuda et al. 2004) below:
Chlorophyll was extracted with 80% acetone at 50 °C for 20 min (Wellburn 1994) and measured by spectrophotometry. The total chlorophyll (Chl a & Chl b) was calculated using the following equation (Porra 2002):
Nitrate nitrogen concentration was measured with an ultraviolet spectrophotometer at the wavelength of 220 nm by establishing standard curve.
Results
Effect of inoculation amount of old cultures on growth of H. pluvialis
Inoculum size of non-motile cells had a significant effect in the semi-continuous cultivation of H. pluvialis. As is shown in Fig. 1a, recovery from non-motile cells to motile cells was reduced with an increase in inoculum size. When the inoculum size of non-motile cells was not more than 15%, the culture was bright green after 5 days and the concentration of motile cells rose with a larger inoculation amount. When the inoculum size was between 20 and 30%, the culture was nearly yellowish brown, indicating that many non-motile cells remained. Finally, when the inoculum size exceeded 40%, H. pluvialis appeared to have stopped producing motile cells, and the culture was reddish brown. Furthermore, settling of non-motile cells was obvious in the reddish brown suspension. When the cultures above were re-inoculated at a level of 10% in fresh medium and cultivated, the green cells from the cultures with 30% or less inoculum size grew energetically. Re-inoculation of the 40 to 60% inoculum size cultures resulted in a few motile cells, but re-inoculation of the 80 to 100% inoculum size cultures did not result in growth.
For expanded cultivation in 100 mL fresh medium, non-motile cell cultures were inoculated at 5, 10, 50, and 100% for 10-day culture (Fig. 1b). Compared with the control group grown from a 10% motile cell culture, the cultures with 5 or 10% old culture inoculum transformed to motile cells, with the cultures changing from light red to pale yellow to light green and to dark green in sequence during the cultivation. However, the culture inoculated with 50% non-motile cells declined conspicuously and became tawny in color, while the 100% non-motile cell culture showed no obvious change.
Microscopical observation showed that in the culture inoculated with the 10% motile cell culture, the cells exhibited strong motility. The color of red cells began to be lighter and a green margin appeared on the 2nd day. Furthermore a part of non-motile cells fractured and zoospores were released on the 3rd day and a group of four motile cells which grew from the zoospores was observed on the 4th day, although there was a little red in the center of each green cell. In the cultures inoculated with the 50 or 100% non-motile cultures, the cells began to decline on the 6th day.
Growth status of H. pluvialis during the cultivation from non-motile cells to motile cells
Considering the effect of inoculation size on cell number and growth, non-motile fresh medium was inoculated with 10% of a non-motile cell culture and cultivated under moderate condition. The proportion of non-motile cells and motile cells during the cultivation is shown in Fig. 2. In the first 3 days, the percentage of non-motile cells decreased and motile cells increased sharply. From the 4th day, the proportion of motile cells was almost stable around 90% until the 8th day, when the non-motile cell percentage rose slightly due to increased biomass and nutrition consumption in the medium.
No significant differences in dry cell weight and chlorophyll content were detected between the control group inoculated with 10% motile cell culture, and the experimental group inoculated with 10% non-motile culture (Fig. 3a) with the non-motile cell culture having slightly higher dry cell weight from the 3rd day. In the last 2 days of cultivation, the dry cell weight of motile culture was still increasing, while the non-motile culture had ceased growth, showing distinct differences in growth activity from different inocula in the late phase of cultivation. A similar tendency was observed in the change of chlorophyll content (Fig. 3b). After inoculation, the chlorophyll content of non-motile-cell-inoculum culture increased to the same level of motile-cell-inocum culture within 3 days and on the 9th day, chlorophyll content was lower than that of the motile-cell-inoculum culture.
Based on the experiment results above, cultivation from non-motile cells to motile cells of H. pluvialis is shown in Online Resource 1. In moderate culturing environment, H. pluvialis cells grew and proliferated in the form of motile cells, while under stress culturing environment, the cells transformed into non-motile cells. When the non-motile cell culture was inoculated (inoculation amount ≤ 15%) in fresh medium and cultivated in moderate environment, astaxanthin in the non-motile cells was metabolized and zoospores were produced. Zoospores developed into motile cells, and the red parent cells declined. Thus, cultivation of H. pluvialis from non-motile cells to motile cells was accomplished, and this process was generally within 3 days after inoculation.
