Abstract
The Matanza Riachuelo Basin is one of the most polluted watersheds in the world. We assessed the relationships between land use, stream-water quality, and the structure of the fish assemblages on a subbasin scale from data obtained in 44 sampling sites. A gradient of change from the rural upper sector to the urbanized and industrialized lower basin in conjunction with water-quality impoverishment was recorded. Redundancy analysis revealed that dissolved oxygen concentration, heavy metals, detergents, and Escherichia coli were the most explanatory variables of that gradient. Fish assemblages manifested the highest richness, abundance, and diversity in the rural areas; in the urbanized and industrialized sectors, the fish community became severely modified, represented only by tolerant species. Multivariate analyses indicated that the fish assemblage structure was highly impacted by water-quality deterioration. The combined study of the impacts of land use on water quality and fish assemblages enabled a detailed diagnosis of the consequent effects on a subbasin scale, thus substantiating the usefulness of fish assemblages as a model for that purpose. Field studies over large-scale gradients of environmental impact are essential for understanding the mechanisms of ecological deterioration and biotic responses as a first step in developing remediation policies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The biodiversity of rivers and streams—the most threatened ecosystems on Earth—is declining at a much faster rate than any other ecosystem (Dudgeon et al., 2006; Strayer & Dudgeon, 2010; McGarrigle, 2014; Albert et al., 2020). Land use has strong effects on the ecological integrity of river ecosystems therein (Roth et al., 1996; Allan, 2004). In urban areas, the natural land cover is replaced by impervious surfaces and pluvial-drainage networks that discharge vast quantities of untreated water into streams (Groffman et al., 2003; Allan, 2004), while the channelization modifies the hydrology of the system, disrupts connectivity with groundwater and leads to a reduction in the complexity of the watercourses (Sliva & Williams, 2001; Allan, 2004; Walsh et al., 2005; Jacobson, 2011). In rural areas, inadequate agricultural practices cause a diffuse entry of sediments, nutrients, herbicides, or pesticides (Cooper, 1993; Allan, 2004; Nessimian et al., 2008) thus affecting stream morphology while cattle raising increases the suspended matter, temperature, and bacterial populations (Kauffman & Krueger, 1984; McKergow et al., 2003).
Fish assemblages in streams draining urbanized basins often exhibit low richness and diversity (Steedman, 1988; Gafny et al., 2000; Helms et al., 2005), low biomass, and the absence of sensitive species (Lenat & Crawford, 1994; Scott & Helfman, 2001), leading to a low biotic integrity (Karr, 1981; Wang et al., 2000). Agricultural land use has been related to the decrease in these biotic attributes in fish assemblages (Roth et al., 1996; Casatti et al., 2009) resulting from habitat loss associated with substrate modification (Burcher & Benfield, 2006; Blann et al., 2009), increases in water turbidity, and reductions in macrophyte coverage (Jeppesen et al., 2000; Giorgi et al., 2015). Indeed, fish assemblages are sensitive indicators of land use effects on the basin scale (Fitzpatrick et al., 2001).
The Matanza Riachuelo Basin (MRB), located in the pampean region of Argentina is considered one of the most polluted areas in Latin America and, in fact, in the world (Olson et al., 1998; SAIJ, 2008; Kramarz, 2018). The land use in this basin ranges from rural to urbanized and highly industrialized. Environmental studies in the MRB revealed a gradient of serious deterioration in water quality and bottom sediments towards the lower sector (Magdaleno et al., 2001; Rendina & Fabrizio de Iorio, 2012), where the watercourses receive runoff and discharges from the most urban and industrial conglomerate of the country, around the Buenos Aires city (Fernández et al., 2009; Matteucci & Morello, 2009). Biological assessments by means of diatoms, macroinvertebrates, and riparian-vegetation assemblages reported a degradation pattern that followed a rural-to-urban gradient (Gómez, 1998, 1999; Rodrigues Capítulo et al., 2004; Guida-Johnson & Zuleta, 2019). The status of the ichthyofauna of the MRB, however, remains poorly understood, with only a few technical reports devoted to that question.
Since the studies on the fish assemblages of the MRB could constitute a valuable tool for evaluating the environmental status of the basin in long-term monitoring, we undertook the investigation reported here to assess how land use gradient from ruralization to urbanization and industry affects the water quality and fish assemblages in the hydrologic subbasins of the MRB.
Materials and methods
Study area
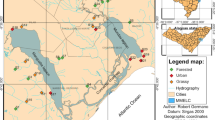
The MRB is located in the pampean region in the eastern-central sector of Argentina, covering an area of 2,240 km2 (Fig. 1). The watercourses from this region are naturally characterized by the lack of a forested riparian zone and run through low-sloping grassy meadows. The bottom of these water bodies is thus composed of fine sediments (Gómez et al., 2003; Feijoó & Lombardo, 2007; Rodrigues Capítulo et al., 2010). These features were once consistent throughout the region, but the natural land cover of the grassland is being progressively replaced by crops and urbanizations or areas modified by livestock (Soriano et al., 1991). The MRB crosses 14 municipalities and the Buenos Aires city, before flowing into the Río de la Plata estuary. Buenos Aires is considered one of the megacities of the world, inhabited by more than 8 million people, of which more than a half are settled in the MRB watershed (INDEC, 2010). Approximately 7,200 industries are located throughout the basin, of which 220 were classified by the government entity Matanza Riachuelo Basin Authority (Autoridad de Cuenca Matanza Riachuelo, ACUMAR) as critical industries (CI), owing to their harmful levels of environmental pollution. This classification was determined on the basis of the mass load of pollutants in each individual industrial discharge; according to the flow volume and pollutant concentration (ACUMAR, 2018). The MRB can be divided into 14 subbasins (Fig. 1) from the upper basin towards the mouth: Cañuelas (CANU), Rodríguez (RODZ), Cebey (CBEY), Morales (MORA), Chacón (CHCN), De La Cañada Pantanosa (CPAN), Aguirre (AGUI), Ortega (ORTG), Barreiro (BARR), Río Matanza (RMAT), Don Mario (DMAR), Del Rey (DREY), Santa Catalina (SCAT), and Riachuelo (RICH). The subbasins Del Rey and Santa Catalina were not considered in this study because they are located within the boundaries of social conflict areas and did not guarantee the necessary levels of security for the field team.
