Abstract
WIN 55212–2 is an endocannabinoids analogue that has been reported to have anti-inflammatory and anti-fibrosis effects on different models. In this study, we investigated the protective effects of WIN 55212–2 on paraquat (PQ)-induced poison on mice especially on lung injury. Mice were administrated with different dose of PQ and thereafter treated with 0.2 mg/kg or 1 mg/kg WIN 55212–2. The survival of mice was recorded during 4 weeks of observation. Twenty-eight days after PQ treatment, the cell population and inflammatory factors IL-6, IL-10, and TNF-α were measured in bronchoalveolar lavage fluid (BALF). Pulmonary fibrosis was evaluated by Masson staining. Our results showed that WIN 55212–2 treatment reduced PQ-induced mortality of mice in a dose dependent manner. It decreased the number of inflammation-associated cells, as well as the level of pro-inflammatory factors in BALF (P < 0.05). WIN 55212–2 increased M2 cells in BALF (P < 0.05), improved the lung histology, reduced fibrosis formation, and decreased TGF-β, α-SMA and PDGFRa expression. The protective effects of WIN 55212–2 on PQ-induced lung injury and fibrosis were associated with an increase inM2 cells and increased expressions of IL-10, CD163, and CD206, suggesting that polarization of M2 macrophages may be involved in WIN 55212–2 protective effects on PQ-induced lung injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Paraquat (PQ, 1,1′-dimethyl-4,4′-bipyridinium) is an excellent herbicide which has been widely used up to 130 years [1]. Although the usage of PQ is rigorously restricted in many countries including China because of the poisoning to human beings, every year, several patients taking PQ from rural areas are referred to provincial hospitals for treatment. PQ exposure, either accidental or intentional, is associated with high mortality rates. The incidence rate of PQ intoxication is at least 3.8 cases per 100,000 inhabitants annually [2]. The pathogenesis of PQ toxicity to human lung goes through two stages. During the early phase (or acute phage), clinical manifestations include lung injury and acute respiratory distress syndrome [3]. Death may occur during this period, especially when PQ intake is excessive. When surviving from the first stage, patients generally show a period of improvement, but in most cases, this is merely the prelude to the second stage, i.e., pulmonary fibrosis after the acute phase. Extensive pulmonary fibrosis results in dyspnea, cyanosis, and eventually death from respiratory failure [4]. Preventing or inhibition the fibrosis seems a promising strategy to reduce pulmonary fibrosis-related death of PQ poisoning.
WIN 55212–2 is a cannabinoid receptor agonist that acts on cannabinoid receptors, CB1R and CB2R with Ki values 62.3 and 3.3 nM respectively. It is reported WIN 55212–2 could abrogate dermal fibrosis in scleroderma bleomycin model paralleled by a strong inhibition of TGFβ, CTGF, and PDGF-BB expression [5] WIN 55212–2 also protects against oxidized LDL-induced inflammatory response in murine macrophages signaling though CB2 receptor [6]. There are many other reports indicate endocannabinoids involve in the progression of fibrosis of multiple organs, including the liver [7,8,9,10], kidney [11, 12], heart [13], and skin [14]. CB2 receptor is highly expressed on inflammatory cells, such as B and T lymphocytes, macrophages, neutrophils, and mast cells [15]. Recent works have related it with immunosuppressive effects by modulating lymphocyte cell activation and differentiation [16]. The anti-inflammatory and protective properties of WIN 55212–2 may be used to control inflammation and tissue damage during SARS-CoV-2 infection (COVID-19) in heart cells [17]. It is noteworthy that cannabinoids have anti-inflammatory properties and exert their biological effect mainly by interaction with the cannabinoid receptors type 1 (CB1R) and/or type 2 (CB2R), to both of which WIN 55212–2 has high affinity[18, 19]. Also, high-cannabidiol (CBD) Cannabis sativa extracts presented anti-inflammatory properties in the epithelia pre-treated with TNF-α and IFN-γ [20]. Since PQ-induced pulmonary injury is a pro-inflammatory process, could the consequences of lung injury in a PQ-induced mouse model be affected if CB2R is activated with WIN 55212–2? In this work, we evaluated the protective role of WIN 55212–2 on PQ-induced lung injury. Our observations suggest that WIN 55212–2 inhibits PQ-induced lung fibrosis and regulates the polarization of M2 macrophages in mice. This supports the therapeutic potential of targeting CB2R system for PQ-induced pulmonary fibrosis.
MATERIALS AND METHODS
Animals and Treatments
Male C57BL/6 mice, 6–8 weeks old, weighing 22–25 g, were obtained from the Experiment Animal Center of Kunming medical university and they were housed in normal laboratory conditions at 22 ± 2 °C with a 12 h light/12 h dark cycle. The mice had free access to water and standard rodent chow. The study was conducted in accordance with the Basic & Clinical Pharmacology & Toxicology policy for experimental and clinical studies [21]. All the animals were treated humanely according to the guideline approved by the Animal Experimentation Ethics Committee of the First People’s Hospital of Yunnan Province.
