Abstract
Effects of n-propyl gallate, a plastid terminal oxidase inhibitor involved in chlororespiration, on photosystem II efficiency in Dunaliella bardawil under low or high illumination was investigated. Rapid chlorophyll a fluorescence transients were recorded and analyzed according to JIP-test, which can quantify the photosystem II performance. The fluorescence transients O-J-I-P drastically decreased and almost reached a plateau when low light-grown cells were exposed for 96 h to 1, 2 and 4 mM n-propyl gallate. Very similar reductions in the efficiency of quantum yield of primary photochemistry (Φpo), the quantum yield for electron transport (ΦEo) and the inferred water-splitting complex activity (Fv/Fo) were found in the same inhibitor concentrations. However, no statistically significant change in fluorescence intensity and photosystem II efficiency was found when algal cells were exposed to the inhibitor concentrations up to 2 mM under high light intensity. The results indicated that inhibitory effects of n-propyl gallate on photosystem II electron flow in D. bardawil cells are dependent on environmental conditions. It is also demonstrated that n-propyl gallate is a multi-target inhibitor of growth kinetics as well as photosynthesis. In addition, we found that the donor side of photosystem II acts as main target place of the inhibitor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Photosynthesis in chloroplasts involves a vectorial electron transfer from water in the lumen to NADP+ in the stroma, by means of redox carriers. High illumination is a complex stress in which the inactivation of photosystem II (PSII) reactions is manifested as a decrease in the quantum yield of photochemistry. Exposure of plants to excess light frequently results in PSII photoinactivation due to reactions on both the PSII acceptor and donor sides and also in the degradation of the reaction center D1 protein (Powles 1984; Barber and Andersson 1992; Prasil et al. 1992; Aro et al. 1993; Asada 1996, 1999; Niyogi 2000). Under such environmental conditions, plants have developed several mechanisms to dissipate excess absorbed energy (Quiles 2006; Diaz et al. 2007; Miyake et al. 2004, 2005). Thus, PSII electron flow can change to cope with stress (Quiles 2006; Diaz et al. 2007; Gamboa et al. 2009; Ibanez et al. 2010) which is easily detectable by fluorescence measurements.
Chlorophyll (Chl) a fluorescence kinetic is an informative tool for investigating PSII electron transport (For review see e.g. Krause and Weis 1991; Govindjee 1995; Strasser et al. 2000, 2004; Fricke and Peters 2002). When dark-adapted photosynthetic samples are excited with light, the fluorescence intensity rises rapidly from an initial low value, Fo (the O level), to a high value, Fp (the P level). Two intermediary steps designated as FJ (the J level) and FI (the I level) normally appear under high light illumination conditions. The sequence of phases is labeled as O, J, I and P (Strasser et al. 1995). The Chl a fluorescence rise from minimum fluorescence level (Fo) to J level (FJ) at around 2 ms is due to reduction of QA by PSII. The fluorescence rise is followed to the I level (FI) at around 30 ms, due to the filling up of the PQ pool. Finally, there is a rise from FI to P level, due to traffic jam on the electron acceptor side of PSI (Strasser et al. 2004). The performance of PSII is quantified through a series of functional and structural parameters derived from the fluorescence transients (O-J-I-P) according to JIP-test (Strasser and Strasser 1995; Stirbet et al. 2001; Strasser and Stirbet 2001). The JIP-test offers simple equations expressing the equilibrium between the inflow and outflow of the entire energy flux within PSII. Therefore, this analysis enables us to understand the relationships between PSII activity, fluorescence signals, and their analytical expressions in various photosynthetic organisms under different stress conditions (Strasser et al. 2000; Tsimilli-Michael et al. 2000; Appenroth et al. 2001; Lu and Vonshak 2002; Bussotti et al. 2007).
n-Propyl gallate (PG), a free radical scavenger with antioxidant properties, displays wide biological effects in plants (Elich et al. 1997; Nagata et al. 2004; Raghavan and Hultin 2005; Chaudhuri and Kar 2008; Zurita et al. 2007). It is widely used as a specific inhibitor of plastid terminal oxidase (PTOX) involved in chlororespiration, in photosynthetic studies (Cournac et al. 2000; Kuntz 2004; Gamboa et al. 2009; Einali and Shariati 2012). Previous studies have demonstrated that PG concentration of 1 mM inhibits PTOX and decreases PSII quantum yield in stressed plants, but has no effect on PSII under favorable conditions (Cournac et al. 2000; Rizhsky et al. 2002; Aluru and Rodermel 2004; Quiles 2006; Diaz et al. 2007; Gamboa et al. 2009; Ibanez et al. 2010). Thus, the inhibitor might affect photosynthetic electron flow within PSII (Einali and Shariati 2012). However, most of these studies have focused on the effects of PG, as a chlororespiratory inhibitor in stressed higher plants, on PSII electron transport capacity and relatively few have examined these effects in algal classes. Furthermore, direct effects of different PG concentrations on PSII electron flow and growth kinetic under both stable and unstable conditions have not yet been investigated extensively.