Geographic location of the Matanza Riachuelo Basin, Buenos Aires, Argentina, subbasins, and sampling sites. The abbreviations for the 14 subbasins are: AGUI: Aguirre, BARR: Barreiro, CANU: Cañuelas, CBEY: Cebey, CHCN: Chacón, CPAN: De La Cañada Pantanosa, DMAR: Don Mario, MORA: Morales, ORTG: Ortega, RMAT: Río Matanza, RODZ: Rodríguez, DREY: Del Rey, SCAT: Santa Catalina, and RICH: Riachuelo
Data collection
Land use categories and the corresponding coverage values (km2) were obtained by the geoprocessing of three vectorial shapes by means of the software Quantum-GIS v2.18.22. Files were collected from two governmental databases (ACUMAR, 2018; IGN, 2018). The watershed land uses were divided into four categories and their percent coverage calculated for each subbasin as follows: rural areas covered by crop cultivation and livestock (%RUR), artificial forestation (%FOR), urbanized areas (%URB), and other land uses with scarce representation in the watershed—e.g., wetlands, stream floodplains, dunes—(%Others). The influence of the industries was quantified by dividing the total number of CI recorded in each subbasin by the area of each one (nCI/km2).
A total of 44 river and streams stretches were selected as sampling sites, according to a water-quality and biotic-monitoring program of ACUMAR (Online Resource 1). The physical and chemical values of the sampling sites were extracted from public technical reports of bimonthly water-quality assessment carried out by ACUMAR (2018). Sampling sites data from 2012 to 2014 period (18 measurements per variable) were grouped by subbasin and averaged to obtain a physical and chemical characterization. The following environmental variables were considered in the statistical analysis: dissolved oxygen concentration (DO), pH, temperature, conductivity, turbidity, biochemical and chemical oxygen demand (BOD and COD, respectively), nitrates (NO3–), nitrites (NO2–), ammonium (NH4+), total N; total P; total organic carbon (TOC), total suspended solids (TSS), detergents, Escherichia coli, fat and oils, hydrocarbons, phenols, sulfides (S–), total chromium (Cr), total nickel (Ni), total copper (Cu), total cadmium (Cd), lead (Pb), and cyanide (CN−).
The fish were collected in the autumn and spring of 2015. Three fishing gears were used along with the same sampling effort in all the surveys: (a) a seine net with a bag (length, 15 m; height, 1.1 m 10 mm; bag width, 2 m; and mesh size, 5 mm between opposite knots); (b) a D-shaped hand net (width, 0.36 m; height, 0.46 m; mesh size, 1 × 1 mm; handle extension, 2 m); and c) a square frame drop net (frame width 0.48 m and height, 0.48 m; mesh size, 1 × 1 mm; rope, 5 m). For seine net operation, at each sampling site, the stream was closed with a block net with a cod end (mesh size, 10 × 5 mm) and two people hauled the seine net to traverse 20 m upstream toward the block net, where both nets were recovered together. D-shaped hand net and square frame drop net were used in very shallow (< 0.3 m) or highly vegetated areas where the seine net could not be used, an effort of five fishing attempts at each site was made with each type of net. The fish species were identified, counted, weighed, and released. The nonidentifiable species in the field were euthanized with an overdose of anesthesia (benzocaine in excess), preserved with a solution of 10% (v/v) aqueous formaldehyde that was later replaced with 70% (v/v) aqueous ethanol for routine laboratory determination.
The fish were identified following Ringuelet et al. (1967), Azpelicueta & Braga (1991), López & Miquelarena (1991), Braga (1993, 1994), Aquino (1997), Reis & Pereira (2000), Casciotta et al. (2005), Miquelarena & Menni (2005), Říčan & Kullander (2008), and Almirón et al. (2015). Updates in the taxonomy were revised according to Mirande & Koerber (2020), Rosso et al. (2018), and Terán et al. (2020). Voucher specimens were stored at the ichthyological collection of the Fundación de Historia Natural Félix de Azara (CFA-IC), Buenos Aires, Argentina. For each sampling site, the number of specimens collected and the total weight per species were recorded by each type of fishing gear.
Data analysis
The captures of the fishing gears were standardized by estimation of the abundance and biomass per m2 (N/m2 and W/m2, respectively) and then averaged to give a single value for each sampling site by season. This procedure was carried out on the basis of the area covered efficiently by each type of fishing gear as the main criterion. Since the D-shaped hand net and the square frame drop net were employed to study exclusively vegetated and shallow areas, their N/m2 and W/m2 were corrected according to the percentage of these areas in each stream stretch analyzed. The species richness (S) and Shannon–Wiener diversity index (H′; Shannon & Weaver, 1949) were calculated for each sampling site. The values of N/m2, W/m2, S, and H’ were averaged for each subbasin (hereafter referred to as the biotic attributes).
A CLUSTER analysis was performed to explore the relationships among subbasins with respect to the land use categories and nCI/km2. The Euclidean-distance method was used to build the resemblance matrix. The presence of statistically different groups was tested by a similarity-profile analysis (SIMPROF test; P < 0.05), used to determine the presence of groups in a priori unstructured dendrograms from hierarchical CLUSTER analyses (Clarke et al., 2008). The subbasin groupings obtained in this analysis were used as a basis for the next analyses.
The physical and chemical variables and univariate indices obtained for each group in the CLUSTER land use analysis were tested by the Kruskal–Wallis nonparametric analysis of variance. Pairwise multiple comparison procedures between groups were carried out according to the Dunn method (P < 0.05). In addition, the CI were divided into four categories according to their activity: food industry, chemical industry, mixed industry, and others; and the percentage of each type of industry was calculated for the groups obtained in the CLUSTER land use analysis.
A Redundancy Analysis (RDA) was carried out through the use of the average values of biotic attributes as response variables and the physical and chemical parameters of the subbasins as explanatory variables. Beforehand, a Detrended Correspondence Analysis was performed to assess the gradient length for the first axis and thereafter select a linear- or unimodal- response model. Since the gradient was 1.1 standard deviation units, a linear response model (RDA) was used (Ter Braak & Smilauer, 1998; Lepš & Šmilauer, 2003). The biotic attributes of N/m2, W/m2, and S were ln(x + 1) transformed. The environmental variables were selected by means of the forward selection method and the Variance Inflation Factor values (VIF < 20) to avoid biases due to multicollinearity (Ter Braak & Smilauer, 1998).
A classification of the subbasins according to the structure of the fish assemblages was performed by CLUSTER analyses based on presence-absence and abundance data (determined from Jaccard and Bray–Curtis similarity indices, respectively). The abundance data were previously log10(x + 1) transformed. A one-way analysis of similarities (ANOSIM) was carried out to test the significance of the grouping (P < 0.05). An analysis of the percent similarity (SIMPER) was performed to identify the qualitative and quantitative contribution of species to the similarity of each group of fish assemblages.
The Spearman rank-correlation method was performed to evaluate the similarity between the subbasin groups obtained in the three CLUSTER analyses by comparing the distance matrix obtained from the land use with the similarity matrices calculated from the presence-absence and abundance data of the fish assemblages.
