Abstract
The purpose of the present study was to evaluate the protective effects of astragaloside IV (AS IV) against paraquat (PQ)-induced pulmonary injury in vivo. Fifty BALB/C mice were randomized into five groups: (1) control, (2) PQ, (3) PQ + dexamethasone (Dex, 5 mg/kg), (4) PQ + AS IV (50 mg/kg), and (5) PQ + AS IV (100 mg/kg). A single dose of PQ (50 mg/kg, i.p.) was intraperitoneally given to induced acute lung injury. Then, mice were treated with AS IV (50 and 100 mg/kg/day, orally) for 5 days. At the end of the experiment, animals were euthanized; then, the bronchoalveolar lavage fluid (BALF) and lung tissues were collected for histological observation, biochemical assay, and Western blot analysis. Malondialdehyde (MDA), myeloperoxidase (MPO), catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GSH-Px) in lung tissues, and interleukin-6 (IL-6), IL-1β, tumor necrosis factor-α (TNF-α) levels in BALF were determined. Histological examination indicated that AS IV attenuated lung damage caused by PQ. Biochemical results showed that AS IV treatment significantly reduced the levels of MDA, MPO, and inflammatory cytokines while increased the levels of SOD, CAT, and GSH-Px compared with those in PQ group. Western blot results revealed that AS IV attenuated the Txnip/Trx expressions and inhibited Rho/ROCK/nuclear factor kappaB (NF-κB) signaling pathway in PQ-challenged mice. These findings suggested the protective effect of AS IV as a natural product on PQ-induced pulmonary injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
Paraquat (PQ, 1-10-dimethyl-40-bipyridylium dichloride), a quaternary nitrogen herbicide, is one of the most widely used herbicides and a highly toxic compound for both animals and humans [1]. PQ toxication contributes to aggressive tissue damage in the lung, kidney, and liver tissues. It is noteworthy that the major target organ is the lung in PQ poisoning. PQ-induced lung injury is characterized by edema, hemorrhage, inflammatory cell infiltration, and alveolar spaces [2]. It is acknowledged that oxidative stress plays a crucial role in the pathogenesis of PQ-stimulated pulmonary injury [3]. In addition, evidence emerged that inflammatory cascade was closely associated with the progression of lung disorders [4].
It has been widely acknowledged that reduction–oxidation (redox) responses at the intracellular and extracellular levels are the critical biological phenomena during the progression of various diseases. Thioredoxin (Trx) system, mainly located in the cytoplasm and mitochondria, is an essential antioxidant system for cellular survival and function through its disulfide reductase activity. As an important Trx-binding protein, thioredoxin-interacting protein (Txnip) has the reciprocal function with Trx. Substantial researches suggested that Trx/Txnip regulation could be an attractive target in the pathogenesis of a variety of diseases including cerebral ischemia injury [5], Alzheimer’s disease [6], and inflammation [7].
Rho and its downstream effector, Rho-associated coiled-coil forming protein kinase (ROCK), are implicated in the cytoskeletal contractile response through their influence on myosin ATPase activity. Notably, RhoA/ROCK pathway is highly related to the regulation of the inflammatory response [8]. Nuclear factor-kappaB (NF-κB), a major nuclear transcription factor, is a regulator which drives the generation of cytokines in inflammatory processes. Santos et al. have reported that NF-κB pathway plays an important role in the development of lung diseases [9].
To date, various nature products have been used for the intervention of disease [10, 11]. Astragaloside IV (AS IV) is a small molecular saponin found in Astragalus membranaceus (Fisch.) Bge which is a widely used herb in China. The herb exerted antioxidative activities by the elevation of antioxidants enzymes, inhibition of free radicals, and reduction of lipid peroxidation [12]. The published literatures showed the diverse pharmacological activities such as antiinflammation [13], antiinfarction [14], antihypertension [15], myocardial protection, and antiheart failure [16]. However, no available study has evaluated the effects of AS IV treatment on PQ-induced lung injury in a mouse model as yet. Herein, we sought to investigate whether AS IV could protect against pulmonary inflammation in mice. Our experimental results might provide a pharmacological basis on its folkloric use for the treatment of PQ-induced lung injury.
