Abstract
The aim of this study is to evaluate the protective effects of chrysophanol (CH) against paraquat (PQ)-induced pulmonary injury. Fifty BALB/C mice were randomized into five groups: (1) control, (2) PQ, (3) PQ + dexamethasone (Dex, 2 mg/kg), (4) PQ + CH (10 mg/kg), and (5) PQ + CH (20 mg/kg). A single dose of PQ (50 mg/kg, i.p.) was intraperitoneally given to induce acute lung injury. Then mice were treated with CH (10 and 20 mg/kg/day, orally) for 7 days. At the end of the experiment, animals were euthanized and then bronchoalveolar lavage fluid (BALF) and lung tissues were collected for histological observation, biochemical analysis, and Western blot analysis. Malondialdehyde (MDA), myeloperoxidase (MPO), superoxide dismutase (SOD), interleukin-6 (IL-6), IL-1β, and tumor necrosis factor-α (TNF-α) levels in BALF were determined. The levels of SOD and MDA in the lung were also detected. The peroxisome proliferator-activated receptor (PPAR)-γ and nuclear factor-kappaB (NF-κB) pathway proteins in the lung were determined by Western blot. Histological examination indicated that CH attenuated lung inflammation caused by PQ. Biochemical results showed that CH treatment significantly reduced the levels of MDA, MPO, and inflammatory cytokines and increased the level of SOD, compared to those in the PQ group. Meanwhile, Western Blot results revealed that CH increased PPAR-γ expression and inhibited NF-κB pathway activation after PQ challenge. These findings suggested the potential therapeutic effects of CH which is derived from a natural product on PQ-induced pulmonary injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
As one of the most widely used herbicides, paraquat (PQ, 1-10-dimethyl-40-bipyridylium dichloride), a quaternary nitrogen herbicide, is a highly toxic compound for both animals and humans [1]. PQ contributes to aggressive tissue damage in the lung, kidney, and liver in serious PQ intoxication. It is noteworthy that the major target organ in PQ poisoning is the lung. PQ-induced lung injury is characterized by edema, hemorrhage, inflammatory cell infiltration, and alveolar spaces [2]. Meanwhile, it is acknowledged that oxidative stress plays a crucial role in the pathogenesis of PQ-stimulated pulmonary injury [3]. On the other hand, there are also sufficient studies reporting that inflammatory cascade was closely associated with the progression of lung disorders [4].
Peroxisome proliferator-activated receptors (PPARs) belonging to ligand-activated transcription factors possess several biological functions such as regulation of lipid metabolism in acute lung injury [5]. When activated by their ligands, PPARs undergo a conformational change, leading to recruitment of distinct coactivators and corepressors. Then, the PPAR complex combining with cis-retinoid X receptor (RXR) would be translocated into the nucleus, where it regulates downstream gene transcription by binding to the peroxisome proliferator response element. In cells, there are three PPAR isoforms (PPAR-α, PPAR-β/δ, and PPAR-γ) existed [6]. Previous studies have indicated that PPAR-γ agonists are capable of inhibiting LPS-induced inflammatory responses in airway epithelial cells, neutrophilia, and alveolar macrophages by suppressing the release of pro-inflammatory cytokines and nuclear factor-kappaB (NF-κB) activation [7]. Remarkably, NF-κB, a major nuclear transcription factor, is a regulator which drives the generation of cytokines in inflammatory processes [8, 9]. Chen et al. have reported that NF-κB pathway plays an important role in the development of pulmonary diseases [10]. Therefore, compounds activating PPAR-γ expression or inhibiting NF-κB pathway presumably have a protective effect against inflammatory diseases such as lung injury.
During the few past decades, various nature products have been used for the intervention of disease [11, 12]. Chrysophanol (CH), a member of the anthraquinone family, was originally extracted from plants of the Rheum genus [13]. At present, CH has been shown to have multiple pharmacological effects, such as anti-diabetic [14], anti-depressive [15], and anti-inflammatory [16] activities. However, no available study has evaluated the effects of CH treatment on PQ-induced lung injury in a mouse model as yet. Herein, we sought to investigate whether CH could protect against pulmonary inflammation caused by PQ stimulation and to explore the potential mechanism in mice.