Regulation of nitrate nitrogen concentration for the semi-continuous cultivation of H. pluvialis
Compared with other nutrients, nitrogen is required much more by H. pluvialis and nitrogen concentration in the medium is one of the main factors that affect its life cycle. Nitrogen lack leads to astaxanthin accumulation (Scibilia et al. 2015), thus, nitrogen concentration was enhanced in this semi-continuous cultivation method of H. pluvialis. In the motile stage, KNO3 content was 600 mg L−1, and in semi-continuous cultivation with an inoculation amount of 10% non-motile cells, KNO3 content was increased to 900, 1200 or 1500 mg L−1 with no change of other conditions. At the same time, lower nitrate nitrogen groups were also set for comparison, in which KNO3 content was 300 or 200 mg L−1. Change of dry cell weight in semi-continuous cultivation with different initial nitrate nitrogen is shown in Fig. 4a. High nitrogen groups has slightly lower dry cell weight than the control group (600 mg L−1 KNO3), indicating that high initial nitrogen had an inhibiting effect on cell growth. Dry cell weights of low nitrogen groups increased slowly in the logarithmic phase, indicating that nitrogen lack inhibited cell growth severely. Therefore, higher biomass requires the addition of nitrogen, such as by a batch-fed method (Lababpour et al. 2005; Sun et al. 2015).
Chlorophyll content reflects cell activity of H. pluvialis (Orosa et al. 2005). Chlorophyll content in this semi-continuous cultivation with different initial nitrate nitrogen is shown in Fig. 4b. The effect of initial nitrogen in the medium on chlorophyll content was much more significant than that on dry cell weight, indicating that regulation of nitrate nitrogen concentration played a more important role in activity recovery than in biomass during the semi-continuous cultivation from non-motile cells to motile cells. On the 10th day, chlorophyll content of high nitrogen groups exceeded the control group (600 mg L−1), while low nitrogen groups had a lower content. Among them, 1200 mg L−1 KNO3 contributed most in enhancing chlorophyll content.
The concentration of nitrate nitrogen in the medium with different initial quantities and average consumption of nitrate nitrogen by 1 g dry cells of H. pluvialis is given in Online Resource 2. Nitrate nitrogen was consumed by H. pluvialis cells gradually. In high nitrogen group of 1200 mg L−1 KNO3, the average consumption was the least, reflecting the highest nitrogen utilization efficiency and cell activity among all the experimental groups. It is also found that the cells had strong motility in high initial nitrogen medium, while the cells tended to clump and yellow in low initial nitrogen medium. Under the microscope, a red center appeared in each motile cell in low nitrogen groups showing weak growth ability. Hence, raising nitrogen concentration within an appropriate range contributed to enhancing growth activity and postponing the next round of astaxanthin accumulation in this semi-continuous cultivation of H. pluvialis.
Regulation of light intensity for the semi-continuous cultivation
Light intensity is an important factor for photosynthesis and a determinant for cell growth of H. pluvialis in autotrophic cultivation. High light intensity resulted in sluggish cell proliferation and astaxanthin synthesis (Hagen et al. 2001). In semi-continuous cultivation with an inoculation of 10% non-motile cell culture, the effect of light intensity was studied. Dry cell weight and chlorophyll content of H. pluvialis cells under different light intensities are shown in Fig. 5. Compared with the 34 μmol photons m−2 s−1 group the dry cell weight in the 27 and 20 μmol photons m−2 s−1 groups was a little lower in the logarithmic phase, but not significantly, on the 10th day. In the 14 and 7 μmol photons m−2 s−1 groups both dry cell weight and chlorophyll content declined. When light intensity was adjusted to 20 μmol photons m−2 s−1, chlorophyll content was the highest among the experimental groups on the 10th day. Therefore, reducing light intensity within a certain range contributed to enhancing growth in the late phase and postponing the second round of astaxanthin accumulation during the semi-continuous cultivation. However, excessively low light intensity was related to growth retardation and a long adaptive phase, which counted against cell growth.