MATERIALS AND METHODS
Reagents
AS IV was purchased from the National Institutes for Food and Drug Control (Beijing, China). Dexamethasone (Dex) was purchased from Xiansheng Drug Store (Nanjing, China). PQ was provided by Sigma. tumor necrosis factor-α (TNF-α), interleukin-1 beta (IL-1β), and IL-6 enzyme-linked immunosorbent assay (ELISA) kits were supplied by BioLegend (San Diego, CA, USA). All antibodies were purchased from Cell Signaling Technology.
Animals
A total of 50 female BALB/c mice (18–22 g), acquired from Jiangning Qinglongshan Animal Cultivation Farm (Nanjing, China), were maintained in an animal facility under standard laboratory conditions for 5 days prior to experiments and provided with water and standard chow ad libitum. All experimental procedures were carried out in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Experimental Design
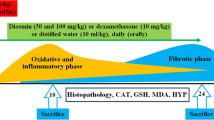
The animals were randomly divided into five groups with ten mice in each group as follows: (1) normal control group; (2) PQ group, mice received PQ (50 mg/kg, i.p.), (3) PQ + Dex group, mice received PQ (50 mg/kg, i.p.) and Dex (5 mg/kg/day, i.p.); (4) PQ + AS IV (50 mg/kg) group, mice received PQ (50 mg/kg, i.p.) and AS IV (50 mg/kg/day, i.p.); (5) PQ + AS IV (100 mg/kg) group, mice received PQ (50 mg/kg, i.p.) and AS IV (100 mg/kg/day, i.p.). PQ was dissolved in saline solution (NaCl 0.9 %) and intraperitoneally given to mice at a single toxic dose of 50 mg/kg. AS IV was also dissolved in saline (NaCl 0.9 %) and administered intragastrically. Two hours after PQ stimulation, AS IV was intragastrically treated at the dose of 50 and 100 mg/kg/day for 5 days. Dex was intragastrically treated as a positive control.
Bronchoalveolar Lavage
Two days after PQ challenge, the animals were sacrificed and bronchoalveolar lavage was collected three times through a tracheal cannula with 0.5 ml of autoclaved PBS to obtain the bronchoalveolar lavage fluid (BALF). The total leukocyte count was determined with a hemocytometer. BALF samples were centrifuged at 3000 rpm for 10 min at 4 °C; the cell-free supernatants were stored in −80 °C for the detection of cytokine concentrations, superoxide dismutase (SOD) activity. The pellets were resuspended in 100 μl of saline, centrifuged onto slides, and stained with Wright–Giemsa staining (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) for neutrophil counting.
Cytokine in BALF
The levels of TNF-α, IL-1β, and IL-6 in BALF were determined using ELISA kits according to the instructions recommended by the manufacturers. The optical density (OD) of each well was determined at 450 nm by a microplate spectrophotometer.
Lung Wet-to-Dry Weight Ratios
The right lungs were excised at the end of the experiment, and the wet weight was determined. Subsequently, the lungs were placed at 60 °C for 48 h to remove all moisture. Then, the dry lungs were weighted, and the lung wet-to-dry (W/D) ratio was calculated.
Pulmonary Histopathology
The lungs were removed at the end of the experiment. Afterward, the samples were fixed in 4 % neutral buffered formalin for 48 h, embedded in paraffin, and cut into 4-μm sections. Then, hematoxylin–eosin staining was performed according to the standard protocol. After that, pulmonary pathological changes were observed under a light microscope.
Western Blot Analysis
Lung tissues were harvested and froze in liquid nitrogen immediately until homogenization. Proteins were extracted with lysis buffer (RIPA with protease and phosphatase inhibitor) for 15 min on ice. The total protein concentrations were determined by BCA protein assay kit. Equal amounts of protein were mixed with five times loading dye (Laemmli buffer) and 2-mercaptoethanol followed by heating for 5 min at 95 °C. The samples were loaded per well on a 10 % sodium dodecyl sulfate polyacrylamide gel and transferred to PVDF membranes. The blots were blocked with 5 % bovine serum albumin (BSA) (5 g BSA was dissolved in 100 ml TBST) for 2 h at room temperature and incubated with primary antibody overnight at 4 °C. After washing, the bound antibodies were incubated with peroxidase-conjugated secondary anti-rabbit antibodies The proteins were visualized by a ECL Key-GEN system (KeyGEN Biotechnology, Nanjing, China) and scanned with a Clinx ChemiScope chemiluminescence imaging system (Gel Catcher 2850, China). GAPDH was detected as an internal control of protein loading.