Three groups of mice (45 mice per group) at different PQ doses (20 mg/kg, 30 mg/kg, and 45 mg/kg) were further divided into nine subgroups according to the administration of WIN 55212–2 (MedChemexpress Co., China), namely the PQ20 (PQ 20 mg/kg) subgroups: [PQ20-WIN 55212–2 (WIN)-0] (the control, n = 15), PQ20-WIN 0.2 mg (n = 15), and PQ20-WIN 1 mg (n = 15); PQ30 (PQ 30 mg/kg) subgroups: PQ30-WIN-0 (the control, n = 15), PQ30-WIN 0.2 mg (n = 15), and PQ30-WIN 1 mg (n = 15); and PQ45 (PQ 45 mg/kg) subgroups: PQ45-WIN 0 (the control, n = 15), PQ45-WIN 0.2 mg (n = 15), and PQ45-WIN 1 mg. A single dose of PQ was given trough intraperitoneal (i.p.) injection and following with WIN 55212–2 i.p. injection after 3 h and then every 2 days, for a total of 28 days of WIN 55212–2 treatment. The death of mice is recorded daily. The dead mice were also dissected to observe the lung. The left lung was taken for histological observation and the remaining right lung for molecular detection. After 28 days of PQ administrating, all of the mice were anesthetized by injecting 10 mg/ml barbital sodium (i.p., 80ug/g body weight). BALFs were collected from 10 mice by intratracheal instillation and draining of 0.5 ml normal saline at 37 °C. The removed left and right lungs were immediately frozen in liquid nitrogen and cryopreserved at −80 °C for later histological and molecular index testing.
Cell Component Analysis of BALFs
For HE staining, freshly collected BALFs were centrifuged at 800 g for 10 min, the pellets were re-suspended with 0.5 mL PBS (containing 1% BSA), and then, 20 µl cell pellets were smeared on slide for hematoxylin–eosin (HE) staining (Solarbio, China). The total and differential leukocyte counts were determined under × 400 magnification.
For immunofluorescence staining, the remaining 480 μl suspension of each sample was divided into two tubes with equal volume, and one for F4/80 and CD206 double staining, the other for F4/80 and CCR7 double staining. Add 5 μl primary antibody, anti-F4/80 antibody [BM8] (ab16911) into each tube, mix well, and incubate with cells for 15 min; cells were then washed with PBS for three times by centrifugation. The pellets were suspended with 200 μl secondary antibody solution (PBS containing 1% BSA and 1 μl goat anti-Rat IgG H&L (Alexa Fluor® 555)), incubated for 10 min, and washed with PBS for three times; the result pellets were than suspended with 200 μl 1% BSA-PBS containing 5 μl anti-Mannose Receptor(CD206) antibody (ab64693) or anti-CCR7 antibody[Y59] (ab32527) for double staining for 15 min; after washing three times with PBS, the cells were staining with another secondary antibody, goat anti-Rabbit IgG H&L (Alexa Fluor® 488) (ab150077) as above. Cell pellets were suspended with 500 μl 1% BSA-PBS, and analysis with FACSCalibur (BD Biosciences), and the results were analyzed with FlowJo software (TreeStar). Primary antibodies and secondary antibodies used were purchased from Abcam Company (US).
Total Protein Analysis of BALFs and Inflammatory Factor Level Determination
BALF total protein was assessed using Bradford Protein Assay Kit (Tiangen, Beijing, China) following the manufacturer’s protocols. Inflammatory factors TNF-a, IL-6, and IL-10 levels in BALFs were determined by enzyme-linked immune sorbent assay (ELISA) with TNF-a, IL-6, and IL-10 ELISA kits (Elabscience. Wuhan, China).
Quantitative Real-time PCR
Thirty- to fifty-milligram cryopreserved lung tissues were ground into powder in liquid nitrogen using an RNase-free mortar. The lung tissues were then removed into 1.5 mL PE tube and the total RNA was extract by TRNzol-A + Reagent (Tiangen, Beijing, China). Two-microgram RNA was reverse transcribed to cDNA using the FastQuant RT Kit (With gDNase) (Tiangen, Beijing, China). TNF-a, IL-16, IL-10, CD163, CD06, TGF-b, a-SMA, Fibronectin, collagen I, collagen III, PDGFRa, and GAPDH mRNA levels were determined using the SuperReal PreMix Plus (SYBR Green) kit (Tiangen, Beijing, China) and the Rotor-Gene Q 2plex HRM platform (Qiagene, DE). Gene expressions were calculated using the 2^-ΔΔCt method. The housekeeping gene GAPDH was used as loading control.
Western Blot Analysis
Twenty-milligram lung tissue was lysed by 150 μl RIPA Lysis Buffer (Beyotime,China) containing 1 mM PMSF protease inhibitor (Beyotime,China). The concentration of protein in the lysate was determined by BCA protein assay kit (Tiangen, Beijing, China). Mix the protein lysate with SDS-PAGE sample loading buffer (Beyotime, China) and degenerate the proteins at 95℃ for 10 min. Fifty-microgram proteins were separated on 10% SDS–polyacrylamide gels and transferred onto PVDF membranes. The membranes were blocked with QuickBlock™ Blocking Buffer (Beyotime, China), and incubated with primary antibodies overnight at 4℃ (antibodies were 1:1000 diluted with blocking buffer). Wash the membranes with TBST buffer for 5 times, each time last 5 min. Incubate the membranes with appropriate secondary antibody. Wash the unconjugated antibody with TBST buffer for 5 times, each time last 5 min. Develop the plots with the SuperSignal chemiluminescence reagents (Pierce, US) and Tanon automatic chemiluminescence image analysis system (Tanon, Shanghai, China). Primary antibodies used are as follows: anti-Mannose Receptor (CD206) antibody (ab64693, Abcam, US); Anti-CD163 antibody [EPR19518] (ab182422, Abcam, US), anti-aSMA antibody(#14968,CST,US) anti-TGF-β Antibody (#3711,CST,US), anti-collagen I antibody (ab34710, Abcam, US), anti-Fibronectin antibody (ab199056, Abcam, US), PDGF Receptor α antibody (#3174, CST, US), and beta actin Monoclonal Antibody (E-AB-20031, Elabscience, China). Secondary antibodies used are as follows: goat Anti-Rabbit IgG H&L (HRP) (ab6721, Abcam, US) and goat Anti-Mouse IgG-HRP (E-AB-1001, Elabscience, China).