Discussion
The non-motile-cell-inoculum culture enter the astaxanthin accumulating stage quicker than the motile-cell-inoculum culture, and this may be related to the different growth status with different inoculumn sizet of old culture of H. pluvialis. It is suggested that larger inoculum size of non-motile cells led to a greater residue of extracellular metabolites. It appears that an inducer of astaxanthin synthesis is released into the medium by the H. pluvialis cells under stress conditions, which triggered accumulation of astaxanthin and change of growth stage. The existence of the astaxanthin inducer also explains why red particuless remained in the center of motile cells and that non-motile cells declined. After the non-motile culture was inoculated at low density in fresh medium, the concentration of the inducer was diluted, most astaxanthin in the non-motile cells was metabolized and zoospores were dispersed. The metabolism of astaxanthin in the formation of the zoospores meant that the culture changed from red to green. However, since a small quantity of astaxanthin inducer remained, astaxanthin particles were accumulated in the center of the motile cells. When the non-motile cells were inoculated at high density (50 to 100%), cell metabolism was affected heavily by the inducer. The H. pluvialis cells failed to regulate their metabolism under moderate culturing environment with a high concentration of the inducer, leading to cell decline.
Dilution of astaxanthin inducer in the medium was the direct cause of zoospore formation. If the astaxanthin inducer of H. pluvialis could be identified and separated, regulation of cell growth stage could be achieved. Removing the inducer promotes cell division and enhances biomass, while adding the inducer increases astaxanthin synthesis, which is quite important for cell regulation and large scale cultivation of H. pluvialis. However, few studies have focused on extracellular metabolites of H. pluvialis. Borowitzka (2016) summarized the chemically mediated interactions between algae and algae and other organisms, and describes autoinhibition. Autoinhibition is where an alga inhibits itself. Auto-inhibitors of Parietochloris incisa have been found in old culture supernatant, and this substance inhibited cell growth and synthesis of chlorophyll, carotene, fatty acid, and protein (Liu et al. 2002). 15-Hydroxyeicosapentaenoic acid was separated both in Skeletonema costatum cell and culture medium, which inhibited its own cell growth (Imada et al. 1991, 1992).
Sun et al. (2001) supplemented equivalent nutrients as refresh medium into old medium of H. pluvialis, the results indicated that some unknown substances, like auto-inhibitors, existed in the old culture, reducing cell growth by decreasing motile cell growth and inducing transformation from motile cells into non-motile cells. Liu et al. (2004) found that the auto-regulators in Haematococcus old culture supernatant were organic soluble substances, and that process of DNA replication in the motile cells did not stop, but that the process of the cell division was heavily blocked by the old culture supernatant.
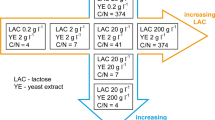
Due to the effect of extracellular metabolites, non-motile-cell-inoculum culture of H. pluvialis accumulate astaxanthin better than the motile-cell-inoculum culture. By regulation of nitrate nitrogen concentration and light intensity, semi-continuous cultivation from non-motile cells to motile cells of H. pluvialis was modified, especially in growth activity. Compared with growth condition of motile-cell-inoculum culture (additional data in Online Resource 3), initial nitrate nitrogen concentration was enhanced and light intensity was decreased. This semi-continuous cultivation method of H. pluvialis verified the integrality and continuity of its life cycle, which is beneficial for the artificial control of growth stage. If applied in large scale production, this method will help the cultures improve adaptability to environmental variability. When green cultures cease to grow and begin to accumulate astaxanthin unexpectedly due to condition fluctuation or long time storage, the cell activity could be adjusted and recovered, which will promote the productive stabilization significantly and avoid waste of time and cost. Thus, a new method of semi-continuous cultivation is provided for H. pluvialis production, in which “motile cell growth-astaxanthin accumulation-next round of motile cell growth and astaxanthin accumulation” model is used (Fig. 6). After motile cells of H. pluvialis have reached prospective biomass, they will be regulated to accumulate astaxanthin. Then, 90% non-motile cells are collected for astaxanthin extraction and purification, while the remaining10% are directly used as inocula for the next batch of biomass production.
By enhancing initial nitrate nitrogen concentration, decreasing light intensity and controlling inoculum size, this semi-continuous cultivation method for H. pluvialis from non-motile cells to motile cells could be promising in a larger cultivation system of cell growth and astaxanthin accumulation. The semi-continuous production will also abridge inoculum cultivation and shorten the production cycle of H. pluvialis enhancing the economics of the process.