The analyses of CLUSTER, SIMPROF, SIMPER, ANOSIM, and the Spearman Rank Correlation were performed with the software Primer-E v6.0 (Clarke & Warwick, 2001) and the Kruskall-Wallis analysis and pairwise multiple comparison with the software Statistica 7.0 (Stat-Soft, 2009).
Results
Land use
Distribution of each land use category throughout the MRB is shown in Fig. 2a. The CLUSTER analysis based on land use categories and nCI/km2 revealed an arrangement of subbasins in three statistically different groups (Fig. 2b). Group 1 (G1) consisted of subbasins with a %RUR > 70 with small, scattered urbanization patches (%URB < 10) and 0.01 nCI/km2 (Fig. 2a, b). In Group 2 (G2) the subbasins also involved mainly rural land use (%RUR < 70), with a %URB > 10 and a nCI/km2 of 0.06 (Fig. 2a, b). Group 3 (G3) evidenced the highest %URB (> 80%), the lowest values of %RUR (< 10%), and a nCI/km2 of 0.44 (Fig. 2a, b).
Land use analysis in the Matanza Riachuelo Basin, Buenos Aires, Argentina (a). CLUSTER analysis of the subbasins according to the land use variables (b). The groupings G1 (white) rural subbasins, G2 (gray) rural and/or urbanized and industrialized subbasins, and G3 (black) urbanized and industrialized subbasins are demarcated above. Key to the abbreviations: AGUI: Aguirre, BARR: Barreiro, CANU: Cañuelas, CBEY: Cebey, CHCN: Chacón, CPAN: De La Cañada Pantanosa, DMAR: Don Mario, MORA: Morales, ORTG: Ortega, RMAT: Río Matanza, RODZ: Rodríguez, RICH: Riachuelo
An analysis of the percentages of each category of industrial activity (Fig. 3) indicated a predominance of food industries in G1 and the chemical industry in G3. The G2 group contained similar percentage values for both categories of industrial activity, thus representing an intermediate situation between G1 and G3.
Values of percentages of each category of critical industry (food industry, chemical industry, mixed industry, and others) for the rural subbasins (G1), the rural and/or urbanized and industrialized subbasins (G2), and the urbanized and industrialized subbasins (G3) in the Matanza Riachuelo Basin, Buenos Aires, Argentina
Physical and chemical variables
The subbasins belonging to G1 evidenced the highest values of DO, BOD, COD, TSS, and turbidity, whereas heavy metals, detergents, E. coli, and nitrites manifested significantly higher values in G3. In G2, the lowest values of nitrites and highest values of total P and N were registered, but G2 was similar to both G1 and G3 with respect to the rest of the variables, constituting an intermediate situation between those groups. Table 1 summarizes the parameters that varied significantly between the groups, detailing the average values and results of pairwise analysis.
Fish assemblages
A total of 19,516 fish specimens were collected, representing 22 species, 12 families, and 5 orders (Table 2). Characiformes and Siluriformes contained the highest richness with 9 species each (37.5%), followed by Cyprinodontiformes with 3 (12.5%), Cichliformes with 2 (8.3%), and Synbranchiformes with 1 (4.2%). The order Cyprinodontiformes contributed the highest proportion of abundance (73.3%), followed by Siluriformes (15.4%) and Characiformes (10.5%). Siluriformes contributed the highest proportion of biomass (51.4%), followed by Characiformes (33.3%) and Cyprinodontiformes (11.6%).
Cnesterodon decemmaculatus (Jenyns 1842) was the most frequently recorded species in the watershed (11 subbasins), followed by Cheirodon interruptus (Jenyns 1842), Corydoras paleatus (Jenyns 1842), and Jenynsia lineata (Jenyns 1842) at 7 subbasins each. Three species—Hyphessobrycon meridionalis Ringuelet, Miquelarena & Menni 1978, Callichthys callichthys (Linnaeus 1758), and Loricariichthys anus (Valenciennes 1836)—were recorded in only one subbasin.
According to Kruskal–Wallis test, the biotic attributes were significantly higher (P < 0.01) in G1 than in G3, while G2 exhibited intermediate values (Fig. 4). Note that in G3 only the species C. decemmaculatus was recorded (Table 2).
Average values and standard deviations of fish assemblage attributes for the rural subbasins (G1), the rural and/or urbanized and industrialized subbasins (G2), and the urbanized and industrialized subbasins (G3) in the Matanza Riachuelo Basin, Buenos Aires, Argentina. The differences between the groups (Kruskal–Wallis) are expressed by the letters a, b, and c (P < 0.05). Key to the abbreviations: AGUI: Aguirre, BARR: Barreiro, CANU: Cañuelas, CBEY: Cebey, CHCN: Chacón, CPAN: De La Cañada Pantanosa, DMAR: Don Mario, MORA: Morales, ORTG: Ortega, RMAT: Río Matanza, RODZ: Rodríguez, RICH: Riachuelo
Multivariate analysis
The RDA analysis (Fig. 5) revealed a significant relationship between the environmental variables and the attributes of the fish assemblages according to the significance obtained for the first canonical axis (P = 0.013) and all the canonical axes (P = 0.022). The two first axes of the RDA explained 75.8% of the relationship between biotic attributes and environmental variables. The first axis was the more explanatory, accumulating 70.4% of the explained variance. The variables E. coli (0.94), detergents (0.56), Cr (0.57), and NH4+ (0.24) were positively related to that first axis; while DO (− 0.78) was negatively related. For the second axis, DO (0.20) was positively related, while detergents (− 0.68) and Cr (− 0.48) were negatively related. The variables E. coli (0.02) and NH4+ (0.06) evidenced a weak relation to the second axis (Fig. 5). The subbasin ordination was consistent with the CLUSTER analysis of the land use grouping. The rural subbasins (G1) were positively correlated with high DO and biotic attributes values, whereas the urbanized and industrialized subbasins (G3) were positively correlated with high values of E. coli, detergents, Cr, and NH4+, but negatively with biotic attributes. The subbasins from G2 were located in an intermediate position on the gradient (Fig. 5).