Masson Staining and Immunohistochemistry
Frozen lung tissues were sliced into 5 mm thick sections with a Leica microtome. Sections were carefully removed and spread onto the slide. Sections were stained with Masson’s Trichrome Stain Kit (Solarbio, China) to assess collagen fibers. For IL-10 and CD206 staining, sections were blocked with normal goat serum for 30 min, and then incubated with 1:100 diluted IL-10 antibody (ab189392, Abcam, US) or 1:200 diluted CD206 antibody (ab64693, Abcam, US) at 4℃ overnight. Sections were washed with PBST. Incubate sections with HRP conjugated secondary antibodies (1:1000 dilute with 1% BSA-PBS) for 1 h at room temperature. Sections were stained with DAB stain for 10 min, rinsed under running tap water for 5 min, and counterstained with hematoxylin. The slides were examined and photographed at × 200 magnifications use a light microscope.
Statistical Analysis
The quantitative data were analysis with IBM SPSS software and showed as average ± SD in column graphs (error bars on histogram represent SD). The ImageJ software was used for Masson and immunohistochemistry (IHC) staining quantification. The differences between two groups were analyzed by one-way ANOVA followed by the Bonferroni T test. The differences in survival rate were analyzed by chi-square test. Overall survival at 28 days in PQ-administrated mice was determined using the Kaplan–Meier function and the significance of the differences was evaluated by log-rank test; P < 0.05 was considered to be statistically significant.
RESULTS
WIN 55212–2 Reduce PQ-Induced Mortality of Mice
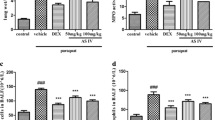
The result showed that PQ20 subgroups had almost no effect on the mortality after 28 days. PQ30 and PQ45 subgroups showed 40% and 46.7% fatality, respectively. In contrast, administration of WIN 55212–2 (i.p.) reverse this situation, resulting in increased survival in both the PQ30 and PQ45 subgroups. They have a significant effect on mice survival status (Table 1, P < 0.05). The overall survival rate of mice was showed in Fig. 1A. Figure 1B shows the survival rates of all mice administered PQ. This indicated that WIN 55212–2 successfully improved the survival rate of PQ-administrated mice (log-rank P = 0.005).
Survival rates of PQ-injected mice. A Survival rates of PQ-injected mice at 20 mg/kg, 30 mg/kg, and 45 mg/kg administrated with 0 mg/kg, 0.2 mg/kg, or 1 mg/kg WIN 55212–2 (each group, n = 15). B Kaplan–Meier function for overall survival of WIN 55212–2 administered mice at different doses, followed up to 28 days after PQ treatment (three PQ dose combinations in this experiment and, n = 45, *P < 0.05).
WIN 55212–2 Reduces PQ-Induced Pro-inflammatory Cells and Increases M2 Macrophage Cells
BALFs collected from different groups were analyzed. Macrophages, neutrophils, and leukocytes were identified by HE staining and microscopy. The total number of inflammatory-related cells and the number of each inflammatory cell types were showed in Fig. 2A&B. The total protein levels were showed in Fig. 2C. The results showed that the total number of inflammation- associated cells increased after PQ injection but lowered after WIN 55212–2 administration(P < 0.05). The total protein levels showed the same trend with the total inflammatory cells(P < 0.05). Since macrophage activation was associated with inflammation response, different subtypes of macrophages serve their respective inflammatory regulatory signaling pathways. Two well-known subtypes of macrophage, M1 and M2, were analyzed by flow cytometry analysis in this work. M1 macrophages were usually related to pro-inflammation pathways, while M2 macrophages serve inflammation suppressing pathway. The percentages of F4/80 + /CD206 + cells (M2 macrophages) and F4/80 + /CCR7 + cells (M1 macrophages) in mice BALFs were quantified by flow cytometry analysis. The results showed that PQ-induced increase of macrophages was mainly due to an increase in F4/80 + /CCR7 + M1 cells (P < 0.05, Fig. 2D). When WIN 55212–2 was used, there was an increase in the F4/80 + /CD206 + M2 macrophages and a decrease in M1 cells (P < 0.05, Fig. 2D). This indicated that WIN 55212–2 may influence inflammatory response by altering the polarization macrophages.
Analysis of inflammatory cells and protein levels in BALFs collected from control mice and mice injected with PQ with or without administration of WIN 55212–2. A Typical figure of HE staining of BALFs cells. Black arrow, macrophage; green arrow, neutrophil; yellow arrow, leukocyte. B The number of different inflammatory cell subtypes in BALFs. C Total protein levels of different groups. In C and B, *P < 0.05 compared to PQ30-WIN-0 group; #P < 0.05 indicates significant difference compared to control group. D Analysis of M1 and M2 macrophages by cell surface antigen labeling and flow cytometry assay. For all of the assays, dose of PQ was 30 mg/kg, n = 5. * or # P < 0.05 indicates a significant difference.