References
Ahmed F, Li Y, Fanning K, Netzel M, Schenk PM (2015) Effect of drying, storage temperature and air exposure on astaxanthin stability from Haematococcus pluvialis. Food Res Int 74:231–236
Borowitzka MA (2016) Chemically-mediated interactions in microalgae. In: Borowitzka MA, Beardall J, Raven JA (eds) The physiology of microalgae. Springer, Dordrecht, pp 321–357
Borowitzka MA, Huisman JM, Osborn A (1991) Culture of the astaxanthin-producing green alga Haematococcus pluvialis. 1. Effects of nutrients on growth and cell type. J Appl Phycol 3:295–304
Boussiba S (2000) Carotenogenesis in the green alga Haematococcus pluvialis: cellular physiology and stress response. Physiol Plant 108:111–117
Boussiba S, Bing W, Yuan J, Zarka A, Chen F (1999) Changes in pigments profile in the green alga Haematococcus pluvialis exposed to environmental stresses. Biotechnol Lett 21:601–604
Bustos-Garza C, Yáñez-Fernández J, Barragán-Huerta BE (2013) Thermal and pH stability of spray-dried encapsulated astaxanthin oleoresin from Haematococcus pluvialis using several encapsulation wall materials. Food Res Int 54:641–649
Fabregas J, Otero A, Maseda A, Dominguez A (2001) Two-stage cultures for the production of astaxanthin from Haematococcus pluvialis. J Biotechnol 89:65–71
Fuentes-Grünewald C,·Garcés E,·Alacid E,·Sampedro N,·Rossi S, Camp J (2012) Improvement of lipid production in the marine strains Alexandrium minutum and Heterosigma akashiwo by utilizing abiotic parameters. J Ind Microbiol Biotechnol 39: 207–216
Guerin M, Huntley ME, Olaizola M (2003) Haematococcus astaxanthin: applications for human health and nutrition. Trends Biotechnol 21:210–216
Hagen C, Grünewald K, Xyländer M, Rothe E (2001) Effect of cultivation parameters on growth and pigment biosynthesis in flagellated cells of Haematococcus pluvialis. J Appl Phycol 13:79–87
Hata N, Ogbonna JC, Hasegawa Y, Taroda H, Tanaka H (2001) Production of astaxanthin by Haematococcus pluvialis in a sequential heterotrophic-photoautotrophic culture. J Appl Phycol 13:395–402
Imada N, Kobayashi K, Tahara K, Oshima Y (1991) Production of an autoinhibitor by Skeletonema costatum and its effect on the growth of other phytoplankton. Nippon Suisan Gakkaishi 57:2285–2290
Imada N, Kobayashi K, Isomura H, Saito H, Kimura S, Tahara K, Oshima Y (1992) Studies on the autoinhibitor produced by Skeletonema costatum.2. Isolation and identification of an autoinhibitor by Skeletonema costatum. Nippon Suisan Gakkaishi 58:1687–1692
Kang CD, Han SJ, Choi SP, Sim SJ (2010) Fed-batch culture of astaxanthin-rich Haematococcus pluvialis by exponential nutrient feeding and stepwise light supplementation. Bioprocess Biosyst Eng 33:133–139
Katsuda T, Lababpour A, Shimahara K, Katoh S (2004) Astaxanthin production by Haematococcus pluvialis under illumination with LEDs. Enzym Microb Technol 35:81–86
Kim S, Kim Z, Lee C, Lee H (2006) Enhanced production of astaxanthin by flashing light using Haematococcus pluvialis. Enzym Microb Technol 39:414–419
Kobayashi M, Kakizono T, Nishio N, Nagai S (1992) Effects of light intensity, light quality, and illumination cycle on astaxanthin formation in a green alga, Haematococcus pluvialis. J Ferment Bioeng 74:61–63
Kobayashi M, Kurimura Y, Kakizono T, Nishio N, Tsuji Y (1997) Morphological changes in the life cycle of the green alga Haematococcus pluvialis. J Ferment Bioeng 84:94–97
Lababpour A, Shimahara K, Hada K, Kyoui Y, Katsuda T, Katsuda T, Katoh S (2005) Fed-batch culture under illumination with blue light emitting diodes (LEDs) for astaxanthin production by Haematococcus pluvialis. J Biosci Bioeng 100:339–342
Lee YK, Ding SY (1995) Of dissolved oxygen partial pressure on the accumulation of astaxanthin in chemostat cultures of Haematococcus lacustris (Chlorophyta). J Phycol 31:922–924