Redundancy analysis. Triplot of biotic attributes, environmental variables, and subbasins in the Matanza Riachuelo Basin, Buenos Aires, Argentina. G1 (in white) rural subbasins, G2 (in gray) rural and/or urbanized and industrialized subbasins, and G3 (in black) urbanized and industrialized subbasins. The numerical code indicates the following MRB subbasins: 1: Aguirre, 2: Barreiro, 3: Cañuelas, 4: Cebey, 5: Chacón, 6: De La Cañada Pantanosa, 7: Don Mario, 8: Morales, 9: Ortega, 10: Río Matanza, 11: Rodríguez, 12: Riachuelo. Abbreviations: S: specific richness, W: biomass, N: abundance, H’: Shannon–Wiener diversity index, NH4+: ammonium, Cr: chromium, DO: dissolved oxygen concentration
A CLUSTER analysis with presence-absence and abundance data sorted the subbasins into three different groups (FG1, FG2, and FG3): ANOSIM (P = 0.001) global R = 0.62 and R = 0.69, respectively (Fig. 6). The Spearman rank correlation between the groups obtained from the land use and fish assemblage presence-absence data evidenced a high correlation (rho = 0.84; P = 0.001). Similarly, the comparison of land use and the fish assemblage–abundance grouping exhibited a rho = 0.77 (P < 0.001), thus revealing a clear agreement between the subbasin groupings obtained from the land use and fish data.
Results of CLUSTER, SIMPER, and ANOSIM analyses from presence-absence (a) and abundance (b) data of the fish assemblages in Matanza Riachuelo Basin, Buenos Aires, Argentina. Key to the abbreviations: Bry ihe: Bryconamericus iheringii, Che int: Cheirodon interruptus, Cne dec: Cnesterodon decemmaculatus, Cor pal: Corydoras paleatus, Cyp voga: Cyphocharax voga, Hop arg: Hoplias argentinensis, Hyp com: Hypostomus commersoni, Jen lin: Jenynsia lineata, Pim lat: Pimelodella laticeps, Psa eig: Psalidodon eigenmanniorum. AGUI: Aguirre, BARR: Barreiro, CANU: Cañuelas, CBEY: Cebey, CHCN: Chacón, CPAN: De La Cañada Pantanosa, DMAR: Don Mario, MORA: Morales, ORTG: Ortega, RMAT: Río Matanza, RODZ: Rodríguez, RICH: Riachuelo
SIMPER analysis revealed a downstream decrease in the contribution of species in the subbasin groups. From the presence-absence data, FG1 registered the highest number of species (Fig. 6). Nine species contributed to 93% of the similarity: C. paleatus, Psalidodon eigenmanniorum (Cope 1894), C. decemmaculatus, and C. interruptus accumulated 65%; while Cyphocharax voga (Hensel 1870), Hoplias argentinensis Rosso, González-Castro, Bogan, Cardoso, Mabragaña, Delpiani & Díaz de Astarloa 2018, Bryconamericus iheringii (Evermann & Kendall 1906), Hypostomus commersoni Valenciennes, 1836, and Pimelodella laticeps Eigenmann 1917 were less prevalent. FG2 evidenced a sharp cut in the contribution to similarity. Cnesterodon decemmaculatus and C. paleatus attained 80% of the contribution, whereas in FG3 C. decemmaculatus accumulated 100%.
From the abundance data, C. decemmaculatus attained the 74% of the similarity in FG1, while in FG2 and FG3 this species accounted for 100%.
Discussion
The land use in the MRB gradually changed from rurality to an increased urbanization and industrialization in the downstream direction. That pattern was also evidenced by gradients in physical and chemical water quality. Accordingly, DO concentrations were significantly higher in rural areas, exhibiting values above 6 mg/l, as has been recorded in other lowland streams elsewhere, with the same land use (Wilcock et al., 1998; Chalar et al., 2013; Paracampo et al., 2020). In agreement with Gómez (1998) and Magdaleno et al. (2001), a decrease in the DO concentration was observed toward the downstream sector of the MRB, even reaching anoxia in the lowermost urbanized sector. The variables BOD, COD, and TSS were significantly higher in the rural areas, likely due to raw effluents discharges from food industries, slaughterhouses, and dairies, established in this area. The wastewater from such industries has a high concentration of organic matter (Marchaim & Klinger, 1987; Shete & Shinkar, 2013; Singh et al., 2014). Similar observations were made in the Carabassa Stream, close to the MRB, where the highest values of BOD, COD, and TSS were found in sampling sites located downstream from a dairy industry (Padulles et al., 2017). The decomposition of high organic matter loads produces oxygen depletion in the water; but, quite paradoxically, G1 nevertheless displayed the highest concentrations of DO. This observation could be explained upon considering that the points of impact by the food industries are widely spaced apart in rural areas; and though BOD could be very high at certain sites, the oxygen depletion occurs in a localized area and thus affects only limited stretches of rural streams. Accordingly, these organic effluents can be considered the most significant human impact occurring within the upper MRB.
The E. coli, detergents, and heavy metals in the water were significantly higher in the urbanized areas. Studies have reported an increase in the levels of those contaminants as the percent of urbanization rises. According to Young & Thackston (1999), the number of fecal bacteria is directly related to the percentage of impervious surface and the density of the housing and population. Furthermore, Sekabira et al. (2010a, b) and Rimoldi et al. (2018) reported a high concentration of heavy metals such as Cu and Cr in the water of streams with high degrees of urbanization and industrialization in their basins. Moreover, we need to emphasize that the concentrations of Cr and Cu recorded in the most urbanized lower sectors of the MRB were an order of magnitude above the guideline levels for the protection of the aquatic biota indicated in Argentina by Law N° 24.051 (National Decree 831/93: “http://www.ambiente.gob.ar/”). In agreement with this pattern, the RDA analysis revealed that, as the DO decreased and the NH4+, Cr, detergent, and E. coli increased, the fish assemblages clearly diminished their richness, diversity, abundance, and biomass downstream. Even more, CLUSTER arrangement obtained from the land use data were consistent with corresponding data for fish assemblages, thus supporting the idea that the structure of the fish assemblages in the MRB is closely related to land use.