Our recent study displayed that responses of Dunaliella salina cells to different PG concentrations is dependent on stress conditions (Einali and Shariati 2012). Therefore, PG-induced changes of PSII electron transport activity is influenced by stress situation. Due to the fact, however, the hypothesis can be developed if the dependence of PG effects to stress situation actually applies in other species. In the present study, Dunaliella bardawil, a unicellular and halotolerant green alga, was used to examine the effects of PG on PSII function as probed by rapid Chl a fluorescence measurement and JIP-test analysis. Additionally, we determined the inhibitory site of PG on PSII electron transport chain.
2 Materials and methods
2.1 Algal cultures and experimental conditions
Dunaliella bardawil Ben-Amotz et Avron, UTEX 2538 was obtained from UTEX, The Culture Collection of Algae at the University of Texas at Austin. The cells were grown in a culture medium (pH = 7.5) with concentration of 1 M NaCl as described before (Shariati and Lilley 1994). Cultures were incubated in a culture room at 25 °C and 70 µmol photons m−2 s−1 of light under a 16 h/8 h light/dark photoperiod with continuous shaking (100 rpm). Exponentially growing cultures were transferred into the aseptic 250-ml Erlenmeyer flasks in final volume of 100 ml where initial cell density was approximately 5 × 106 cells ml−1. The algal samples (in triplicate) were treated with n-propyl gallate (PG) (purchased from Fluka company) at five levels of 0.1, 0.5, 1, 2 and 4 mM. PG-treated cultures were exposed for 96 h to 70 µmol photons m−2 s−1 as low light (LL) and 400 µmol photons m−2 s−1 as high light (HL) conditions. Cultures at the low or high light in the absence of inhibitor were used as control.
2.2 Cell growth and pigment determination
The cell number was determined using a hemocytometer under a light microscope (Schoen 1988). For total Chl and β-Car extraction, an aliquot (1 ml) of algal suspension was precipitated by centrifugation (10,000×g for 5 min) followed by the addition of 1 ml of 80 % (v/v) acetone and recentrifuged (10,000×g for 2 min) after vortexing. Chl content was spectrophotometrically determined by method of Arnon (1949 g). β-Car was assayed according to Ben-Amotz and Avron (1983). E 1%1cm of 2,273 at 480 nm has been used to calculate of β-Car concentration.
2.3 Measurement of Chl fluorescence
To evaluate the effects of PG on the electron transport of PSII, the fast Chl a fluorescence transients in D. bardawil exposed 96 h to different inhibitor concentrations under LL and HL conditions were measured. Chl a fluorescence induction was measured using the Plant Efficiency Analyzer (Handy PEA fluorimeter, Hansatech Instruments Ltd., Pentney, King’s Lynn, Norfolk, England). The cell suspensions (10 µg Chl a ml−1) were pipetted into the glass vials and pre-darkened for 10 min at room temperature. Rapid Chl a fluorescence induction from 10 µs to 1,000 ms was measured when the dark-adapted cells were exposed to a strong light pulse (3,500 µmol photons m−2 s−1). The data were analyzed and the so-called JIP-test parameters was calculated using BiolyzerHP3 software (Laboratory of Bioenergetics, University of Geneva, Switzerland) (Strasser et al. 2000). The JIP-test parameters used in the study is listed in Table 1. The fluorescence intensity at 50 µs was considered as O value (F50 µs) and the maximum fluorescence intensity was attained as P(Fm), according to Strasser et al. (2004). The PSII water-splitting complex activity was determined by the ratio between variable fluorescence (Fv, fluorescence intensity between O and P transients) and minimal (Fo, fluorescence intensity at O transient), Fv/Fo, according to Schreiber et al. (1994) and Pereira et al. (2000). The Performance index (PIABS) was calculated according to Strasser et al. (2000). The parameter describes overall photosynthetic performance, combines several parameters that depict three main functional characteristics of PSII reaction center, namely density of reaction centers per PSII antenna chlorophyll (RC/ABS), ratio of trapping and dissipation fluxes (TRo/DIo) and efficiency of the conversion of excitation energy to electron transport (ETo/(TRo − ETo)) (Strasser et al. 2000).