WIN 55212–2 Regulated Inflammatory Factors TNF-a, IL-6, IL-10, CD206, and CD163 Expression
Expression of TNF-α and IL-6 is induced in many acute injury-associated inflammatory response, while the expression of IL-10, CD206, and CD163 was reported to be associated with the activation of M2 macrophage and to play a role in inflammation suppressing. In this work, the expression of inflammatory factors TNF-a, IL-6, and IL-10 in BALFs was analyzed by ELISA. The results in Fig. 3 showed that PQ-induced TNF-a and IL-6 up expression was attenuated by WIN 55212–2 (P < 0.05), while IL-10 expression was not changed by PQ but was increased by WIN 55212–2 treatment. The Q-PCR results of TNF-a, IL-6, and IL-10 in the right lung further verified this altered expression (Fig. 3B). More importantly, WIN 55212–2 increased CD163 and CD206 expression in the right lung at both the mRNA and protein levels (Fig. 3C–D, P < 0.05), indicating M2 macrophages were activated.
Expression assay of inflammatory factors. A ELISA analysis of inflammatory cytokines levels in BALFs collected from control mice and PQ injected mice with or without WIN 55212–2 administration. B Fold change in mRNA expression of TNF-a, IL-6, and IL-10 in lung tissues. C Fold change of mRNA expression of CD163 and CD206 in lung tissues. D Western blot analysis of CD163 and CD206 in lung tissues. PQ dose was 30 mg/kg. n = 5. #P < 0.05 compared with PQ30-WIN-0 group, *P < 0.05 indicates significant difference compared with control group.
WIN 55212–2 Reduced PQ-Induced Fibrosis While Enhancing M2 Macrophage Maker Gene Expression
In this work, the fibrosis-related factors and fibrosis maker genes were analyzed. The expression of fibrosis-related genes TGF-β, α-SMA, PDGFRa, Fibronectin, and collagen I was suppressed by WIN 55212–2 (Fig. 4A–E, P < 0.05), while collagen III expression was not significantly altered by PQ or WIN 55212–2 administration in our results (Fig. 4C). Masson staining of the left lung also showed a significant improvement in PQ-induced fibrosis status. Also, WIN 55212–2 caused upregulation of IL-10 and CD206 expression, and these changes were confirmed by immunohistochemical results (Fig. 4C). This was further consistent with the western blot and Q-PCR results.
WIN 55212–2 reduced PQ-induced fibrosis and enhance IL-10 and CD206 expression. A–C Q-PCR analysis of TGF-b, a-SMA, Fibronectin, PDGFRα, collagen I, and collagen III exp-ression in different groups of lung tissues. #P < 0.05 compared to the PQ30-WIN-0 group; *P < 0.05 compared to the control group indicates a significant difference; D–E western blot analysis of TGF-b, a-SMA, Fibronectin, Col-I, and PDGFRα expression in different groups of lung tissues; F–I Masson staining and immunohistochemical staining of IL-10 and CD 206 and their relative staining percentage analysis. Collagen fiber was blue staining in Masson staining. IL-10 and CD 206 positive cells were brown staining in IHC DBA development. For each sample, 5 different microscopic fields were quantified by the ImageJ software. PQ dose is 30 mg/kg, n = 5, *or #P < 0.05 indicates a significant difference.
DISCUSSIONS
PQ poisoning can cause damage to many organs, mainly in the lungs. The lethality of its toxicity is mainly caused by lung damage, with the main lesions being proliferative bronchiolitis and alveolitis. The morphological changes of the lungs after PQ poisoning depend on the length of the life after ingestion. In the first week after poisoning, type I and type II alveolar epithelial cell swell, degenerate and necrosis, with pathological changes such as pulmonary congestion, edema, and increased lung weight characteristic of oxygen toxicity. After 1 week of survival, alveolar exudates (including alveolar epithelial debris, macrophages, erythrocytes, and clear membranes), mononuclear infiltration, hemorrhage, and interstitial fibroblast proliferation, alveolar interstitial thickening, extensive fibrosis, and cellular lung and bronchiectasis were observed. Oxidative stress, inflammation, and pulmonary fibrosis are for the cause of lung injury. PQ poisoning can also cause renal tubular necrosis, central lobule cell damage, necrosis, myocarditis, pulmonary arterial thickening, and adrenal cortical necrosis. The mechanism of PQ poisoning is relatively clear, but the investigation related to the treatment of PQ poison and prevention of PQ-induced injury to organs is always insufficient.
Our results revealed that WIN 55212–2 treatment improved the survival of PQ-administrated mice. Cellular component analysis of BALF showed that WIN 55212–2 reduced PQ-induced pro-inflammatory cells but increased F4/80 + /CD206 + M2 macrophages. WIN 55212–2 attenuated PQ-induced expression of pro-inflammatory factors IL-6 and TNF-a expression, but increased IL-10 expression in lung tissues and BALF. IL-10 is one of well researched inflammation regulatory factor. It inflammatory suppress function has been revealed by many reports [22,23,24,25,26]. IL-10 is considered to be a marker of M2 macrophage activation. In this research, we found that not only IL-10 expression was increased but also F4/80 + /CD206 + M2 macrophages were increased by WIN 55212–2 treatment. Another M2 maker gene CD163 also upregulated by WIN 55212–2. These findings indicated that WIN 55212–2 may initiate a pro-/anti-inflammatory transfer regulatory process associated with macrophage polarization.