Statistical Analysis
The results were expressed as mean value ± SD and analyzed with one-way analysis of variance (ANOVA) with Tukey multiple comparison test. A P value less than 0.05 was considered statistically significant.
RESULTS
Effects of AS IV on PQ-Induced Lung Wet-to-Dry Ratio
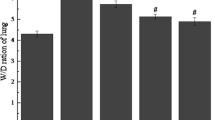
The index of lung edema was measured by calculating the W/D ratio of lung tissue. As revealed in Fig. 1, the lung W/D ratios in PQ-stimulated mice were evidently higher versus those in control mice. On the contrary, the W/D ratios in AS IV (50 and 100 mg/kg) groups and the Dex (5 mg/kg) group significantly decreased compared with those in PQ group. The data clearly indicated the obvious reduction of pulmonary edema content with the treatment of AS IV.
The effects of AS IV on PQ-induced acute lung injury: a lung W/D ratio, b lung MPO activity, c the number of total cells in BALF, d the number of neutrophils in BALF. Values are expressed as mean ± SD. # P < 0.05, ## P < 0.01, and ### P < 0.001 compared with control group. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with PQ group.
Effects of AS IV on Inflammatory Cells in BALF and MPO Activity in Lung Tissues
The myeloperoxidase (MPO) activity was measured to elucidate the neutrophil accumulation in pulmonary tissues. Meanwhile, the number of total cells and neutrophils were analyzed to the migration and infiltration of pulmonary cells. As illustrated in Fig. 1, the number of total cells, neutrophils, and MPO activity in PQ group significantly increased as compared with those in control group. Treatment with AS IV (50 and 100 mg/kg) and Dex (5 mg/kg) effectively decreased the number of total cells, neutrophils, and MPO level compared with those in PQ group. Our results suggested that AS IV exhibited an inhibitory effect on cell infiltration.
Effects of AS IV on Cytokines in BALF
Cytokines participate in the initiation and amplification of inflammatory cascade of acute lung injury (ALI). As shown in Fig. 2, it was proved that the levels of TNF-α, IL-1β, and IL-6 in BALF were significantly increased in PQ group compared with those in control group. AS IV (50 and 100 mg/kg) and Dex (5 mg/kg) treatment remarkably decreased the generations of TNF-α, IL-1β, and IL-6 in dose-dependent manners. Herein, AS IV reduced the synthesis and release of inflammatory cytokines in PQ-induced ALI.
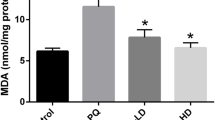
The effects of AS IV on the oxidative stress of PQ-induced mice. The levels of a MDA, b SOD, c GSH-Px, and d CAT in lung tissues. Values are expressed as mean ± SD. # P < 0.05, ## P < 0.01, and ### P < 0.001 compared with control group. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with PQ group.
Effects of AS IV on Oxidative Stress
Lipid peroxidation in lung tissues was determined by assaying the generations of malondialdehyde (MDA), SOD, glutathione peroxidase (GSH-Px), and catalase (CAT). As revealed in Fig. 3, PQ stimulation significantly declined the SOD, CAT activities, and GSH-Px content, while AS IV administration effectively restored the levels of GSH-Px, CAT, and SOD. Meanwhile, exposure to PQ displayed a strikingly high MDA level, whereas the treatment with AS IV remarkably ameliorated this condition. Our experimental data demonstrated that AS IV was capable of ameliorating oxidative stress in PQ-stimulated mice.
Effect of AS IV on PQ-Induced Pathological Changes of the Lung
Hematoxylin and eosin (H&E) staining was performed to evaluate the protective effects of AS IV physiological impairment. Scarce obvious histological alteration was observed in lung specimen. By contrast, in PQ group, histological evaluation of lungs by light microscopy showed alveolar wall hyperemia and excessive neutrophil infiltration around the pulmonary vessel after the simulation of PQ. AS IV treatment groups obviously attenuated the severity of lung injury. These findings suggested that AS IV significantly attenuated the histopathology conditions in PQ-induced ALI (Fig. 4).