2.4 Statistical analysis
The experiments were done in three independent replicates for all treatments. Means and standard deviations (SD) were calculated for each treatment. Statistically significant differences between control and PG-treated samples at P < 0.05 were determined using Analysis of Variance (ANOVA) with a Holm-Sidak post hoc test. The ANOVA information for all physiological and JIP-test parameters has been addressed in Table 2. Correlation coefficients and significance level of some physiological and JIP-test parameters was determined. All statistical analyses were performed using SigmaStat 3.0, Systat Software, San Jose, California.
3 Results
3.1 Effects of PG on cell density and pigment content
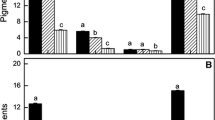
Cell mortality was severe in the Dunaliella cultures in the presence of PG concentrations higher than 0.1 mM, so that about 90 % of the cells died in LL-grown cells subjected 96 h to 4 mM of the inhibitor (Fig. 1a). No significant change was found in cell number when algal cultures were exposed for 96 h to PG concentrations up to 1 mM under HL conditions. However, cell density was decreased in these cultures at 2 and 4 mM PG by 23 and 33 % as compared to the control, respectively (Fig. 1a).
Effect of PG on cell density (a), Total Chl (b) and β-Car (c) of D. bardawil suspensions under LL and HL conditions after 96 h of incubation with the inhibitor. Suspensions grown without PG were taken as control. Each point represents the mean value of three separate samples ± SD. Asterisks represent significant difference from control sample (absence of PG) at P < 0.05
Pigment content showed a pattern similar to each other in PG-treated cells subjected 96 h to both LL and HL conditions (Fig. 1b, c). Chl and β-Carotene (β-Car) content increase in LL-grown cells treated with 0.1 mM PG but decreased significantly under higher PG concentrations. HL-grown cells exhibited a significant decrease in pigment content in the presence of the inhibitor concentrations up to 2 mM. However, pigment content of these cells increased pronouncedly at 4 mM PG (Fig. 1b, c).
3.2 Effects of PG on Chl a fluorescence induction curves
Dunaliella bardawil exhibited a typical polyphasic rise of fluorescence induction (O-J-I-P) (Fig. 2). Fluorescence transient curves represent no change in rise of fluorescence induction (O-J-I-P) in LL-grown cells subjected to PG concentrations of 0.1 and 0.5 mM when compared to the control (absence of PG) (Fig. 2a). However, these transients almost reached a plateau when these cells subjected to PG concentrations of 1, 2 and 4 mM (Fig. 2a). In fact, very low PSII electron transport capacity was detectable due to exposure of Dunaliella cells to the concentrations more than 0.5 mM PG. Experiments on HL-grown cells revealed a lesser polyphasic rise of fluorescence when compared to LL conditions (Fig. 2). Nevertheless, no significant change in photosynthetic electron transport was detectable between HL-grown cells incubated with PG concentrations up to 2 mM (Fig. 2b). In addition, electron transport capacity in these cultures subjected to 4 mM PG was appreciable although that is much lower than other fields.
3.3 Measurement of electron transport capacity between PSII components
The efficiency of quantum yield of primary photochemistry (Φpo) declined significantly when LL-grown cells were exposed for 96 h to the inhibitor concentrations higher than 0.5 mM (Fig. 3a). Similar results were obtained in the oxidation content of \( {\rm Q}_{{\rm A}^{-}} \) to QA (Ψo) as well as the quantum yield of electron transport (ΦEo) (Fig. 3a). In contrast to LL, these parameters started to increase in HL-grown cells treated with 1 and 2 mM PG (Fig. 3b). However, a significant decline in all parameters was found for HL-grown cells incubated with 4 mM PG (Fig. 3b).