The inhibitory effect of WIN 55212–2 on pulmonary fibrosis was also evident in our results. Collagen deposition, a-SMA, Fibronectin, PDGFRa, and TGF-b expression were reduced by WIN 55212–2, but whether this reduction is a consequence of anti-inflammatory process or some other process relate to fibrogenesis regulation is unclear. Endocannabinoids may behave as pro- or anti-fibrogenic substances depending on their interaction with CB1 or CB2 receptors, respectively. CB2 receptor activation with CB2 agonist JWH-133 or WIN 55212–2 could prevent the pro-fibrotic effect of bleomycin on the skin [27]. In a mouse model of systemic sclerosis, CB2 agonist also counteracted the pro-fibrotic effects of HOCl on skin and lung [28], and inhibitions of CB2 receptor or CB2R knocking out had an opposite effect in the same model. In contrast to CB2, the overactivity of the cannabinoid CB1 receptor contributes to the pathogenesis of idiopathic pulmonary fibrosis [29]. Cannabinoid CB1 receptor also mediates signaling in the onset and progression of radiation-induced pulmonary fibrosis (RIF) [30]. WIN 55212–2 is a nonselective CB1 and CB2 agonist, but has a greater affinity with CB2. Considering the contributions of CB2R to the regulation of fibrosis in other studies, the antifibrotic effect of WIN 55212–2 in a model of PQ intoxication may be direct and may be independent of the anti-inflammatory effect.
PQ-induced lung injury is a ROS response process. As an electron acceptor, PQ induces numerous ROS upon entry into the cell [31]. High levels of ROS disrupt cell function, induce apoptosis, and cause tissue damage through the induction of oxidative stress [31, 32]. The mitogen-activated protein kinase (MAPK) pathway, which regulates cell apoptosis, is vulnerable to ROS attack. High levels of ROS and activation of MAPK signaling pathways are important causes of PQ-induced lung injury [32,33,34]. Modulation of ROS production and AMPK activation has been described as therapeutic strategy to ameliorate PQ poisoning. Although the mechanism is not clearly defended, there are increasing evidences confirm cannabinoid regulates ROS and MAPK signaling pathway. It was reported that WIN 55212–2 could reduce oxLDL-induced TNF-alpha and ROS levels in RAW264.7 mouse macrophages through inhibition of MAPK (ERK1/2) signaling and NF-kappa B activity [6], while the effects of WIN 55212-2 were attenuated by the selective CB2 receptor antagonist AM630 [6]. WIN 55212–2 reduces ROS formation in cortical neuron cultures treated with FeCl2 [35] and protects against I/R damage by decreasing inflammation and oxidative stress [36, 37]. There are also conflicting results from I/R damage researches that did not observe a ROS clearance effect of CB2 agonist which may related to the time-points at which evaluations were performed [38, 39]. PQ-induced oxidative stress and early inflammatory response are responsible for the early phase (or acute phage) of lung injury. Though we did not detect these indexes in this stage, it is possible that WIN 55212–2 plays roles at an earlier stage (on day 7 or 14) before fibrosis onset.
In this work, we observed the recruitment of M2 macrophages and the upregulation of IL-10 in PQ-poisoning lung after WIN 55212–2 administration, but whether the accumulation of M2 macrophages is responsible for the reduction of pulmonary fibrosis is need to be further addressed. Immunoregulatory M2 macrophage and IL-10 have also been reported to have pro-fibrosis activities when loss control in many other researches [40, 41]. IL-10 is a biomarker of M2 macrophages, but it is also released by other cell types such as dendritic cells when treated with a pro-tolerogenic allergens [42], eosinophils that derived from hypereosinophilic syndrome patients [43], neutrophils during the initial phase of immune priming [44], and B cells in human diseases such as multiple sclerosis, osteoarthritis, and bullous pemphigoid [45]. IL-10 is also produced by CD8 + T cells, TH1, TH2, and TH17 and regulatory T cells and has distinct functions in immune response [46]. These types of immune cells may have involved in the WIN 55212–2 anti-fibrosis effects considering the ubiquitous expression of cannabinoid receptors on the surface of immune cells. Although WIN 55212–2 has a greater affinity with CB2 than CB1, the nonselective activation of both CB2 and CB2 indicates CB1 may be involved in the anti- inflammatory/fibrosis effects of WIN 55212–2. Angelina et al. demonstrated that WIN 55212-2 exerts anti-inflammatory effects by generating tolerogenic dendritic cells and Tregs, and this was not depend on CB2R but on the activation of CB1R and other cannabinoid ligand targets such as PPARα [47]. In this work, we observed the protective effects of WIN 55212–2 on PQ-induced lung damage and fibrosis, but how M2 macrophage balances from immunosuppression/pro-fibrosis activities and whether dendritic cells and Tregs contribute to WIN 55212-2’s anti-inflammatory effects at different PQ poisoning stage were unclear and need further addressing.
Another shortcoming of this paper is that the test period is only 28 days, and the effects of WIN 55212–2 on early phase (or acute phase) of lung injury characterized by clinical acute respiratory distress syndrome (human) cannot be evaluated. Indexes including M2 macrophage polarization, inflammatory factors IL-6, IL-10, and TNF-a, which are closely related to the development of pulmonary fibrosis, may have changed in day 7–14 in mice with PQ administration.