Neotropical fish assemblages are known to be dominated by species of the orders Characiformes and Siluriformes (Lévêque et al., 2008; Reis et al., 2016). The first taxon includes species with a large range of trophic guilds, with most being water-column feeders, sensitive to habitat destruction and persistent water-quality degradation (Pinto & Araújo, 2007; Gonino et al., 2020). Siluriformes species are more closely associated with the bottom and they have also been characterized as sensitive to decreasing dissolved oxygen along with toxic substances in the sediments (Pinto & Araújo, 2007). Thus, under anthropically impacted conditions, decreases in the richness and the abundance of these orders and, at the same time, an increase in the tolerant taxonomic groups can be expected. In the present study, the dominance of the tolerant Cyprinodontiformes reflected a clear modification in the structure of the fish assemblages, regardless of the group of subbasins. The qualitative and quantitative analyses based on SIMPER enabled an assessment of the specific differences among the groups of assemblages detected by the CLUSTER analyses. The presence-absence SIMPER results demonstrated that the rural subbasins attained the highest number of species (nine). The highest contributions to the similarity within this group were represented by two tolerant to anoxia and pollution species (C. paleatus and C. decemmaculatus), and one sensitive to such conditions (P. eigenmanniorum) (Lewis, 1970; Kramer & Mehegan, 1981; Hued & Bistoni, 2005; Plaul et al., 2016; Paracampo et al., 2020). In addition, only in this group was detected a secondary contribution of species that inhabited different sectors of the streams and/or with diverse trophic guilds (Menni, 2004; Hued & Bistoni, 2005; Paracampo et al., 2020). The shared dominance among two pollution tolerant and one sensitive species together with the residual contribution of species commonly recorded in low impacted pampean streams, accordingly, reflected that fish assemblages of the rural areas of MRB were being affected by human activities. For urbanized subbasins, the qualitative SIMPER analysis indicated a simplified assemblage where the species contribution to intragroup similarity was almost restricted to the two tolerant species in FG2 and to only C. decemmaculatus in FG3. The SIMPER analysis from abundance data was the simplest, revealing that the Cyprinodontiformes C. decemmaculatus had the most significant contributions to the similarity in all three subbasin groups, as such accounting for the total similarity in FG2 and FG3. These findings supported the postulate that the fish assemblages of the whole MRB were affected by anthropic impacts and that the progressive reduction in species number and abundance of Characiformes and Siluriformes could be used as indicators of water-quality deterioration.
When the results of the study are considered together, the decrease in fish assemblage richness can be explained largely by the progressive fall in DO values as urbanization-industrialization increased. Indeed, the clear dominance of species tolerant to hypoxia, C. decemmaculatus and C. paleatus, and the absence of sensitive species in FG2 and FG3, were indicative of the strong influence of this variable on the assemblage structure. Similar results had also been registered in other urbanized and industrialized areas of Argentina such as the Suquía River downstream from Córdoba city (Hued & Bistoni, 2005) and the Rodriguez stream, Buenos Aires (Paracampo et al., 2020). Dissolved oxygen is a key control of habitat quality, a measure of stream health, and one of the major parameters affecting fish in polluted environments (Coble, 1982; Franklin, 2014). Under laboratory conditions, DO concentrations below 2.7 mg/l have been reported to be lethal for many freshwater fish species (Gómez, 1993; Smale & Rabeni, 1995; Ostrand & Wilde, 2001). Critical values in nature, however, could be higher because of the interaction with other conditions, such as the presence of organic matter in the water (Small et al., 2014). On the basis of laboratory and field measurements, Franklin (2014) proposed an abrupt threshold limit of 3.5 mg/l to avoid acute effects on New Zealand lowland freshwater fish communities. Moreover, Eklöv et al. (1998) proved that the improvement in the processing and treatment of industrial and municipal sewage resulted in a greater presence of common species due to a consequent increase in the DO concentration above 5 mg/l. Records of DO above this threshold were found in only the rural subbasins, thus substantiating that this variable is a major driver of the fish assemblages structure in the MRB.
According to Marshall et al. (2010), a separation of the relative influence of the different conditions associated with urbanization in the ecologic deterioration of streams is often difficult. In such environments, the complex interaction of coexisting anthropic variables has been called the “urban stream syndrome” (Walsh et al., 2005). In addition to the DO concentration, high values of anthropic pollutants such as detergent, heavy metals, and E. coli were found to be critical for the fish assemblage structure in urban subbasins. Since sediments tend to concentrate pollutants when permanently exposed to contaminants (Yi et al., 2011, Morrissey & Edds, 1994), and the toxicity of heavy metals increases with decreasing DO concentration (de LG Solbé, 1979); a complex synergistic effect could exert a negative impact on the fish assemblage in G2 and especially in G3.
In temperate streams without geomorphologic discontinuities, fish species richness tends to increase from the headwaters to the mouth because of increased water volume and habitat diversity (Matthews, 1998; Roberts & Hitt, 2010). The confluences of the lower reaches in mainstream rivers support a greater species richness along with an abundance of riverine species (Osborne & Wiley, 1992; Hitt & Angermeier, 2008; Roberts & Hitt, 2010). In the present study, the drastic decrease in the specific richness and abundance of fish towards the lower sectors of the MRB follows a pattern opposite to this natural one. The near absence of fish in the urbanized subbasins of the MRB indicates not only that they are clearly restricted from inhabiting this area, but also that their access to it from the Río de la Plata estuary is diminished. Therefore, such a situation represents a loss of relevant habitats within the fish’s life cycles because the lower sectors of pampean streams that flow into this estuary represent nursery areas for a number of fish species (Almirón et al., 2000; Llompart et al., 2012; Paracampo et al., 2015, 2020). In the MRB-subbasin scenarios, the loss of river connectivity due to anthropic impacts isolate populations (Soininen, 2014; Heino et al., 2017), restricting fish from the habitats that comply with their environmental requirements (Heino et al., 2015) as well as limiting their dispersion for feeding and reproductive purposes (Henderson et al., 2017). Certain studies have demonstrated that basin connectivity enables the preservation of the fish assemblage structure to a certain level in urban streams (Czeglédi et al., 2020) and facilitates a recolonization in instances of total assemblage loss (Albanese et al., 2009). Thus, the alteration in connectivity within the MRB could also imply a reduction in the resilience and elasticity of the fish assemblages along with a loss of persistence in the long term.
Conclusion
In this study, we have evidenced an anthropic impact pattern in one of the most populated basins of the world through the combined analysis of land use, physical and chemical stream variables, and fish assemblages on a subbasin scale. In addition, we have identified and described the main environmental variables that lead qualitatively and quantitatively changes in fish assemblages along a rural-to-urban gradient. Hence, this study not only provides relevant clues for designing and managing the environmental recovery of urbanized basins but also points to the usefulness of fish assemblages as a model for diagnosing environmental quality on a broad scale.
Availability of data and material
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
ACUMAR, 2018. Autoridad de la Cuenca Matanza-Riachuelo. http://www.acumar.gob.ar/. Accessed 1 May 2020.
Albanese, B., P. L. Angermeier & J. T. Peterson, 2009. Does mobility explain variation in colonization and population recovery among stream fishes? Freshwater Biology 54: 1444–1460.
Albert, J. S., G. Destouni, S. M. Duke-Sylvester, A. E. Magurran, T. Oberdorff, R. E. Reis, K. O. Winemiller & W. J. Ripple, 2020. Scientists’ warning to humanity on the freshwater biodiversity crisis. Ambio 50: 85–94.
Allan, J. D., 2004. Landscapes and Riverscapes: the influence of land use on stream ecosystems. Annual Review of Ecology, Evolution, and Systematics 35: 257–284.
Almirón, A. E., M. L. García, R. C. Menni, L. Protogino & L. C. Solari, 2000. Fish ecology of a seasonal lowland stream in temperate South America. Marine and Freshwater Research 51: 265–274.