MATERIALS AND METHODS
Reagents
CH was purchased from the National Institutes for Food and Drug Control (Beijing, China). Dexamethasone (Dex) was purchased from Xiansheng Drug Store (Nanjing, China). PQ was provided by Sigma. Tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and IL-6 enzyme-linked immunosorbent assay (ELISA) kits were supplied by BioLegend (San Diego, CA, USA). The levels of superoxide dismutase (SOD) and malondialdehyde (MDA) were evaluated using commercially available kits subscribed from Jiancheng Institute of Biotechnology (Nanjing, China). All antibodies were purchased from Cell Signaling Technology.
Animals
A total of 50 female BALB/c mice (18–22 g), acquired from the Jiangning Qinglongshan Animal Cultivation Farm (Nanjing, China), were maintained in an animal facility under standard laboratory conditions for 7 days prior to experiments with water and standard chow ad libitum. All experimental procedures were carried out in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Experimental Design
The animals were randomly divided into five groups with 10 mice in each group as follows: (1) normal control group; (2) PQ group, mice received PQ (50 mg/kg, i.p.); (3) PQ + Dex group, mice received PQ (50 mg/kg, i.p.) and Dex (2 mg/kg/day); (4) PQ + CH (10 mg/kg) group, mice received PQ (50 mg/kg, i.p.) and CH (10 mg/kg/day); and (5) PQ + CH (20 mg/kg) group, mice received PQ (50 mg/kg, i.p.) and CH (20 mg/kg/day). PQ was dissolved in saline solution (NaCl 0.9 %) and intraperitoneally given to mice at a single toxic dose of 50 mg/kg. CH was also dissolved in saline (NaCl 0.9 %) and administered intragastrically. Two hours after PQ stimulation, CH was intragastrically treated at the dose of 10 and 20 mg/kg/day for 7 days. Dex was intragastrically treated as a positive control.
Bronchoalveolar Lavage
Two days after PQ challenge, the animals were sacrificed and bronchoalveolar lavage was collected three times through a tracheal cannula with 0.5 ml of autoclaved PBS to obtain the bronchoalveolar lavage fluid (BALF). The total leukocyte count was determined with a hemocytometer. BALF samples were centrifuged at 3000 rpm for 10 min at 4 °C, and then the cell-free supernatants were stored in −80 °C for the detection of cytokine concentrations, SOD activity, and MDA content. The pellets were resuspended in 100 μl of saline, centrifuged onto slides, and stained with Wright–Giemsa staining (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) for neutrophil counting.
Lung Wet-to-Dry Weight Ratios
The right lungs were excised at the end of the experiment, and the wet weight was determined. Subsequently, the lungs were placed at 60 °C for 48 h to remove all moisture and then the dry lungs were weighted and the lung wet-to-dry (W/D) ratio was calculated.
Cytokine in BALF
The levels of TNF-α, IL-1β, and IL-6 in BALF were determined using ELISA kits according to the instructions recommended by the manufacturers. The optical density (OD) of each well was determined at 450 nm by a microplate spectrophotometer.
Measurement of SOD and MDA in the Tissue
Lung samples were prepared to make 1:10 (w/v) homogenates followed by centrifugation at 12,000×g (4 °C) for 20 min to obtain supernatants for the next determination of SOD and MDA. Thereafter, SOD activity in the lung was conducted in the light of manufacturer’s recommendation (Jiangcheng Institute of Biotechnology, Nanjing, China). And MDA level was determined with the thiobarbituric acid-reactive substance (TBARS) method using a commercial detection kit (Jiangcheng Institute of Biotechnology, Nanjing, China) according to the manufacturer’s instructions.
Pulmonary Histopathology
The lungs were removed at the end of the experiment. Afterward, the samples were fixed in 4 % neutral-buffered formalin for 48 h, embedded in paraffin, and cut into 3-μm sections. Then, hematoxylin–eosin staining was performed using the standard protocol. After that, pulmonary pathological changes were observed under a light microscope.