CONCLUSION
The protective effects of WIN 55212–2 on PQ-induced lung damage and fibrosis were linked with an increase in M2 cells and higher expressions of IL-10, CD163, and CD206, indicating that M2 macrophages may be involved in WIN 55212–2 protective effects on PQ-induced lung injury. This paper demonstrates that the use of WIN 55212–2 may reduce the formation of pulmonary fibrosis, and whether this effect would also be beneficial in the treatment of COVID-19 or improve the prognosis of COVID-19 deserves further investigation.
AVAILABILITY OF DATA AND MATERIALS
All data and figures analyzed in this study are available from the corresponding author by request.
References
Dinis-Oliveira, R.J., J.A. Duarte, A. Sanchez-Navarro, F. Remiao, M.L. Bastos, and F. Carvalho. 2008. Paraquat poisonings: Mechanisms of lung toxicity, clinical features, and treatment. Critical reviews in toxicology 38 (1): 13–71.
Elenga, N., C. Merlin, R. Le Guern, R. Kom-Tchameni, Y.M. Ducrot, M. Pradier, B. Ntab, K.A. Dinh-Van, M. Sobesky, D. Mathieu, et al. 2018. Clinical features and prognosis of paraquat poisoning in French Guiana: A review of 62 cases. Medicine (Baltimore) 97 (15): e9621.
Xu, L., J. Xu, and Z. Wang. 2014. Molecular mechanisms of paraquat-induced acute lung injury: A current review. Drug and chemical toxicology 37 (2): 130–134.
Smith, L.L. 1982. Young Scientists Award lecture 1981: the identification of an accumulation system for diamines and polyamines into the lung and its relevance to paraquat toxicity. Archives of toxicology Supplement = Archiv fur Toxikologie Supplement 5:1–14.
Balistreri, E., E. Garcia-Gonzalez, E. Selvi, A. Akhmetshina, K. Palumbo, S. Lorenzini, R. Maggio, M. Lucattelli, M. Galeazzi, and J.W. Distler. 2011. The cannabinoid WIN 55, 212–2 abrogates dermal fibrosis in scleroderma bleomycin model. Annals of the rheumatic diseases 70 (4): 695–699.
Hao, M.X., L.S. Jiang, N.Y. Fang, J. Pu, L.H. Hu, L.H. Shen, W. Song, and B. He. 2010. The cannabinoid WIN 55212–2 protects against oxidized LDL-induced inflammatory response in murine macrophages. Journal of Lipid Research 51 (8): 2181–2190.
Teixeira-Clerc, F., B. Julien, P. Grenard, J. Tran Van Nhieu, V. Deveaux, L. Li, V. Serriere-Lanneau, C. Ledent, A. Mallat, and S. Lotersztajn. 2006. CB1 cannabinoid receptor antagonism: A new strategy for the treatment of liver fibrosis. Nature medicine 12 (6): 671–676.
Patsenker, E., M. Stoll, G. Millonig, A. Agaimy, T. Wissniowski, V. Schneider, S. Mueller, R. Brenneisen, H.K. Seitz, M. Ocker, et al. 2011. Cannabinoid receptor type I modulates alcohol-induced liver fibrosis. Molecular medicine 17 (11–12): 1285–1294.
Trebicka, J., I. Racz, S.V. Siegmund, E. Cara, M. Granzow, R. Schierwagen, S. Klein, A. Wojtalla, M. Hennenberg, S. Huss, et al. 2011. Role of cannabinoid receptors in alcoholic hepatic injury: Steatosis and fibrogenesis are increased in CB2 receptor-deficient mice and decreased in CB1 receptor knockouts. Liver international : Official journal of the International Association for the Study of the Liver 31 (6): 860–870.
Cinar, R., M.R. Iyer, Z. Liu, Z. Cao, T. Jourdan, K. Erdelyi, G. Godlewski, G. Szanda, J. Liu, and J.K. Park, et al. 2016. Hybrid inhibitor of peripheral cannabinoid-1 receptors and inducible nitric oxide synthase mitigates liver fibrosis. JCI insight 1(11).
Lin, C.L., Y.C. Hsu, P.H. Lee, C.C. Lei, J.Y. Wang, Y.T. Huang, S.Y. Wang, and F.S. Wang. 2014. Cannabinoid receptor 1 disturbance of PPARgamma2 augments hyperglycemia induction of mesangial inflammation and fibrosis in renal glomeruli. Journal of molecular medicine 92 (7): 779–792.
Lecru, L., C. Desterke, S. Grassin-Delyle, C. Chatziantoniou, S. Vandermeersch, A. Devocelle, A. Vernochet, N. Ivanovski, C. Ledent, S. Ferlicot, et al. 2015. Cannabinoid receptor 1 is a major mediator of renal fibrosis. Kidney international 88 (1): 72–84.
Slavic, S., D. Lauer, M. Sommerfeld, U.R. Kemnitz, A. Grzesiak, M. Trappiel, C. Thone-Reineke, J. Baulmann, L. Paulis, K. Kappert, et al. 2013. Cannabinoid receptor 1 inhibition improves cardiac function and remodelling after myocardial infarction and in experimental metabolic syndrome. Journal of molecular medicine 91 (7): 811–823.
Lazzerini, P.E., M. Natale, E. Gianchecchi, P.L. Capecchi, C. Montilli, S. Zimbone, M. Castrichini, E. Balistreri, G. Ricci, E. Selvi, et al. 2012. Adenosine A2A receptor activation stimulates collagen production in sclerodermic dermal fibroblasts either directly and through a cross-talk with the cannabinoid system. Journal of molecular medicine 90 (3): 331–342.