Almirón, A. E., J. R. Casciotta, L. Ciotek & P. Giorgis, 2015. Guía de los peces del Parque Nacional Pre-Delta. Administración de Parque Nacionales, Buenos Aires.
Aquino, A. E., 1997. Las especies de Hypoptomatinae (Pisces, Siluriformes, Loricariidae) en la Argentina. Revista de Ictiología 5: 5–21.
Azpelicueta, M. M. & L. Braga, 1991. Los Curimátidos en Argentina. In Castellanos, Z. A. (ed.), Fauna de agua dulce de la República Argentina. Profadu CONICET, La Plata: 1–55.
Blann, K. L., J. L. Anderson, G. R. Sands & B. Vondracek, 2009. Effects of agricultural drainage on aquatic ecosystems: a review. Critical Reviews in Environmental Science and Technology 39: 909–1001.
Braga, L., 1993. Los Anostomidae (Pisces, Characiformes) de Argentina. In Castellanos, Z. A. (ed.), Fauna de agua dulce de la República Argentina. Profadu, CONICET, La Plata: 5–45.
Braga, L., 1994. Los Characidae de Argentina de las subfamilias Cynopotaminae y Ancestrorhynchinae. In Castellanos, Z. A. (ed.), Fauna de Agua Dulce de la República Argentina. Profadu, CONICET, La Plata: 21–29.
Burcher, C. L. & E. F. Benfield, 2006. Physical and biological responses of streams to suburbanization of historically agricultural watersheds. Journal of North American Benthological Society 25: 356–369.
Casatti, L., C. P. Ferreira & F. Langeani, 2009. A fish-based biotic integrity index for assessment of lowland streams in southeastern Brazil. Hydrobiologia 623: 173–189.
Casciotta, J. R., A. Almirón & J. A. Bechara, 2005. Peces del Iberá: hábitat y diversidad. Grafikar, La Plata.
Chalar, G., L. Delbene, I. González-Bergonzoni & R. Arocena, 2013. Fish assemblage changes along a trophic gradient induced by agricultural activities (Santa Lucía, Uruguay). Ecological Indicators 24: 582–588.
Clarke, K. R. & R. M. Warwick, 2001. Change in Marine Communities. An Approach to Statistical Analysis and Interpretation. Plymouth Marine Laboratory, Plymouth.
Clarke, K. R., P. J. Somerfield & R. N. Gorley, 2008. Testing of null hypotheses in exploratory community analyses: similarity profiles and biota-environment linkage. Journal of Experimental Marine Biology and Ecology 366: 56–69.
Coble, D. W., 1982. Fish populations in relation to dissolved oxygen in the Wisconsin River. Transactions of the American Fisheries Society 111: 612–623.
Cooper, C. M., 1993. Biological effects of agriculturally derived surface water pollutants on aquatic systems – a review. Journal of Environmental Quality 22: 402–408.
Czeglédi, I., B. Kern, R. Tóth, G. Seress & T. Erős, 2020. Impacts of urbanization on stream fish assemblages: the role of the species pool and the local environment. Frontiers in Ecology and Evolution 8: 137.
de LG Solbé, J. F., 1979. Studies on the effects of pollution on freshwater fish. In Ravera, O. (ed.), Biological Aspects of Freshwater Pollution. Pergamon Press, Oxford: 1–18.
Dudgeon, D., A. H. Arthington, M. O. Gessner, Z. I. Kawabata, D. J. Knowler, C. Lévêque, R. J. Naiman, A. H. Prieur-Richard, D. Soto, M. L. J. Stiassny & C. A. Sullivan, 2006. Freshwater biodiversity: importance, threats, status and conservation challenges. Biological Reviews 81: 163–182.
Eklöv, A. G., L. A. Greenberg, C. Brönmark, P. Larsson & O. Berglund, 1998. Response of stream fish to improved water quality: a comparison between the 1960s and 1990s. Freshwater Biology 40: 771–782.
Feijoó, C. & R. Lombardo, 2007. Baseline water quality and macrophyte assemblages in Pampean streams: a regional approach. Water Research 41: 1399–1410.
Fernández L., A. C. Herrero & I. Martín, 2009. Alteración de servicios ecológicos del urbanismo privado en la región metropolitana de Buenos Aires. In XI Jornadas de Investigación del Centro de Investigaciones Geográficas y del Departamento de Geografía 12 y 13 de noviembre de 2009 La Plata, Argentina.. http://www.fuentesmemoria.fahce.unlp.edu.ar/trab_eventos/ev.818/ev.818.pdf.
Fitzpatrick, F. A., B. C. Scudder, B. N. Lenz & D. J. Sullivan, 2001. Effects of multi-scale environmental characteristics on agricultural stream biota in eastern Wisconsin. Journal of the American Water Resources Association 37: 1489–1507.
Franklin, P. A., 2014. Dissolved oxygen criteria for freshwater fish in New Zealand: a revised approach. New Zealand Journal of Marine and Freshwater Research 48: 112–126.
Gafny, S., M. Goren & A. Gasith, 2000. Habitat condition and fish assemblage structure in a coastal mediterranean stream (Yarqon, Israel) receiving domestic effluent. Hydrobiologia 422(423): 319–330.
Giorgi, A., J. J. Rosso & E. Zunino, 2015. Efectos de la exclusión de ganado sobre la calidad ambiental de un arroyo pampeano. Biología Acuática 30: 133–140.
Gómez, S. E., 1993. Concentración letal de oxígeno disuelto para Corydoras paleatus y Pimelodella laticeps (Pisces, Siluriformes). Revista del Museo Argentino de Ciencias Naturales 7: 31–45.
Gómez, N., 1998. Use of epipelic diatoms for evaluation of water quality in the Matanza-Riachuelo (Argentina), a pampean plain river. Water Research 32: 2029–2034.
Gómez, N., 1999. Epipelic diatoms from the Matanza-Riachuelo river (Argentina), a highly polluted basin from the pampean plain: biotic indices and multivariate analysis. Aquatic Ecosystem Health & Management 2: 301–309.
Gómez, N., M. Licursi, D. E. Bauer & P. R. Hualde, 2003. Reseña sobre Modalidades de Estudio mediante la Utilización de Microalgas en la Evaluación y Monitoreo de algunos Sistemas Lóticos Pampeanos Bonaerenses. Boletín de la Sociedad Argentina de Botánica 38: 93–101.
Gonino, G., E. Benedito, V. D. M. Cionek, M. T. Ferreira & J. M. Oliveira, 2020. A fish-based index of biotic integrity for Neotropical rainforest sandy soil streams-southern Brazil. Water 12: 1215.