Western Blot Analysis
Lung tissues were harvested and frozen in liquid nitrogen immediately until homogenization. Proteins were extracted with lysis buffer (RIPA with protease and phosphatase inhibitor) for 15 min on ice. The total protein concentrations were determined by a BCA protein assay kit. Equal amounts of protein were mixed with five times loading dye (Laemmli buffer) and 2-mercaptoethanol followed by heating for 5 min at 95 °C. The samples were loaded per well on a 10 % sodium dodecyl sulfate polyacrylamide gel and transferred to PVDF membranes. The blots were blocked with 5 % bovine serum albumin (BSA) (5 g BSA was dissolved in 100 ml TBST) for 2 h at room temperature and incubated with primary antibody overnight at 4 °C. After washing, the bound antibodies were incubated with peroxidase-conjugated secondary anti-rabbit antibodies The proteins were visualized by a ECL Key-GEN system (KeyGEN Biotechnology, Nanjing, China) and scanned with a Clinx ChemiScope chemiluminescence imaging system (Gel Catcher 2850, China). GAPDH was detected as an internal control of protein loading.
Statistical Analysis
The results are expressed as means ± SDs and analyzed with one-way analysis of variance (ANOVA) with the Tukey multiple comparison test. A P value less than 0.05 was considered to be statistically significant.
RESULTS
Effects of CH on PQ-Induced Lung Wet-to-Dry Ratio
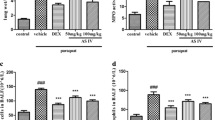
The index of lung edema was measured by calculating the W/D ratio of lung tissue. As revealed in Fig. 1, the lung W/D ratios in PQ-stimulated mice were evidently higher versus those of control mice. On the contrary, the W/D ratios in CH (10 and 20 mg/kg) groups and the Dex (2 mg/kg) group significantly decreased compared to those in the PQ group. The data clearly indicated the obvious reduction of pulmonary edema content with the treatment of CH.
The effects of CH on PQ-induced acute lung injury. a Lung W/D ratio. b MPO activity. c The number of total cells in BALF. d The number of neutrophils in BALF. Values are expressed as mean ± SD. # P < 0.05; ## P < 0.01; ### P < 0.001, compared to the control group. *P < 0.05; **P < 0.01; ***P < 0.001, compared to the PQ group.
Effects of CH on Inflammatory Cells in BALF and Myeloperoxidase Activity in Lung Tissues
The myeloperoxidase (MPO) activity was measured to elucidate the neutrophil accumulation in pulmonary tissues. Meanwhile, the number of total cells and neutrophils was analyzed to reveal the migration and infiltration of pulmonary cells (Fig. 2). As illustrated in Fig. 1, the number of total cells, neutrophils, and MPO activity in the PQ group significantly increased as compared to those in the control group. Treatment with CH (10 and 20 mg/kg) and Dex (2 mg/kg) effectively decreased the number of total cells, neutrophils, and MPO level compared to those in the PQ group. Our results suggested that CH exhibited an inhibitory effect on cell infiltration.
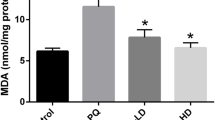
The effects of CH on the oxidative stress of PQ-induced mice. a MDA in the BALF. b SOD in the BALF. c MDA in the tissue. d SOD in the tissue. Values are expressed as mean ± SD. # P < 0.05; ## P < 0.01; ### P < 0.001, compared to the control group. *P < 0.05; **P < 0.01; ***P < 0.001, compared to the PQ group.
Effects of CH on Cytokines in BALF
Cytokines participate in the initiation and amplification of inflammatory cascade of acute lung injury (ALI). As shown in Fig. 3, it was proved that the levels of TNF-α, IL-1β, and IL-6 in BALF were significantly increased in the PQ group compared to those in the control group. The CH (10 and 20 mg/kg) and Dex (2 mg/kg) treatment remarkably decreased the generations of TNF-α, IL-1β, and IL-6. CH reduced the synthesis and release of inflammatory cytokines in PQ-induced ALI.