Sipe, J.C., N. Arbour, A. Gerber, and E. Beutler. 2005. Reduced endocannabinoid immune modulation by a common cannabinoid 2 (CB2) receptor gene polymorphism: Possible risk for autoimmune disorders. Journal of leukocyte biology 78 (1): 231–238.
Castaneda, J.T., A. Harui, and M.D. Roth. 2017. Regulation of cell surface CB2 receptor during human B cell activation and differentiation. Journal of neuroimmune pharmacology : The official journal of the Society on NeuroImmune Pharmacology 12 (3): 544–554.
Aragão, L.G.H.S., J.T. Oliveira, J.R. Temerozo, M.A. Mendes, J.A. Salerno, C.S.G. Pedrosa, T. Puig-Pijuan, C.P. Veríssimo, I.M. Ornelas, T. Torquato, et al. 2021. WIN 55212–2 shows anti-inflammatory and survival properties in human iPSC-derived cardiomyocytes infected with SARS-CoV-2. PeerJ 9: e12262–e12262.
Devane, W.A., F.A. Dysarz 3rd., M.R. Johnson, L.S. Melvin, and A.C. Howlett. 1988. Determination and characterization of a cannabinoid receptor in rat brain. Molecular Pharmacology 34 (5): 605–613.
Munro, S., K.L. Thomas, and M. Abu-Shaar. 1993. Molecular characterization of a peripheral receptor for cannabinoids. Nature 365 (6441): 61–65.
Wang, B., A. Kovalchuk, D. Li, R. Rodriguez-Juarez, Y. Ilnytskyy, I. Kovalchuk, and O. Kovalchuk. 2020. In search of preventive strategies: Novel high-CBD Cannabis sativa extracts modulate ACE2 expression in COVID-19 gateway tissues. Aging (Albany NY) 12 (22): 22425–22444.
Tveden-Nyborg, P., T.K. Bergmann, and J. Lykkesfeldt. 2018. Basic & Clinical Pharmacology & Toxicology Policy for Experimental and Clinical studies. Basic & clinical pharmacology & toxicology 123 (3): 233–235.
Hellenbrand, D.J., K.A. Reichl, B.J. Travis, M.E. Filipp, A.S. Khalil, D.J. Pulito, A.V. Gavigan, E.R. Maginot, M.T. Arnold, A.G. Adler, et al. 2019. Sustained interleukin-10 delivery reduces inflammation and improves motor function after spinal cord injury. Journal of neuroinflammation 16 (1): 93.
Balasingam, V., and V.W. Yong. 1996. Attenuation of astroglial reactivity by interleukin-10. The Journal of neuroscience : The official journal of the Society for Neuroscience 16 (9): 2945–2955.
Bustos, M.L., L. Huleihel, E.M. Meyer, A.D. Donnenberg, V.S. Donnenberg, J.D. Sciurba, L. Mroz, B.J. McVerry, B.M. Ellis, N. Kaminski, et al. 2013. Activation of human mesenchymal stem cells impacts their therapeutic abilities in lung injury by increasing interleukin (IL)-10 and IL-1RN levels. Stem cells translational medicine 2 (11): 884–895.
Hoegl, S., K.A. Boost, H. Czerwonka, A. Dolfen, P. Scheiermann, H. Muhl, B. Zwissler, and C. Hofstetter. 2009. Inhaled IL-10 reduces biotrauma and mortality in a model of ventilator-induced lung injury. Respiratory medicine 103 (3): 463–470.
Xu, S., M. Xu, G.G. Li, C. Wang, H. Song, and J. Bai. 2016. Early recruitment of IL-10-producing B cells into alveoli improved the resolution of acute lung injury. Cellular physiology and biochemistry : International journal of experimental cellular physiology, biochemistry, and pharmacology 38 (5): 1752–1760.
Marquart, S., P. Zerr, A. Akhmetshina, K. Palumbo, N. Reich, M. Tomcik, A. Horn, C. Dees, M. Engel, J. Zwerina, et al. 2010. Inactivation of the cannabinoid receptor CB1 prevents leukocyte infiltration and experimental fibrosis. Arthritis and rheumatism 62 (11): 3467–3476.
Servettaz, A., N. Kavian, C. Nicco, V. Deveaux, C. Chereau, A. Wang, A. Zimmer, S. Lotersztajn, B. Weill, and F. Batteux. 2010. Targeting the cannabinoid pathway limits the development of fibrosis and autoimmunity in a mouse model of systemic sclerosis. The American journal of pathology 177 (1): 187–196.
Cinar, R., B.R. Gochuico, M.R. Iyer, T. Jourdan, T. Yokoyama, J.K. Park, N.J. Coffey, H. Pri-Chen, G. Szanda, Z. Liu, et al. 2017. Cannabinoid CB1 receptor overactivity contributes to the pathogenesis of idiopathic pulmonary fibrosis. JCI insight 2(8).
Bronova, I., B. Smith, B. Aydogan, R.R. Weichselbaum, K. Vemuri, K. Erdelyi, A. Makriyannis, P. Pacher, and E.V. Berdyshev. 2015. Protection from radiation-induced pulmonary fibrosis by peripheral targeting of cannabinoid receptor-1. American journal of respiratory cell and molecular biology 53 (4): 555–562.
Reczek, C.R., K. Birsoy, H. Kong, I. Martínez-Reyes, T. Wang, P. Gao, D.M. Sabatini, and N.S. Chandel. 2017. A CRISPR screen identifies a pathway required for paraquat-induced cell death. Nature Chemical Biology 13 (12): 1274–1279.