The effects of AS IV on PQ-stimulated lung histopathologic changes in lung tissues. a The lung section from the control mice, b the lung section from PQ mice, c the lung section from the mice administered with PQ and dexamethasone (5 mg/kg), d the lung section from the mice administered with PQ and AS IV (5 0 mg/kg), and e the lung section from the mice administered with PQ and AS IV (100 mg/kg).
Effects of AS IV on PQ-Induced Txnip/Trx
It is assumed that protection from oxidative stress is mediated by Trx systems. Thus, we detected the expressions of Trx and Txnip by Western blot analysis. As shown in Fig 5, PQ challenge respectively induced an obvious increase in the expression of Txnip and a pronounced decrease in the expression of Trx. It was proved that the treatment with AS IV significantly activated Trx and restrained Txnip. These observations indicated that Trx system was involved in the inhibitory effect of AS IV on PQ-stimulated acute lung injury.
Effects of AS IV on PQ-Induced RhoA/Rho Kinase Pathway Activation
RhoA/Rho kinase pathway plays a key role in the regulation of inflammatory mediator production. We found that the expressions of RhoA, ROCK1, and ROCK2 were significantly upregulated in lung tissues after PQ stimulation. By contrast, treatment with AS IV (50 and 100 mg/kg) and Dex (5 mg/kg) obviously ameliorated these situations (Fig. 6).
Effects of AS IV on PQ-Induced NF-κB Pathway Activation
To identify the activation of the NF-κB pathway in lung after PQ stimulation, the phosphorylated and nonphosphorylated forms of NF-κBp65 and IкBα were measured by Western blot. Our results implied that the treatment with AS IV (50 and 100 mg/kg) and Dex (5 mg/kg) effectively blocked the phosphorylation of NF-кBp65 and IкBα in lung tissues of PQ-induced ALI. The analytical results suggested that the AS IV was involved in the degradation and the phosphosphorylation of IκBα, which contributed to the activation of NF-κBp65 (Fig. 7).
DISCUSSION
In this study, our results showed that the natural product AS IV could attenuate PQ-induced pulmonary and lung injury in mice. The ability of AS IV to reduce PQ-induced toxicity was due to its antioxidant and antiinflammatory effects in lung tissues.
Paraquat is the widely used herbicide around the world. Since its first application on agriculture in 1962, thousands of people died yearly from the intentional or accidental ingestion of PQ. The lung tissue is the major target organ as PQ is actively taken up by the alveolar epithelium [17]. Therefore, the injection of PQ is usually used as an inducer for acute lung injury in scientific studies.
ALI is a severe clinical syndrome characterized by noncardiogenic pulmonary edema, severe hypoxemia, accumulation of pulmonary cells, and overproduction of inflammatory cytokines [18]. Unfortunately, there has been few effective drugs to treat ALI as yet [19]. Thus, there is an urgent need to find safer medicine for the management of acute lung injury.
A. membranaceus is a longstanding Chinese herbal and traditionally used for multiple disease including inflammatory disorders [20]. As its critical component, astragaloside IV showed the potential effects on infarction, hypertension, heart failure, asthma, and inflammation [21]. However, the therapeutic efficacy as well as the mechanism of astragaloside IV on acute lung injury remains unknown.
Edema is the pivotal feature of systemic and partial inflammation. The W/D ratio was calculated to determine the water content of lung tissues [22]. As suggested by the evident reduction of W/D ratio, our data confirmed that AS IV was capable of attenuating the pulmonary edema. The migration and infiltration of neutrophils into the lung is the characteristic symptom of pulmonary disease. Moreover, the suppression of neutrophils participates in the mediation of oxygen species, cytokines, chemokines, and granular enzyme-mediated lung injury. MPO drives superabundant oxidative production which leads to the tissue damage under inflammatory conditions [23]. The experimental results indicated that AS IV effectively inhibited the MPO activity and the inflammatory cell infiltration. Furthermore, the histopathological observation also confirmed the protective effects of AS IV on PQ-challenged mice.