Effects of PG on the quantum yield of primary photochemistry (ΦPo), the efficiency of electron transfer from \( {\rm Q}_{{\rm A}^{-}} \) to QB (Ψo), and the quantum yield for electron transport (ΦEo) in D. bardawil cells under LL (a) or HL (b) conditions. Cultures grown without PG under LL or HL conditions were taken as control. Data are the means of three separate experiments ± SD. Asterisks indicate result significantly different from control sample (P < 0.05)
The efficiency of the water-splitting complex on the donor side of PSII (as inferred from Fv/Fo) decreased pronouncedly in LL-grown cells subjected 96 h to PG concentrations higher than 0.5 mM (Fig. 4a). However, no significant change in Fv/Fo parameter was found when HL-grown cells were incubated with PG concentrations up to 2 mM as compared to the control, though it declined at 4 mM PG (Fig. 4a). LL-grown cells incubated with PG concentrations higher than 0.5 mM revealed an evident increase in thermal dissipation yield (ΦDo), while HL-grown cells manifested such increase only for 4 mM PG-treated cells (Fig. 4b). Furthermore, the initial slope at the beginning of the relative variable fluorescence transients (dV/dto) and the light absorption flux (for PSII antenna chlorophylls) per reaction center (ABS/RC) increased considerably in LL-grown cells exposed 96 h to the inhibitor concentrations higher than 0.5 mM, while they remained roughly unchanged when PG-treated algal cells were exposed to HL intensity (Fig. 4c, d).
Changes in a water-splitting complex activity (Fv/Fo), b thermal dissipation yield (ΦDo), c the initial slope at the beginning of the relative variable fluorescence transients (dV/dto) and d the amount of light absorption per total number of active reaction center (ABS/RC) in D. bardawil cultures subjected 96 h to different PG concentrations under LL and HL conditions. Suspensions grown without PG under LL or HL conditions were taken as control. Values are means of three separate experiments ± SD. Asterisks represent significant difference from control sample (P < 0.05)
In LL-grown cells exposed to the inhibitor concentrations higher than 0.5 mM, a pronounced decline in values of PIABS and all its components including RC/ABS, TRo/DIo and ETo/(TRo − ETo) was detectable (Fig. 5a, c). However, PG had no negative effect on these parameters when algal cells were incubated with PG up to 2 mM under HL conditions (Fig. 5b, d).
Effects of different PG concentrations on performance index (PIABS) and density of reaction centers per PSII antenna chlorophyll (RC/ABS) (a, b) in D. bardawil cells under LL (a) and HL (b) conditions. Effect of the inhibitor on flux ratio trapping per dissipation (TRo/DIo) and electron transport beyond \( {\rm Q}_{{\rm A}^{-}} \) (ETo/(TRo-ETo)) (c, d) in the cells under LL (c) and HL (d) conditions. Cultures grown without PG under LL or HL conditions were used as control. The bars show standard deviation of the means of the measured parameters of three separate experiments. Asterisks represent result significantly different from control sample (P < 0.05)
3.4 Correlation of growth kinetic with PSII electron transport capacity
There was significant positive correlation between cell density and pigment content with PIABS and Fv/Fo in LL-grown algae, while no significant or a negative association was observed between these traits in HL-grown cells. The Fv/Fo parameter showed significant correlation with Φpo, ΦEo, and PIABS parameters but negative correlation with ΦDo in both LL and HL-grown cells (Table 3).
4 Discussion
Dunaliella dark-adapted cells exhibited a polyphasic Chl a fluorescence rise, as previously reported for higher plants, green algae and cyanobacteria (Strasser et al. 1995; Appenroth et al. 2001; Lu and Vonshak 2002; Xia et al. 2004). The OJIP curves showed that PSII electron flow drastically decreases in LL-grown cells incubated with PG concentrations of 1, 2 and 4 mM (Fig. 2a). It is consistent with Forti and Caldiroli (2005), demonstrating that PG inhibits progressively PSII fluorescence in Chlamydomonas reinhardtii. Such drastic decrease of fluorescence intensity at all fluorescence transients in these cultures (Fig. 2a) can show a diminished PSII capacity for electron transport from water-splitting system toward to PSI. Very similar reduction induced by PG concentrations more than 0.5 mM was also found for the efficiency of quantum yield of primary photochemistry (Φpo), quantum yield for electron transport (ΦEo) (Fig. 3a) and Fv/Fo, a value that is proportional to activity of the water-splitting complex on the donor side of the PSII (Schreiber et al. 1994; Pereira et al. 2000, Kalaji et al. 2011) (Fig. 4a). It has been previously described that the efficiency of the water-splitting complex on the donor side of PSII (inferred from Fv/Fo) is the most sensitive component in the photosynthetic electron transport chain (Kalaji et al. 2011). A decrease in this ratio results from photosynthetic electron transport impairment (Pereira et al. 2000). Correlation analysis showed that there were positive correlation between Fv/Fo with Φpo, ΦEo, and PIABS (Table 3). Therefore, it may be assumed that the decrease of the PSII activity can be induced by PG inhibition of water-splitting system feeding electron transport.