Zhang, Z.D., Y.J. Yang, X.W. Liu, Z. Qin, S.H. Li, and J.Y. Li. 2021. Aspirin eugenol ester ameliorates paraquat-induced oxidative damage through ROS/p38-MAPK-mediated mitochondrial apoptosis pathway. Toxicology 453: 152721.
Hu, X., H. Shen, Y. Wang, and M. Zhao. 2017. Liver X receptor agonist TO901317 attenuates paraquat-induced acute lung injury through inhibition of NF-κB and JNK/p38 MAPK signal pathways. BioMed Research International 2017: 4652695.
Pei, Y.H., X.M. Cai, J. Chen, B.D. Sun, Z.R. Sun, X. Wang, and X.M. Qian. 2014. The role of p38 MAPK in acute paraquat-induced lung injury in rats. Inhalation Toxicology 26 (14): 880–884.
Kim, S.H., S.J. Won, X.O. Mao, K. Jin, and D.A. Greenberg. 2005. Involvement of protein kinase A in cannabinoid receptor-mediated protection from oxidative neuronal injury. Journal of Pharmacology and Experimental Therapeutics 313 (1): 88–94.
Bátkai, S., D. Osei-Hyiaman, H. Pan, O. El-Assal, M. Rajesh, P. Mukhopadhyay, F. Hong, J. Harvey-White, A. Jafri, G. Haskó, et al. 2007. Cannabinoid-2 receptor mediates protection against hepatic ischemia/reperfusion injury. The FASEB Journal 21 (8): 1788–1800.
Di Filippo, C., F. Rossi, S. Rossi, and M. D’Amico. 2004. Cannabinoid CB2 receptor activation reduces mouse myocardial ischemia-reperfusion injury: Involvement of cytokine/chemokines and PMN. Journal of Leukocyte Biology 75 (3): 453–459.
Garberg, H.T., M.U. Huun, J. Escobar, J. Martinez-Orgado, E.M. Løberg, R. Solberg, and O. Didrik Saugstad. 2016. Short-term effects of cannabidiol after global hypoxia-ischemia in newborn piglets. Pediatric Research 80 (5): 710–718.
Alonso-Alconada, D., F.J. Álvarez, F. Goñi-de-Cerio, E. Hilario, and A. Álvarez. 2020. Cannabinoid-mediated modulation of oxidative stress and early inflammatory response after hypoxia-ischemia. International Journal of Molecular Sciences 21(4).
Huaux, F. 2021. Interpreting immunoregulation in lung fibrosis: A new branch of the immune model. Frontiers in Immunology 12: 690375.
Cheng, P., S. Li, and H. Chen. 2021. Macrophages in lung injury, repair, and fibrosis. Cells 10(2).
Schülke, S. 2018. Induction of interleukin-10 producing dendritic cells as a tool to suppress allergen-specific T helper 2 responses. Frontiers in Immunology 9: 455.
Lamkhioued, B., A.S. Gounni, D. Aldebert, E. Delaporte, L. Prin, A. Capron, and M. Capron. 1996. Synthesis of type 1 (IFN gamma) and type 2 (IL-4, IL-5, and IL-10) cytokines by human eosinophils. Annals of the New York Academy of Sciences 796: 203–208.
Li, L., H. Si, S.W. Wu, J.O. Mendez, D. Zarlenga, W. Tuo, and Z. Xiao. 2019. Characterization of IL-10-producing neutrophils in cattle infected with Ostertagia ostertagi. Science and Reports 9 (1): 20292.
Cerqueira, C., B. Manfroi, and S. Fillatreau. 2019. IL-10-producing regulatory B cells and plasmocytes: Molecular mechanisms and disease relevance. Seminars in Immunology 44: 101323.
Bedke, T., F. Muscate, S. Soukou, N. Gagliani, and S. Huber. 2019. Title: IL-10-producing T cells and their dual functions. Seminars in Immunology 44: 101335.
Angelina, A., M. Pérez-Diego, J. López-Abente, B. Rückert, I. Nombela, M. Akdis, M. Martín-Fontecha, C. Akdis, and O. Palomares. 2022. Cannabinoids induce functional Tregs by promoting tolerogenic DCs via autophagy and metabolic reprograming. Mucosal Immunology 15 (1): 96–108.
Funding
This work was supported by the Yunnan Financial Social Security Departments Emergency Fund (2017) (grant number: 116).
Author information
Authors and Affiliations
Contributions
Quan He guaranteed the integrity of the entire study, designed the study, and prepared the manuscript. Wen Zhang and Quan He performed the animal experiments and collected the materials for analyzing. Jinjuan Zhang performed the date analysis and interpreted the western blot and Q-PCR date. Yuanyou Deng interpreted the IHC and flow cytometry date. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
The experimental protocol was established, according to the ethical guidelines of the Helsinki Declaration and was approved by the Animal Experimentation Ethics Committee of the First People’s Hospital of Yunnan Province (No. YYLH106).
Consent for Publication
Not applicable.
Competing Interests
The author declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
He, Q., Zhang, W., Zhang, J. et al. Cannabinoid Analogue WIN 55212–2 Protects Paraquat-Induced Lung Injury and Enhances Macrophage M2 Polarization. Inflammation 45, 2256–2267 (2022). https://doi.org/10.1007/s10753-022-01688-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-022-01688-z