Effects of CH on Oxidative Stress
Lipid peroxidation in BALF as well as lung tissues was determined by assaying the generations of MDA and SOD. As revealed in Fig. 2, the trend of MDA and SOD levels in the BALF between groups is consistent with that of MDA and SOD levels in the tissue. However, the levels of MDA and SOD in the BALF exhibited more significant differences among groups than those in the tissue. PQ stimulation significantly declined the SOD content, while CH administration effectively restored the level of SOD. Meanwhile, exposure to PQ displayed a strikingly high MDA level whereas the treatment with CH remarkably ameliorated this condition.
Effect of CH on PQ-Induced Pathological Changes of the Lung
Hematoxylin and eosin (H&E) staining was performed to evaluate the protective effects of CH on physiological impairment. Scarce obvious histological alteration was observed in the lung specimen. By contrast, in the PQ group, histological evaluation of the lungs by light microscopy demonstrated alveolar wall hyperemia and excessive neutrophil infiltration around the pulmonary vessel after the simulation of PQ. CH treatment groups obviously attenuated the severity of lung injury. These findings suggested that CH significantly attenuated the histopathology conditions in PQ-induced ALI (Fig. 4).
The effects of CH on PQ-stimulated lung histopathologic changes in lung tissues. a The lung section from the control mice; b the lung section from the PQ mice; c the lung section from the mice administered with PQ and dexamethasone (2 mg/kg); d the lung section from the mice administered with PQ and CH (10 mg/kg); e the lung section from the mice administered with PQ and CH (20 mg/kg).
Effect of CH on PQ-Induced PPAR-γ
We detected the expression of PPAR-γ by Western blot analysis. As shown in Fig. 5, PQ challenge respectively induced an obvious decrease in the expression of PPAR-γ. However, it was proved that the treatment with CH significantly activated PPAR-γ.
Effect of CH on PQ-Induced NF-κB Pathway
NF-κB pathway plays a key role in the regulation of inflammatory mediator production. We found that the expressions of p-NF-κBP65, P-IκBα, P-IKKα, and P-IKKβ were significantly upregulated in lung tissues after PQ stimulation. By contrast, treatment with CH (10 and 20 mg/kg) and Dex (2 mg/kg) obviously ameliorated these situations (Fig. 6).
DISCUSSION
Evidence has emerged indicating that CH exerted the anti-inflammatory and anti-oxidative properties [17]. In this study, our results showed that CH could attenuate PQ-induced pulmonary and lung injury in mice. The ability of CH to reduce PQ-induced toxicity was due to its anti-inflammatory property in lung tissues.
Paraquat is the widely used herbicide around the world. Since its first application on agriculture in 1962, thousands of people died yearly from the intentional or accidental ingestion of PQ. As to our knowledge, the lung tissue is the major target organ as PQ is actively taken up by the alveolar epithelium [18]. Therefore, the injection of PQ was usually used as an inducer for acute lung injury in scientific studies [19].
ALI is a severe clinical syndrome characterized by noncardiogenic pulmonary edema, severe hypoxemia, accumulation of pulmonary cells, and overproduction of inflammatory cytokines [20]. Unfortunately, there were few effective drugs to treat ALI as yet [21]. Thus, there is an urgent need to find a safer medicine for the management of acute lung injury. Edema is the pivotal feature of systemic and partial inflammations [22]. The W/D ratio was calculated to determine the water content of lung tissues [23]. As suggested by the evident reduction of W/D ratio, our data confirmed that CH was capable of attenuating the pulmonary edema. The migration and infiltration of neutrophils into the lung is the characteristic symptom of pulmonary disease. Moreover, the suppression of neutrophils participates in the mediation of oxygen species, cytokines, and chemokines and granular enzyme-mediated lung injury [24]. MPO drove superabundant oxidative production which leads to the tissue damage under inflammatory conditions [25]. The experimental results indicated that CH effectively reduced the MPO activity and the inflammatory cell infiltration. Furthermore, the histopathological observation also confirmed the protective effects of CH on PQ-challenged mice.