Groffman, P. M., D. J. Bain, L. E. Band, K. T. Belt, G. S. Brush, J. M. Grove, R. V. Pouyat, I. C. Yesilonis & W. C. Zipperer, 2003. Down by the riverside: urban riparian ecology. Frontiers in Ecology and the Environment 1: 315–321.
Guida-Johnson, B. & G. A. Zuleta, 2019. Environmental degradation and opportunities for riparian rehabilitation in a highly urbanized watershed: the Matanza-Riachuelo in Buenos Aires, Argentina. Wetlands Ecology and Management 27: 243–256.
Heino, J., A. S. Melo, T. Siqueira, J. Soininen, S. Valanko & L. M. Bini, 2015. Metacommunity organization, spatial extent and dispersal in aquatic systems: patterns, processes, and prospects. Freshwater Biology 60: 845–869.
Heino, J., J. Alahuhta, T. Ala-Hulkko, H. Antikainen, L. M. Bini, N. Bonada, T. Datry, T. Erős, J. Hjort, O. Kotavaara, A. S. Melo & J. Soininen, 2017. Integrating dispersal proxies in ecological and environmental research in the freshwater realm. Environmental Reviews 25: 334–349.
Helms, B. S., J. W. Feminella & S. Pan, 2005. Detection of biotic responses to urbanization using fish assemblages from small streams of western Georgia, USA. Urban Ecosystems 8: 39–57.
Henderson, C. J., B. L. Gilby, S. Y. Lee & T. Stevens, 2017. Contrasting effects of habitat complexity and connectivity on biodiversity in seagrass meadows. Marine Biology 164: 117.
Hitt, N. P. & P. L. Angermeier, 2008. River-Stream connectivity affects fish bioassessment performance. Environmental Management 42: 132–150.
Hued, A. C. & M. A. Bistoni, 2005. Development and validation of a Biotic Index for evaluation of environmental quality in the central region of Argentina. Hydrobiologia 543: 279–298.
IGN, 2018. Instituto Geográfico Nacional. https://www.ign.gob.ar/NuestrasActividades/InformacionGeoespacial/CapasSIG. Accessed 5 May 2020.
INDEC, 2010. Instituto Nacional de Estadística y Censos. https://www.indec.gob.ar/indec/web/Nivel4-Tema-2-41-135. Accessed 1 May 2020.
Jacobson, C. R., 2011. Identification and quantification of the hydrological impacts of imperviousness in urban catchments: a review. Journal of Environmental Management 92: 1438–1448.
Jeppesen, E., J. Peder Jensen, M. Søndergaard, T. Lauridsen & F. Landkildehus, 2000. Trophic structure, species richness and biodiversity in Danish lakes: changes along a phosphorus gradient: a detailed study of Danish lakes along a phosphorus gradient. Freshwater Biology 45: 201–218.
Karr, J. R., 1981. Assessment of biotic integrity using fish communities. Fisheries 6: 21–27.
Kauffman, J. B. & W. C. Krueger, 1984. Livestock impacts on riparian ecosystems and streamside management implications… A review. Journal of Range Management Archives 37: 430–438.
Kramarz, T., 2018. Using the courts to protect the environment in Argentina: accountability pitfalls when judges have the last word. Case Studies in the Environment. https://doi.org/10.1525/cse.2017.000992.
Kramer, D. L. & J. P. Mehegan, 1981. Aquatic surface respiration, an adaptive response to hypoxia in the guppy, Poecilia reticulata (Pisces, Poeciliidae). Environmental Biology of Fishes 6: 299–313.
Lenat, D. R. & J. K. Crawford, 1994. Effects of land use on water quality and aquatic biota of three North Carolina Piedmont streams. Hydrobiologia 294: 185–199.
Lepš, J. & P. Šmilauer, 2003. Multivariate Analysis of Ecological Data Using CANOCO. Cambridge University Press, London.
Lévêque, C., T. Oberdorff, D. Paugy, M. L. J. Stiassny & P. A. Tedesco, 2008. Global diversity of fish (Pisces) in freshwater. Hydrobiologia 595: 545–567.
Lewis, W. M., 1970. Morphological adaptations of cyprinodontoids for inhabiting oxygen deficient waters. Copeia 1970: 319–326.
Llompart, F. M., A. Paracampo, P. J. Solimano & I. García, 2012. Peces de la Reserva Natural Punta Lara. In Roesler, I. & M. G. Agostini (eds), Inventario de vertebrados de la Reserva Natural Punta Lara, Provincia de Buenos Aires, Argentina: 57–70.
López, H. L. & A. M. Miquelarena, 1991. Peces loricaridos de la cuenca del Plata, Argentina. I: El género Cochliodon Heckel, 1854 (Pisces: Siluriformes). Gayana Zoologia 55: 3–11.
Magdaleno, A., A. Puig, L. de Cabo, C. Salinas, S. Arreghini, S. Korol, S. Bevilacqua, L. López & J. Moretton, 2001. Water pollution in an urban Argentine river. Bulletin of Environmental Contamination and Toxicology 67: 408–415.
Marchaim, U. & I. Klinger, 1987. Biological aspects of waste control in slaughter-houses. In Nielson, V. C., J. H. Voorburg & P. L. Hermite (eds), Volatile Emissions from Livestock Farming and Sewage Operations. Elsevier Applied Science, London: 217–222.
Marshall, S., V. Pettigrove, M. Carew & A. Hoffmann, 2010. Isolating the impact of sediment toxicity in urban streams. Environmental Pollution 158: 1716–1725.
Matteucci, S. D. & J. Morello, 2009. Environmental consequences of exurban expansion in an agricultural area: the case of the Argentinian Pampas ecoregion. Urban Ecosystems 12: 287–310.
Matthews, W., 1998. Patterns in Freshwater Fish Ecology. Springer, Oklahoma.
McGarrigle, M., 2014. Assessment of small water bodies in Ireland. Biology and Environment: Proceedings of the Royal Irish Academy 114B: 119–128.
McKergow, L. A., D. M. Weaver, I. P. Prosser, R. B. Grayson & A. E. G. Reed, 2003. Before and after riparian management: sediment and nutrient exports from a small agricultural catchment, Western Australia. Journal of Hydrology 270: 253–272.
Menni, R. C., 2004. Peces y ambientes en la Argentina continental. Monografías del Museo Argentino de Ciencias Naturales 5: 1–316.
Miquelarena, A. M. & R. C. Menni, 2005. Astyanax tumbayaensis, a new species from northwestern Argentina highlands (Characiformes: Characidae) with a key to the Argentinean species of the genus and comments on their distribution. Revue Suisse de Zoologie 112: 661–676.