Li Y, Sommerfeld M, Chen F, Hu Q (2008) Consumption of oxygen by astaxanthin biosynthesis: a protective mechanism against oxidative stress in Haematococcus pluvialis (Chlorophyceae). J Plant Physiol 165:1783–1797
Liu J, Yin M, Zhang J, Meng Z, Bourne WF (2000) Studies of cell cycle in Haematococcus pluvialis. Oceanol Limnol Sinica 31:145–150
Liu J, Zhang C, Cohen Z, Richmond A (2002) Physiological inhibitory effect of OCS in arachidonic acid-rich Parietochloris incisa (Trebouxiophyceae, Chlorophyta). Chin J Oceanol Limnol 20:248–255
Liu J, Sun Y, Yin M, Liu W, Zhang Z (2004) Inorganic carbon and the cell growth regulator in micro-alga Haematococcus pluvialis. Oceanol Limnol Sinica 35:459–466
Olaizola M (2000) Commercial production of astaxanthin from Haematococcus pluvialis using 25,000-liter outdoor photobioreactors. J Appl Phycol 12:499–506
Orosa M, Franqueira D, Cid A, Abalde J (2005) Analysis and enhancement of astaxanthin accumulation in Haematococcus pluvialis. Bioresour Technol 96:373–378
Pérez-López P, González-García S, Jeffryes C, Agathos SN, McHugh E, Walsh D, Murray P, Moane S, Feijoo G, Moreira M (2014) Life cycle assessment of the production of the red antioxidant carotenoid astaxanthin by microalgae: from lab to pilot scale. J Clean Prod 64:332–344
Porra RJ (2002) The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth Res 73:149–156
Scibilia L, Girolomoni L, Berteotti S, Alboresi A, Ballottari M (2015) Photosynthetic response to nitrogen starvation and high light in Haematococcus pluvialis. Algal Res 12:170–181
Steinbrenner J, Linden H (2003) Light induction of carotenoid biosynthesis genes in the green alga Haematococcus pluvialis: regulation by photosynthetic redox control. Plant Mol Biol 52:343–356
Sun YN, Yin MY, Liu JG (2001) Auto-signals in Haematococcus pluvialis. Trans Oceanol Limnol 3:22–28
Sun H, Kong Q, Geng Z, Duan L, Yang M, Guan B (2015) Enhancement of cell biomass and cell activity of astaxanthin-rich Haematococcus pluvialis. Bioresour Technol 186:67–73
Tjahjono A, Hayama Y, Kakizono T, Terada Y, Nishio N, Nagai S (1994) Hyper-accumulation of astaxanthin in a green alga Haematococcus pluvialis at elevated temperatures. Biotechnol Lett 16:133–138
Wan M, Zhang Z, Wang W, Wang J, Huang J, Fan J, Yu A, Li Y (2015) Sequential heterotrophy-dilution-Photoinduction cultivation of Haematococcus pluvialis for efficient production of astaxanthin. Bioresour Technol 198:557–563
Wang N, Guan B, Kong Q, Sun H, Geng Z, Duan L (2016) Enhancement of astaxanthin production from Haematococcus pluvialis mutants by three-stage mutagenesis breeding. J Biotechnol 236:71–77
Wayama M, Ota S, Matsuura H, Nango N, Hirata A, Kawano S (2013) Three-dimensional ultrastructural study of oil and astaxanthin accumulation during encystment in the green alga Haematococcus pluvialis. PLoS One 8(1):e53618.
Wellburn AR (1994) The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144:307–313
Zhang XW, Gong X, Chen F (1999) Kinetic models for astaxanthin production by high cell density mixotrophic culture of the microalga Haematococcus pluvialis. J Ind Microbiol Biotechnol 23:691–696
Acknowledgements
This research was supported by the Development of Science and Technology Project of Shandong Province of China (2014GSF121029) and National Natural Science Foundation of China (31471657), for which the authors are grateful.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(PDF 99 kb)
Rights and permissions
About this article
Cite this article
Wang, N., Guan, B., Kong, Q. et al. A semi-continuous cultivation method for Haematococcus pluvialis from non-motile cells to motile cells. J Appl Phycol 30, 773–781 (2018). https://doi.org/10.1007/s10811-017-1337-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-017-1337-6