Inflammatory cytokines appear in the early stage of the inflammatory response and contribute to the severity of lung dysfunction [26]. IL-1β conduces to the alveolar epithelial repairment and induces the release of other cytokines [27]. IL-6 is highly related to the acute-phase response of inflammation and the initiation of inflammatory cascade [28]. TNF-α, playing an important role in the motivation of innate immune reaction, is responsible for the pathogenesis of inflammation [29]. As expected, CH was found to effectively downregulate the contents of IL-1β, IL-6, and TNF-α, which indicated that the protective effects of CH on PQ-induced acute lung injury was partly attributed to the suppression of inflammatory mediators.
Accumulating evidences proposed the common link between acute lung injury and oxidative stress [30]. The excessive oxygen-free radical, which occurs during the pathogenesis of pulmonary lesion, also contributes to biological membrane lipid peroxidation and severe cell damage [31]. Thus, the attenuation of oxidative stress might be beneficial to the treatment of pulmonary disease. MDA is the end product of polyunsaturated fatty acid and usually used as an indicator for the lipid peroxidation [32]. SOD exhibits various physiological activities including anti-inflammatory and anti-oxidative effects [33]. As the analytical results shown, CH evidently decreased the content of MDA and restored SOD activity in both BALF and lung tissues. Our results suggested that the therapeutic effects of CH might result from the anti-oxidative activity.
It was indicated that PPAR-γ exhibits a cytoprotective effect against cellular stress and inflammatory insults. Above all, our study firstly demonstrated that CH increases the expression/activity and nuclear translocation of PPAR-γ in the lungs of ALI. Next, PQ challenge brings about the activation of NF-κB through the phosphorylation and degradation of the IκBα [34]. Substantial researches also elicited that NF-κB pathway was implicated in the lipid peroxidation and inflammatory process [35]. Kim et al. displayed that CH exhibited the anti-inflammatory effects through the NF-κB signaling [36]. Our Western blotting data ascertained that CH, exerting an ameliorative effect on ALI, significantly increased the expression of PPAR-γ and inhibited the NF-κB pathway in response to PQ challenge, which demonstrated our hypothesis before.
In conclusion, the results of the present study revealed that CH could effectively attenuate PQ-induced ALI. The potential mechanism of CH might be involved in the amelioration of inflammatory cytokines and oxidative stress through the activated PPAR-γ and inhibited NF-κB pathway. Despite this, we elaborate the protective effect of CH against paraquat-induced lung injury in the aspect of pharmacodynamics instead of pharmacokinetics in the present study. Adverse effect of CH and its pharmacokinetic situation in vivo remain to be investigated. Further studies are warranted to capitalize on the protective effects of CH on ALI caused by PQ in the clinical practice.
References
Silva, R., H. Carmo, V. Vilas-Boas, D.J. Barbosa, M. Monteiro, P.G. de Pinho, et al. 2014. Several transport systems contribute to the intestinal uptake of paraquat, modulating its cytotoxic effects. Toxicology Letters 232: 271–283.
Choi, J.S., S.S. Jou, M.H. Oh, Y.H. Kim, M.J. Park, H.W. Gil, et al. 2013. The dose of cyclophosphamide for treating paraquat-induced rat lung injury. The Korean Journal of Internal Medicine 28: 420–427.
Nguyen, V., D.S. Malik, and M.A. Howland. 2014. Methylene blue protects against paraquat-induced acute lung injury in rats. International Immunopharmacology 20: 358.
Chen, T., Y. Mou, J. Tan, L. Wei, Y. Qiao, T. Wei, et al. 2015. The protective effect of CDDO-Me on lipopolysaccharide-induced acute lung injury in mice. International Immunopharmacology 25: 55–64.
Lucas, R., A.D. Verin, S.M. Black, and J.D. Catravas. 2009. Regulators of endothelial and epithelial barrier integrity and function in acute lung injury. Biochemical Pharmacology 77: 1763–1772.
Lin, M.H., M.C. Chen, T.H. Chen, H.Y. Chang, and T.C. Chou. 2015. Magnolol ameliorates lipopolysaccharide-induced acute lung injury in rats through PPAR-gamma-dependent inhibition of NF-kB activation. International Immunopharmacology 28: 270–278.
Wei, D., and Z. Huang. 2014. Anti-inflammatory effects of triptolide in LPS-induced acute lung injury in mice. Inflammation 37: 1307–1316.