Previous studies on higher plants have shown that PG decreases PSII quantum yield under stress conditions (Quiles 2006; Diaz et al. 2007; Gamboa et al. 2009). However, we found that PG did not have any negative effect on electron transport yield and oxygen evolving complex in HL-grown cells (Figs. 2b, 3b, 4a). We suggest that PTOX does not affect PSII electron flow in D. bardawil cells under HL intensity. Evidence in support of this suggestion comes from a study showing that PTOX does not act as a stress-induced safety valve in the protection of the photosynthetic apparatus in Arabidopsis (Rosso et al. 2006). In agreement to the suggestion, Ibanez et al. (2010) discovered that in Chrysanthemum morifolium, a sun species, chlororespiratory pathway is not stimulated in response to stress and importance of this way in photosynthesis may differ in each plant species. In addition, our recent work on D. salina also showed that PG-induced PTOX inhibition did not affect PSII and PSI quantum yields under HL conditions (Einali et al. 2013), which further confirm our suggestion. Therefore, the supposition of existence of another alternative pathway(s) rather than PTOX in Dunaliella cells for oxidizing of the electron transport chain to protection of the photosynthetic systems against reactive oxygen species (ROS) generated during high illumination does not seem unlikely. The latter suggestion might be supported by a study showing that acclimation to high light enhance the Mehler reaction in wheat (Savitch et al. 2000), an alternative method of keeping the electron transport chain oxidized. Although we could recently detect presence of PTOX genes in Dunaliella cells (submitted on Genbank, PCR results not shown), however, role of PTOX in Dunaliella cells remains to be elucidated by molecular and biochemical instruments.
In addition to PG effects on PSII electron flow, we found a severe increase in cell mortality and a pronounced decrease in pigment content in LL-grown cells subjected 96 h to 4 mM of the inhibitor (Fig. 1). This event might be due to a formation of ROS because of high PG concentration. Therefore, PG inhibition of PSII activity may also be related to its photoinhibitory effects. It has been previously determined that inhibition of water-splitting complex increases the susceptibility of PSII to photoinhibition (Wang et al. 1992) and it can accelerate degradation of the D1 protein in PSII (Jegershold et al. 1990). It has also been confirmed that PG inhibits phosphorylation of light-harvesting Chl a/b proteins (LHCII) (Elich et al. 1997; Georgakopoulos and Argyroudi-Akoyunoglou 1998). Inhibition of LHCII phosphorylation could be resulted in imbalance in excitation rates of PSII and PSI (Allen and Forsberg 2001) and oxidative stress occurrence. Thus, it seems that high concentration of PG could affect PSII by direct influence on photosynthetic components. Interestingly, it has also been demonstrated that PG inhibits high light-induced D1 degradation (Georgakopoulos and Argyroudi-Akoyunoglou 1998) and protects PSI against photoinhibition (Sonoike 1995). Therefore, the protective effect of PG on D1 protein might be due to resistance of Dunaliella cells against the inhibitor under HL conditions. It is also likely that PG could affect nature of PSII and or nature of cell membrane and chloroplast envelope under LL conditions. However, PG could not photodegrade under HL conditions because our experiments on algal cultures under salinity stress showed that high PG concentration does not also have any negative effect on PSII electron transport (Einali and Shariati 2012).
As mentioned above, Chl a fluorescence transients showed a very low potential of electron transport activity in LL-grown cells exposed to 1, 2 and 4 mM PG (Fig. 2a). The described differences in the shape of OJIP curves were reflected on values of performance index (PIABS) in LL-grown cells exposed to different concentrations of the inhibitor (Fig. 5a). Under this conditions, the main decreasing contributions of performance index (PIABS) were lowering of RC/ABS and TRo/DIo than ETo/(TRo - ETo) (Fig. 5). In addition, under LL conditions, PG resulted in an increase of the light absorption flux per reaction center (ABS/RC), which was associated with the increase in the antenna size or a decrease in the number of active reaction centers (Fig. 4d). A significant increase in dV/dto parameter (Fig. 4c), suggests a decrease in the number of active reaction centers. This means that amount of closed reaction centers has enhanced. The increase in ABS/RC together decrease in RC/ABS, indicate the presence of non-QA reducing reaction centers (Lepedus et al. 2011) and can also be related to irreversible PSII damage (Kalaji et al. 2011). Such non-QA reducing reaction centers are also called silent reaction centers and act as heat sinks (Strasser et al. 2004). The accumulation of inactive reaction centers is associated with the increased efficiency of dissipation of absorbed light as heat, as shown by the high values of thermal dissipation yield (ΦDo) (Fig. 4b). The increase in dV/dto (Fig. 4c) and more decrease in TRo/DIo than ETo/(TRo-ETo) parameters (Fig. 5c) in PG-treated cells under LL conditions, further indicate PG inhibitory effects on the donor side of PSII. However, different inhibitor concentrations had no significant negative effect on none of dV/dto, ABS/RC and PIABS parameters under HL conditions (Figs. 4c, d, 5b), suggesting that algal cells respond to PG depending on environmental conditions.