Inflammatory cytokines appear in the early stage of the inflammatory response and contribute to the severity of lung dysfunction [24]. IL-1β conduces to the alveolar epithelial repairment and induces the release of other cytokines [25]. IL-6 is highly related to the acute-phase response of inflammation and the initiation of inflammatory cascade [26]. TNF-α, an important molecule in the motivation of innate immune reaction, is responsible for the pathogenesis of inflammation [27]. As expected, AS IV was found to effectively downregulate the contents of IL-1β, IL-6, and TNF-α, which indicated that the protective effects of AS IV on PQ-induced acute lung injury were partly attributed to the suppression of inflammatory mediators.
Accumulating evidences proposed the common link between acute lung injury and oxidative stress [28]. The excessive oxygen-free radical which occurs during the pathogenesis of pulmonary lesion also contributes to biological membrane lipid peroxidation and severe cell damage [29]. Thus, the attenuation of oxidative stress might be beneficial to the treatment of pulmonary disease. MDA is the end product of polyunsaturated fatty acid and usually used as an indicator for the lipid peroxidation [30]. SOD exhibits various physiological activities including antiinflammatory and antioxidative effects [31]. GSH-Px is a critical nonprotein antioxidant and participates in scavenging the lipid peroxide radicals [32]. Previous reports demonstrated that CAT was implicated in the pulmonary lesion [33]. The analytical results suggested that AS IV evidently decreased the content of MDA and restored SOD, GSH-Px, and CAT activity. Our results suggested that the therapeutic effects of AS IV might result from the antioxidative activity.
Txnip, combined with thioredoxin in physiological condition, is considered as the negative regulator of Trx and redox. A recent report described Txnip as the sensitive target to oxidative stress [34]. Excessive ROS promoted the dissociation of Txnip from Trx [35]. Mounting evidence suggested the critical role of the oxidoreductase thioredoxin (Trx) in the mediation of NF-κB activity [36]. It is noteworthy that Txnip may be a therapeutic target for pulmonary diseases. In this study, the data demonstrated that AS IV participated in the mediation of Trx system, which governed the lipid peroxidation.
The Rho family of small GTPases is an important regulator which is involved in various intracellular signaling pathways; ROCKs are the downstream events of Rho guanosine triphosphatases [37]. ROCK is involved in various cellular processes, such as actomyosin contractility, cell adhesion, and inflammatory response [38]. There are two known ROCK isoforms: ROCK1 and ROCK2. As two isoforms of ROCK in mammalian cells, ROCK1 and ROCK2 share 65 % overall identity and 92 % identity in the kinase domain [39]. Former researches proposed the effects of ROCK1/ROCK2 in inflammatory pathogenesis [40]. NF-κB plays a crucial role in the regulation of immune and inflammatory progressions including acute lung injury [41]. PQ challenge brings about the activation of NF-κB through the phosphorylation and degradation of the IκBα. Evidence has emerged indicating that Rho/ROCK/NF-κB pathway was involved in the pulmonary disorder [42]. Substantial researches also elicited that NF-κB pathway was implicated in the lipid peroxidation and inflammatory process [43]. Our Western blotting data ascertained that AS IV significantly attenuated the expressions of Rho/ROCK/NF-κB cascade in response to PQ challenge. Simplified overview of the above signaling pathways was as illustrated in Fig. 8.
In conclusion, the results of the present study revealed that AS IV could effectively attenuate PQ-induced ALI in vivo. The potential mechanism of AS IV might be involved in the ameliorations of inflammatory cytokines and oxidative stress through the Trx/Txnip regulation and the blockade of Rho/ROCK/NF-κB signalings. However, there still remain several limitations in the current work. The future investigation might be focused on the critical role of Trx/Txnip and Rho/ROCK/NF-κB molecules with knockdown or overexpression methods in vitro. More studies are warranted to capitalize on the protective effects of AS IV in humans and to make it an effective functional medicine in the prevention of ALI caused by PQ.
References
Silva, R., H. Carmo, V. Vilas-Boas, D.J. Barbosa, M. Monteiro, P.G. de Pinho, et al. 2014. Several transport systems contribute to the intestinal uptake of Paraquat, modulating its cytotoxic effects. Toxicology Letters 232: 271–283.