Chang, X., F. Luo, W. Jiang, L. Zhu, J. Gao, H. He, et al. 2015. Protective activity of salidroside against ethanol-induced gastric ulcer via the MAPK/NF-kappaB pathway in vivo and in vitro. International Immunopharmacology 28: 604–615.
Zhu, L., T. Chen, X. Chang, R. Zhou, F. Luo, J. Liu, et al. 2016. Salidroside ameliorates arthritis-induced brain cognition deficits by regulating Rho/ROCK/NF-κB pathway. Neuropharmacology 103: 134–142.
Chen, T., Q. Guo, H. Wang, H. Zhang, C. Wang, P. Zhang, et al. 2015. Effects of esculetin on lipopolysaccharide (LPS)-induced acute lung injury via regulation of RhoA/Rho kinase/NF-small ka, CyrillicB pathways in vivo and in vitro. Free Radical Research 49: 1459–1468.
Chen, T., J. Gao, P. Xiang, Y. Chen, J. Ji, P. Xie, et al. 2015. Protective effect of platycodin D on liver injury in alloxan-induced diabetic mice via regulation of Treg/Th17 balance. International Immunopharmacology 26: 338–348.
Chen, T., L. Xiao, L. Zhu, S. Ma, T. Yan, and H. Ji. 2015. Anti-asthmatic effects of ginsenoside Rb1 in a mouse model of allergic asthma through relegating Th1/Th2. Inflammation 38: 1814–1822.
Lin, F., C. Zhang, X. Chen, E. Song, S. Sun, M. Chen, et al. 2015. Chrysophanol affords neuroprotection against microglial activation and free radical-mediated oxidative damage in BV2 murine microglia. International Journal of Clinical and Experimental Medicine 8: 3447–3455.
Lee, M.S., and C.B. Sohn. 2008. Anti-diabetic properties of chrysophanol and its glucoside from rhubarb rhizome. Biological & Pharmaceutical Bulletin 31: 2154–2157.
Zhang, K., J. Liu, X. You, P. Kong, Y. Song, L. Cao, et al. 2016. P2X7 as a new target for chrysophanol to treat lipopolysaccharide-induced depression in mice. Neuroscience Letters 613: 60–65.
Zhang, N., and X. Zhang. 2014. Chrysophanol inhibits NALP3 inflammasome activation and ameliorates cerebral ischemia/reperfusion in mice. 2014:370530.
Rim, H.K., P.D. Moon, I.H. Choi, E.H. Lee, H.M. Kim, and H.J. Jeong. 2013. SoSoSo or its active ingredient chrysophanol regulates production of inflammatory cytokines & adipokine in both macrophages & adipocytes. The Indian Journal of Medical Research 137: 142–150.
Orito, K., Y. Suzuki, H. Matsuda, M. Shirai, and F. Akahori. 2004. Chymase is activated in the pulmonary inflammation and fibrosis induced by paraquat in hamsters. The Tohoku Journal of Experimental Medicine 203: 287–294.
Ahmed, A.A. 2009. Protective effect of montelukast on paraquat-induced lung toxicity in rats. Bioscience Trends 3: 63–72.
Jiang, W., F. Luo, Q. Lu, J. Liu, P. Li, X. Wang, et al. 2016. The protective effect of Trillin LPS-induced acute lung injury by the regulations of inflammation and oxidative state. Chemico-Biological Interactions 243: 127–134.
Xie, X., S. Sun, W. Zhong, L.W. Soromou, X. Zhou, M. Wei, et al. 2014. Zingerone attenuates lipopolysaccharide-induced acute lung injury in mice. International Immunopharmacology 19: 103–109.
Chen, T., Ma, Z., Zhu, L., Jiang, W., Wei, T., Zhou, R., et al. 2015. Suppressing receptor-interacting protein 140: a new sight for salidroside to treat cerebral ischemia. Molecular Neurobiology.
Huang, X., Y. Liu, Y. Lu, and C. Ma. 2015. Anti-inflammatory effects of eugenol on lipopolysaccharide-induced inflammatory reaction in acute lung injury via regulating inflammation and redox status. International Immunopharmacology 26: 265–271.