In conclusion, data of the present study suggest that algal cells display different responses to PG under stress or non-stress conditions. It was found for PG to induce inhibitory effects on water-splitting complex on the donor side of PSII, decreasing its electron transport capacity. Therefore, PG-induced inhibition of the water-splitting complex on the donor side of PSII affects the conversion of light energy from antenna complex to electron transport within photosystem II. It was also demonstrated here that PG is a multi-target inhibitor of algal growth as well as photosynthesis. Indeed, PG in concentrations higher than 0.5 mM act as an inhibitor in LL-grown cells, while in such concentrations it can play a protective role as an antioxidant in HL-grown cells. These two different responses can be attributed to molecular basis of direct effect of PG on PSII components although the direct evidence as to how PG inhibits PSII components requires to be further studied.
References
Allen JF, Forsberg J (2001) Molecular recognition in thylakoid structure and function. Trends Plant Sci 6:317–326
Aluru MR, Rodermel SR (2004) Control of chloroplast redox by the IMMUTANS terminal oxidase. Physiol Plant 120:4–11
Appenroth KJ, Stockel J, Srivastava A, Strasser RJ (2001) Multiple effects of chromate on the photosynthetic apparatus of Spirodela polyrhiza as probe by OJIP chlorophyll a fluorescence measurements. Environ Pollut 115:49–64
Arnon D (1949) Copper enzymes in isolated chloroplasts: polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1–15
Aro E-M, Virgin I, Andersson B (1993) Photoinhibition of photosystem II. Inactivation, protein damage and turnover. Biochim Biophys Acta 1143:113–134
Asada K (1996) Radical production and scavenging in the chloroplasts. In: Baker NR (ed) Photosynthesis and the Environment. Kluwer Academic Publishers, Dordrecht, pp 128–150
Asada K (1999) The water–water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50:601–639
Barber J, Andersson B (1992) Too much of a good thing: light can be bad for photosynthesis. Trends Biochem Sci 17:61–66
Ben-Amotz A, Avron M (1983) On the factors which determine massive β-Carotene accumulation in the halo-tolerant alga Dunaliella bardawil. Plant Physiol 72:593–597
Bussotti F, Strasser RJ, Schaub M (2007) Photosynthetic behavior of woody species under high ozone exposure probed with the JIP-test: a review. Environ Pollu 147:430–437
Chaudhuri A, Kar RK (2008) Inhibition of seed germination by propyl gallate, a free radical scavenger and recovery of germination by hydrogen peroxide and ethylene in Vigna Radiata. World J Agr Sci 4:914–921
Cournac L, Redding K, Ravenel J, Rumeau D, Josse EM, Kuntz M, Peltier G (2000) Electron flow between photosystem II and oxygen in chloroplasts of photosystem I-deficient algae is mediated by a quinol oxidase involved in chlororespiration. J Biol Chem 275:17256–17262
Diaz M, Haro VD, Munoz M, Quiles MJ (2007) Chlororespiration is involved in the adaptation of Brassica plants to heat and high light intensity. Plant Cell Environ 30:1578–1585
Einali A, Shariati M (2012) Effects of n-propyl gallate on photosynthesis and physiological parameters in Dunaliella salina are affected by stressful conditions. Braz J Plant Physiol 24:193–202
Einali A, Shariati M, Sato F, Endo T (2013) Cyclic electron transport around photosystem I and its relationship to non-photochemical quenching in unicellular green alga Dunaliella salina under nitrogen deficiency. J Plant Res 126:179–186
Elich TD, Edelman M, Mattoo AK (1997) Evidence for light-dependent and light independent protein dephosphorilation in chloroplasts. FEBS Lett 411:236–238
Forti G, Caldiroli G (2005) state transitions in Chlamydomonas reinhardtii. The role of the Mehler reaction in state 2-to-state 1 transition. Plant Physiol 137:492–499