Choi, J.S., S.S. Jou, M.H. Oh, Y.H. Kim, M.J. Park, H.W. Gil, et al. 2013. The dose of cyclophosphamide for treating paraquat-induced rat lung injury. The Korean Journal of Internal Medicine 28: 420–427.
Brennan, F.M., D. Chantry, A. Jackson, R. Maini, and M. Feldmann. 1989. Inhibitory effect of TNF alpha antibodies on synovial cell interleukin-1 production in rheumatoid arthritis. Lancet 2: 244–247.
Jing, W., M. Chunhua, and W. Shumin. 2015. Effects of acteoside on lipopolysaccharide-induced inflammation in acute lung injury via regulation of NF-kappaB pathway in vivo and in vitro. Toxicology and Applied Pharmacology 285: 128–135.
Hua, K., X. Sheng, T.T. Li, L.N. Wang, Y.H. Zhang, Z.J. Huang, et al. 2015. The edaravone and 3-n-butylphthalide ring-opening derivative 10b effectively attenuates cerebral ischemia injury in rats. Acta Pharmacologica Sinica 36: 917–927.
Gao, J., H. He, W. Jiang, X. Chang, L. Zhu, F. Luo, et al. 2015. Salidroside ameliorates cognitive impairment in a d-galactose-induced rat model of Alzheimer's disease. Behavioural Brain Research 293: 27–33.
Kim, S.J., and S.M. Lee. 2013. NLRP3 inflammasome activation in D-galactosamine and lipopolysaccharide-induced acute liver failure: role of heme oxygenase-1. Free Radical Biology & Medicine 65: 997–1004.
Segain, J.P., D. Raingeard de la Bletiere, V. Sauzeau, A. Bourreille, G. Hilaret, C. Cario-Toumaniantz, et al. 2003. Rho kinase blockade prevents inflammation via nuclear factor kappa B inhibition: evidence in Crohn's disease and experimental colitis. Gastroenterology 124: 1180–1187.
Santos, L.A., E.L. Ribeiro, K.P. Barbosa, I.T. Fragoso, F.O. Gomes, M.A. Donato, et al. 2014. Diethylcarbamazine inhibits NF-kappaB activation in acute lung injury induced by carrageenan in mice. International Immunopharmacology 23: 153–162.
Chen, T., J. Gao, P. Xiang, Y. Chen, J. Ji, P. Xie, et al. 2015. Protective effect of platycodin D on liver injury in alloxan-induced diabetic mice via regulation of Treg/Th17 balance. International Immunopharmacology 26: 338–348.
Chen, T., L. Xiao, L. Zhu, S. Ma, T. Yan, and H. Ji. 2015. Anti-asthmatic effects of ginsenoside rb1 in a mouse model of allergic asthma through relegating Th1/Th2. Inflammation 38(5): 1814–1822.
Ko, J.K., F.Y. Lam, and A.P. Cheung. 2005. Amelioration of experimental colitis by Astragalus membranaceus through anti-oxidation and inhibition of adhesion molecule synthesis. World Journal of Gastroenterology 11: 5787–5794.
Zhang, W.J., P. Hufnagl, B.R. Binder, and J. Wojta. 2003. Antiinflammatory activity of astragaloside IV is mediated by inhibition of NF-kappaB activation and adhesion molecule expression. Thrombosis and Haemostasis 90: 904–914.
Luo, Y., Z. Qin, Z. Hong, X. Zhang, D. Ding, J.H. Fu, et al. 2004. Astragaloside IV protects against ischemic brain injury in a murine model of transient focal ischemia. Neuroscience Letters 363: 218–223.
Zhang, W.D., C. Zhang, X.H. Wang, P.J. Gao, D.L. Zhu, H. Chen, et al. 2006. Astragaloside IV dilates aortic vessels from normal and spontaneously hypertensive rats through endothelium-dependent and endothelium-independent ways. Planta Medica 72: 621–626.
Li, Z.P., and Q. Cao. 2002. Effects of astragaloside IV on myocardial calcium transport and cardiac function in ischemic rats. Acta Pharmacologica Sinica 23: 898–904.