Tianzhu, Z., Y. Shihai, and D. Juan. 2014. The effects of morin on lipopolysaccharide-induced acute lung injury by suppressing the lung NLRP3 inflammasome. Inflammation 37: 1976–1983.
Bettelli, E., Y. Carrier, W. Gao, T. Korn, T.B. Strom, M. Oukka, et al. 2006. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441: 235–238.
Tianzhu, Z., and W. Shumin. 2015. Esculin inhibits the inflammation of LPS-induced acute lung injury in mice via regulation of TLR/NF-kappaB pathways. Inflammation 38: 1529–1536.
Wang, J., Y.T. Liu, L. Xiao, L. Zhu, Q. Wang, and T. Yan. 2014. Anti-inflammatory effects of apigenin in lipopolysaccharide-induced inflammatory in acute lung injury by suppressing COX-2 and NF-kB pathway. Inflammation 37: 2085–2090.
Gao, J., H. He, W. Jiang, X. Chang, L. Zhu, F. Luo, et al. 2015. Salidroside ameliorates cognitive impairment in a d-galactose-induced rat model of Alzheimer’s disease. Behavioural Brain Research 293: 27–33.
He, H., X. Chang, J. Gao, L. Zhu, M. Miao, and T. Yan. 2015. Salidroside mitigates sepsis-induced myocarditis in rats by regulating IGF-1/PI3K/Akt/GSK-3beta signaling. Inflammation 38: 2178–2184.
Ma, C.H., J.P. Liu, R. Qu, and S.P. Ma. 2014. Tectorigenin inhibits the inflammation of LPS-induced acute lung injury in mice. Chinese Journal of Natural Medicines 12: 841–846.
Tao, W., Q. Su, H. Wang, S. Guo, Y. Chen, J. Duan, et al. 2015. Platycodin D attenuates acute lung injury by suppressing apoptosis and inflammation in vivo and in vitro. International Immunopharmacology 27: 138–147.
Ma, C., L. Zhu, J. Wang, H. He, X. Chang, J. Gao, et al. 2015. Anti-inflammatory effects of water extract of Taraxacum mongolicum hand.-Mazz on lipopolysaccharide-induced inflammation in acute lung injury by suppressing PI3K/Akt/mTOR signaling pathway. Journal of Ethnopharmacology 168: 349–355.
Chang, X., H. He, L. Zhu, J. Gao, T. Wei, Z. Ma, et al. 2015. Protective effect of apigenin on Freund’s complete adjuvant-induced arthritis in rats via inhibiting P2X7/NF-kappaB pathway. Chemico-Biological Interactions 236: 41–46.
Chen, T., Wang, R., Jiang, W., Wang, H., Xu, A., Lu, G., et al. 2015. Protective effect of astragaloside IV against paraquat-induced lung injury in mice by suppressing Rho signaling. Inflammation.
Zhu, L., T. Wei, X. Chang, H. He, J. Gao, Z. Wen, et al. 2015. Effects of salidroside on myocardial injury in vivo in vitro via regulation of Nox/NF-kappaB/AP1 pathway. Inflammation 38: 1589–1598.
Kim, S.J., M.C. Kim, B.J. Lee, D.H. Park, S.H. Hong, and J.Y. Um. 2010. Anti-inflammatory activity of chrysophanol through the suppression of NF-kappaB/caspase-1 activation in vitro and in vivo. Molecules (Basel, Switzerland) 15: 6436–6451.
Acknowledgments
This study was supported by grants of the Natural Science Foundation of Jiangsu Province of China (BK20150707) and the Fundamental Research Funds for the Central Universities (JKZD2013009 and ZJ15030). This project also funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), Research Innovation Program Project for Graduate Students in Jiangsu Province (CXZZ13_03), and National Undergraduate Training Programs for Innovation and Entrepreneurship (G13034).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Li, A., Liu, Y., Zhai, L. et al. Activating Peroxisome Proliferator-Activated Receptors (PPARs): a New Sight for Chrysophanol to Treat Paraquat-Induced Lung Injury. Inflammation 39, 928–937 (2016). https://doi.org/10.1007/s10753-016-0326-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-016-0326-2