Fricke W, Peters WS (2002) The biophysics of leaf growth in salt-stressed barley. A study at the cell level. Plant Physiol 129:374–388
Gamboa J, Munoz M, Quiles MJ (2009) Effects of antimycin a and n-propyl gallate on photosynthesis in sun and shade plants. Plant Sci 177:643–647
Georgakopoulos JH, Argyroudi-Akoyunoglou JH (1998) Thylakoid protein phosphorilation is suppressed by free radical scavengers. Correlation between PSII core protein degradation and thylakoid protein phosphorylation. Photosynth Res 58:269–280
Govindjee (1995) Sixty-three years since Kautsky: chlorophyll a fluorescence. Aust J Plant Physiol 22:131–160
Ibanez H, Ballester A, Munoz R, Quiles MJ (2010) Chlororespiration and tolerance to drought, heat and high illumination. J Plant Physiol 167:732–738
Jegershold C, Virgin I, Stryring S (1990) Light-dependent degradation of the D1 protein in photosystem II is accelerated after inhibition of the water splitting reaction. Biochemistry 29:6179–6186
Kalaji HM, Govindjee Bosa K, Koscielniak J, Zuk-Golaszewska K (2011) Effects of salt stress on photosystem II efficiency and CO2 assimilation of two Syrian barley landraces. Environ Exp Bot 73:64–72
Krause GH, Weis E (1991) Chlorophyll fluorescence and photosynthesis: the basics. Annu Rev Plant Physiol Plant Mol Biol 42:313–349
Kuntz M (2004) Plastid terminal oxidase and its biological significance. Planta 218:896–899
Lepedus H, Begovic L, Mlinaric S, Cimic D, Stolfa I, Paradikovic N, Uzarevic Z, Jurkovic V, Cesar V (2011) Physiology and biochemistry of leaf bleaching in prematurely aging maple (acer saccharinum l.) trees. II. Functional and molecular adjustment of PSII. Acta Bot Croat 70:133–146
Lu CM, Vonshak A (2002) Effects of salinity stress on photosysten II function in cyanobacterial Spirulina platensis cells. Physiol Plant 114:405–413
Miyake C, Shinzaki Y, Miyata M, Tomizawa K (2004) Enhancement of cyclic electron flow around PSI at high light and its contribution to the induction of non-photochemical quenching of chl fluorescence in intact leaves of tobacco plants. Plant Cell Physiol 45:1426–1433
Miyake C, Miyata M, Shinzaki Y, Tomizawa K (2005) CO2 response of cyclic electron flow around PSI (CEF-PSI) in tobacco leaves—Relative electron fluxes through PSI and PSII determine the magnitude of non-photochemical quenching (NPQ) of Chl fluorescence. Plant Cell Physiol 46:629–637
Nagata T, Todoriki S, Kikuchi S (2004) Radial expansion root cells and elongation of root hairs of Arabidopsis thaliana induced by massive doses of gamma irradiation. Plant Cell Physiol 45:1557–1565
Niyogi KK (2000) Safety valves for photosynthesis. Curr Opin Plant Biol 3:455–460
Pereira WE, Desiqueira DL, Martinez CA, Puiatti M (2000) Gas exchange and chlorophyll fluorescence in four citrus root stocks under aluminium stress. J Plant Physiol 157:513–520
Powles SB (1984) Photoinhibition of photosynthesis induced by visible light. Annu Rev Plant Physiol 35:15–44
Prasil O, Adir N, Ohad I (1992) Dynamics of photosystem II: mechanism of photoinhibition and recovery processes. In: Barber J (ed) The photosystems: structure, function, and molecular biology. Topics in photosynthesis, Vol 11. Elsevier Science Publishers, Amsterdam, pp 295–348
Quiles MJ (2006) Stimulation of chlororespiration by heat and high light intensity in Oat plants. Plant Cell Environ 29:1463–1470
Raghavan S, Hultin HO (2005) Model system for testing the efficacy of antioxidants in muscle foods. J Agr Food Chem 53:4572–4577
Rizhsky L, Hallak-Herr E, Van Breusegem F, Rachmilevitch S, Barr JE, Rodermel S, Inze D, Mittler R (2002) Double antisense plants lacking ascorbate peroxidase and catalase are less sensitive to oxidative stress than single antisense plants lacking ascorbate peroxidase or catalase. Plant J 32:329–342
Rosso D, Ivanov AG, Fu A, Geisler-Lee J, Hendrickson L, Geisler M, Stewart G, Krol M, Hurry V, Rodermel SR, Maxwell DP, Huner NPA (2006) IMMUTANS does not act as a stress-induced safety valve in the protection of the photosynthetic apparatus of Arabidopsis during steady-state photosynthesis. Plant Physiol 142:574–585