Nguyen, V., D.S. Malik, and M.A. Howland. 2014. Methylene blue protects against paraquat-induced acute lung injury in rats. International Immunopharmacology 20: 358.
Wang, J., Y.T. Liu, L. Xiao, L. Zhu, Q. Wang, and T. Yan. 2014. Anti-inflammatory effects of apigenin in lipopolysaccharide-induced inflammatory in acute lung injury by suppressing COX-2 and NF-kB pathway. Inflammation 37: 2085–2090.
Chen, T., Y. Mou, J. Tan, L. Wei, Y. Qiao, T. Wei, et al. 2015. The protective effect of CDDO-Me on lipopolysaccharide-induced acute lung injury in mice. International Immunopharmacology 25: 55–64.
Li, W., Y.N. Sun, X.T. Yan, S.Y. Yang, S.B. Song, Y.M. Lee, et al. 2013. NF-kappaB inhibitory activity of sucrose fatty acid esters and related constituents from Astragalus membranaceus. Journal of Agricultural and Food Chemistry 61: 7081–7088.
Jin, H., Q. Luo, Y. Zheng, M. Nurahmat, J. Wu, B. Li, et al. 2013. CD4 + CD25 + Foxp3+ T cells contribute to the antiasthmatic effects of Astragalus membranaceus extract in a rat model of asthma. International Immunopharmacology 15: 42–49.
Tao, W., Q. Su, H. Wang, S. Guo, Y. Chen, J. Duan, et al. 2015. Platycodin D attenuates acute lung injury by suppressing apoptosis and inflammation in vivo and in vitro. International Immunopharmacology 27: 138–147.
Lee, C.Y., J.J. Yang, S.S. Lee, C.J. Chen, Y.C. Huang, K.H. Huang, et al. 2014. Protective effect of Ginkgo biloba leaves extract, EGb761, on endotoxin-induced acute lung injury via a JNK- and Akt-dependent NFkappaB pathway. Journal of Agricultural and Food Chemistry 62: 6337–6344.
Qian, J., Y. Ye, L. Lv, C. Zhu, and S. Ye. 2014. FTY720 attenuates paraquat-induced lung injury in mice. International Immunopharmacology 21: 426–431.
Huang, G.J., J.S. Deng, C.C. Chen, C.J. Huang, P.J. Sung, S.S. Huang, et al. 2014. Methanol extract of Antrodia camphorata protects against lipopolysaccharide-induced acute lung injury by suppressing NF-kappaB and MAPK pathways in mice. Journal of Agricultural and Food Chemistry 62: 5321–5329.
Lou, T., W. Jiang, D. Xu, T. Chen, and Y. Fu. 2015. Inhibitory effects of polydatin on lipopolysaccharide-stimulated RAW 264.7 cells. Inflammation 38: 1213–1220.
Tianzhu, Z., Y. Shihai, and D. Juan. 2014. The effects of morin on lipopolysaccharide-induced acute lung injury by suppressing the lung NLRP3 inflammasome. Inflammation 37: 1976–1983.
Su, Z.Q., Z.Z. Mo, J.B. Liao, X.X. Feng, Y.Z. Liang, X. Zhang, et al. 2014. Usnic acid protects LPS-induced acute lung injury in mice through attenuating inflammatory responses and oxidative stress. International Immunopharmacology 22: 371–378.
Chen, L., L. Zhao, C. Zhang, and Z. Lan. 2014. Protective effect of p-cymene on lipopolysaccharide-induced acute lung injury in mice. Inflammation 37: 358–364.
Wang, Y.Z., Y.C. Zhang, J.S. Cheng, Q. Ni, P.W. Li, W. Han, et al. 2014. Protective effects of BML-111 on cerulein-induced acute pancreatitis-associated lung injury via activation of Nrf2/ARE signaling pathway. Inflammation 37: 1120–1133.
Xu, L., Y. Li, S. Wan, Y. Wang, and P. Yu. 2014. Protective effects of apocynin nitrone on acute lung injury induced by lipopolysaccharide in rats. International Immunopharmacology 20: 377–382.