Savitch LV, Massacci A, Gray GR, Huner NPA (2000) Acclimation to low temperature or high light mitigates sensitivity to photoinhibition: roles of the Calvin cycle and the Mehler reaction. Aust J Plant Physiol 27:253–264
Schoen M (1988) Cell counting. In: Lobban C, Champan D, Kermer BP (eds) Experimental phycology. Cambridge University Press, Cambridge
Schreiber U, Bilger W, Neubauer C (1994) Chlorophyll fluorescence as a non- intrusive indicator for rapid assessment of in vivo photosynthesis. In: Schulze ED, Caldwell MM (eds) Ecophysiology of photosynthesis. Springer, New York, pp 49–70
Shariati M, McC Lilley (1994) Loss of intracellular glycerol from Dunaliella by electroporation at constant osmotic pressure: subsequent restoration of glycerol content and associated volume changes. Plant Cell Environ 17:1295–1304
Sonoike K (1995) Selective photoinhibition of Photosystem I in isolated thylakoid membranes from cucumber and spinach. Plant Cell Physiol 36:825–830
Stirbet AD, Rosenau P, Stroder AC, Strasser RJ (2001) Parameter optimization of fast chlorophyll fluorescence induction model. Math Comput Simul 56:443–450
Strasser RJ, Stirbet AD (2001) Estimation of the energetic connectivity of PSII centres in plants using the fluorescence rise O-J-I-P fitting of experimental data to three different PSII models. Math Comput Simul 56:451–461
Strasser BJ, Strasser RJ (1995) Measuring fast fluorescence transients to address environmental questions: the JIP test. In: Mathis P (ed) Photosynthesis: from light to biosphere, vol 5. Kluwer Academic Publisher, Dordrecht, pp 977–980
Strasser RJ, Srivastava A, Govindjee (1995) Polyphasic chlorophyll a fluorescence transient in plants and cyanobacteria. Photochem Photobiol 61:32–42
Strasser RJ, Srivastava A, Tsimilli Michael M (2000) The fluorescence transient as a tool to characterize and screen photosynthetic samples. In: Yunus M, Pathre U, Mohanty P (eds) Probing photosynthesis: mechanisms. Regulation and adaptation. Taylor and Francis, London, pp 445–483
Strasser RJ, Tsimilli-Michael M, Srivastava A (2004) Analysis of the chlorophyll fluorescence transient. In: Papageorgiou GC, Govindjee (eds) Chlorophyll a fluorescence: a signature of photosynthesis, advances in photosynthesis and respiration. Vol. 19, Kluwer Academic Publishers, Dordrecht, pp 321–362
Tsimilli-Michael M, Eggenberg P, Biro B, Voros I, Koves-Pechy K, Strasser RJ (2000) Synergistic and antagonistic effects of Arbuscular mycorrhizal fungi and Azospirillum and Rhizobium nitrogen-fixers on the photosynthetic activity of alfalfa, probed by the polyphasic chlorophyll a fluorescence transient O-J-I-P. Appl Soil Ecol 15:169–182
Wang WQ, Chapman DJ, Barber J (1992) Inhibition of water splitting increases the susceptibility of photosystem II to photoinhibition. Plant Physiol 99:16–20
Xia J, Li Y, Zou D (2004) Effects of salinity stress on PSII in Ulva lactuca as probed by chlorophyll fluorescence measurements. Aqua Bot 80:129–137
Zurita JL, Jos A, Peso AD, Salguero M, Lopez-Artiguez M, Repetto G (2007) Ecotoxicological effects of the antioxidant additive propyl gallate in five aquatic systems. Water Res 41:2599–2611
Acknowledgments
This work was supported by Grants from UI Deputy of Research to M. S. We would also like to thank the USB Deputy of Research and Education for supporting A. E. during research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Einali, A., Shariati, M. Effects of propyl gallate on photosystem II efficiency in Dunaliella bardawil under high illumination as investigated by chlorophyll fluorescence measurements. Theor. Exp. Plant Physiol. 27, 61–73 (2015). https://doi.org/10.1007/s40626-015-0032-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40626-015-0032-8