Chen, Y., Y.C. Nie, Y.L. Luo, F. Lin, Y.F. Zheng, G.H. Cheng, et al. 2013. Protective effects of naringin against paraquat-induced acute lung injury and pulmonary fibrosis in mice. Food and Chemical Toxicology 58: 133–140.
Huang, X., Y. Liu, Y. Lu, and C. Ma. 2015. Anti-inflammatory effects of eugenol on lipopolysaccharide-induced inflammatory reaction in acute lung injury via regulating inflammation and redox status. International Immunopharmacology 26: 265–271.
Li, Y., J. Li, S. Li, Y. Li, X. Wang, B. Liu, et al. 2015. Curcumin attenuates glutamate neurotoxicity in the hippocampus by suppression of ER stress-associated TXNIP/NLRP3 inflammasome activation in a manner dependent on AMPK. Toxicology and Applied Pharmacology 286: 53–63.
Spindel, O.N., C. World, and B.C. Berk. 2012. Thioredoxin interacting protein: redox dependent and independent regulatory mechanisms. Antioxidants & Redox Signaling 16: 587–596.
Kelleher, Z.T., Y. Sha, M.W. Foster, W.M. Foster, M.T. Forrester, and H.E. Marshall. 2014. Thioredoxin-mediated denitrosylation regulates cytokine-induced nuclear factor kappaB (NF-kappaB) activation. The Journal of Biological Chemistry 289: 3066–3072.
Wong, C.M., L. Wei, S.L. Au, D.N. Fan, Y. Zhou, F.H. Tsang, et al. 2015. MiR-200b/200c/429 subfamily negatively regulates Rho/ROCK signaling pathway to suppress hepatocellular carcinoma metastasis. Oncotarget 6(15): 13658–13670.
Perey, A.C., I.M. Weishaar, and D.W. McGee. 2015. The effect of ROCK on TNF-alpha-induced CXCL8 secretion by intestinal epithelial cell lines is mediated through MKK4 and JNK signaling. Cellular Immunology 293: 80–86.
Shi, J., X. Wu, M. Surma, S. Vemula, L. Zhang, Y. Yang, et al. 2013. Distinct roles for ROCK1 and ROCK2 in the regulation of cell detachment. Cell death & Disease 4: e483.
Kanno, S., S. Hirano, S. Chiba, H. Takeshita, T. Nagai, M. Takada, et al. 2015. The role of Rho-kinases in IL-1beta release through phagocytosis of fibrous particles in human monocytes. Archives of Toxicology 89: 73–85.
Yu, W.W., Z. Lu, H. Zhang, Y.H. Kang, Y. Mao, H.H. Wang, et al. 2014. Anti-inflammatory and protective properties of daphnetin in endotoxin-induced lung injury. Journal of Agricultural and Food Chemistry 62: 12315–12325.
Chen T, Guo Q, Wang H, Zhang H, Wang C, Zhang P, et al. 2015 Effects of esculetin on lipopolysaccharide(LPS)-induced acute lung injury via regulation of RhoA/Rho Kinase/NF-кB pathways in vivo and in vitro. Free Radical Research:1-21.
Yeh, Y.H., Y.L. Hsieh, and Y.T. Lee. 2013. Effects of yam peel extract against carbon tetrachloride-induced hepatotoxicity in rats. Journal of Agricultural and Food Chemistry 61: 7387–7396.
Acknowledgments
This study was supported by grants of the Natural Science Foundation of Jiangsu Province of China (BK20150707) and the Fundamental Research Funds for the Central Universities JKZD2013009 and ZJ15030). This Project also funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), Research Innovation Program Project for Graduate Students in Jiangsu Province (CXZZ13_03), National Undergraduate Training Programs for Innovation and Entrepreneurship (G13034) and the Representational Achievement Cultivating Project of State Key Laboratory of Natural Medicines (SKLNMBZ201402).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
There is no conflict of interest among authors.
Rights and permissions
About this article
Cite this article
Chen, T., Wang, R., Jiang, W. et al. Protective Effect of Astragaloside IV Against Paraquat-Induced Lung Injury in Mice by Suppressing Rho Signaling. Inflammation 39, 483–492 (2016). https://doi.org/10.1007/s10753-015-0272-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-015-0272-4