Mirande, J. M. & S. Koerber, 2020. Checklist of the Freshwater Fishes of Argentina. 2nd edition (CLOFFAR-2). Ichthyological Contributions of Peces Criollos 72: 1–81.
Morrissey, J. R. & D. R. Edds, 1994. Metal pollution associated with a landfill: concentrations in water, sediment, crayfish, and fish. Transactions of the Kansas Academy of Science 97: 18–25.
National Decree 831/93. https://www.argentina.gob.ar/normativa/nacional/decreto-831-1993-12830/texto Accessed 5 May 2020.
Nessimian, J. L., E. M. Venticinque, J. Zuanon, P. De Marco, M. Gordo, L. Fidelis, J. D’arc Batista & L. Juen, 2008. Land use, habitat integrity, and aquatic insect assemblages in Central Amazonian streams. Hydrobiologia 614: 117–131.
Olson, D., E. Dinerstein, P. Canevari, I. Davidson, G. Castro, V. Morisset, R. Abell & E. Toledo, 1998. Freshwater Biodiversity of Latin America and the Caribbean: A Conservation Assessment. Biodiversity Support Program, Washington DC.
Osborne, L. L. & M. J. Wiley, 1992. Influence of tributary spatial position on the structure of warmwater fish communities. Canadian Journal of Fisheries and Aquatic Sciences 49: 671–681.
Ostrand, K. G. & G. R. Wilde, 2001. Temperature, dissolved oxygen, and salinity tolerances of five prairie stream fishes and their role in explaining fish assemblage patterns. Transactions of the American Fisheries Society 130: 742–749.
Padulles, M. L., V. T. D. Conforti, P. Nannavecchia & I. O’Farrell, 2017. Impacto de la contaminación orgánica sobre el fitoplancton de un arroyo de la llanura pampeana. Ecología Austral 27: 437–448.
Paracampo, A., I. García, H. Mugni, N. Marrochi, P. Carriquiriborde & C. Bonetto, 2015. Fish assemblage of a Pampasic stream (Buenos Aires, Argentina): temporal variations and relationships with environmental variables. Studies on Neotropical Fauna and Environment 50: 145–153.
Paracampo, A., N. Marrochi, I. García, T. Maiztegui, P. Carriquiriborde, C. Bonetto & H. Mugni, 2020. Fish assemblages of Pampean streams (Buenos Aires, Argentina): relationships with abiotic and anthropogenic variables. Anais da Academia Brasileira de Ciências 92: e20190476.
Pinto, B. C. T. & F. G. Araújo, 2007. Assessing of biotic integrity of the fish community in a heavily impacted segment of a tropical river in Brazil. Brazilian Archives of Biology and Technology 50: 489–502.
Plaul, S. E., C. G. Barbeito & A. O. Díaz, 2016. Histochemical differences along the intestine of Corydoras paleatus (Siluriformes: Callichthyidae). Revista de Biología Tropical 64: 327–340.
Reis, R. E. & E. H. L. Pereira, 2000. Three new species of the loricariid catfish genus Loricariichthys (Teleostei: Siluriformes) from southern South America. Copeia 4: 1029–1047.
Reis, R. E., J. S. Albert, F. Di Dario, M. M. Mincarone, P. Petry & L. A. Rocha, 2016. Fish biodiversity and conservation in South America. Journal of Fish Biology 89: 12–47.
Rendina, A. & A. R. Fabrizio de Iorio, 2012. Heavy metal partitioning in bottom sediments of the Matanza-Riachuelo River and main tributary streams. Soil and Sediment Contamination 21: 62–81.
Říčan, O. & S. O. Kullander, 2008. The Australoheros (Teleostei: Cichlidae) species of the Uruguay and Paraná River drainages. Zootaxa 1724: 1–51.
Rimoldi, F., L. Peluso, G. Bulus Rossini, A. E. Ronco & P. M. Demetrio, 2018. Multidisciplinary approach to a study of water and bottom sediment quality of streams associated with mixed land uses: case study Del Gato Stream, La Plata (Argentina). Ecological Indicators 89: 188–198.
Ringuelet, R. A., R. H. Aramburu & A. Aramburu, 1967. Los Peces Argentinos de Agua Dulce. CIC, Buenos Aires.
Roberts J. H. & N. P. Hitt, 2010. Longitudinal structure in temperate stream fish communities: evaluating conceptual models with temporal data. In Gido K. B. & D. A. Jackson (eds), Community Ecology of Stream Fishes: Concepts, Approaches, and Techniques. American Fisheries Society, Symposium 73, Bethesda: 281–299.
Rodrigues Capítulo, A., C. Ocon & M. Tangorra, 2004. Una visión bentónica de los arroyos pampeanos. Biología Acuática 21: 1–18.
Rodrigues Capítulo, A., N. Gómez, A. Giorgi & C. Feijoó, 2010. Global changes in pampean lowland streams (Argentina): implications for biodiversity and functioning. Hydrobiologia 657: 53–70.
Rosso, J. J., M. González-Castro, S. Bogan, Y. P. Cardoso, E. Mabragaña, M. Delpiani & J. M. Díaz de Astarloa, 2018. Integrative taxonomy reveals a new species of the Hoplias malabaricus species complex (Teleostei: Erythrinidae). Ichthyological Exploration of Freshwaters 28: 235–252.
Roth, N. E., J. D. Allan & D. L. Erickson, 1996. Landscape influences on stream biotic integrity assessed at multiple spatial scales. Landscape Ecology 11: 141–156.
SAIJ, 2008. Sistema Argentino de Información Jurídica. http://www.saij.gob.ar/corte-suprema-justicia-nacion-federal-ciudad-autonoma-buenos-aires-mendoza-beatriz-silvia-otros-estado-nacional-otros-danos-perjuicios-danos-derivados-contaminacion-ambiental-rio-matanza-riachuelo-fa08000047-2008-07-08/123456789-740-0008-0ots-eupmocsollaf. Accessed 1 May 2020.
Scott, M. C. & G. S. Helfman, 2001. Native invasions, homogenization, and the mismeasure of integrity of fish assemblages. Fisheries 26: 6–15.
Sekabira, K., H. O. Origa, T. A. Basamba, G. Mutumba & E. Kakudidi, 2010a. Assessment of heavy metal pollution in the urban stream sediments and its tributaries. International Journal of Environmental Science and Technology 7: 435–446.
Sekabira, K., H. O. Origa, T. A. Basamba, G. Mutumba & E. Kakudidi, 2010b. Heavy metal assessment and water quality values in urban stream and rain water. International Journal of Environmental Science and Technology 7: 759–770.
Shannon, C. E. & W. Weaver, 1949. The mathematical theory of communication. University Illinois Press, Urbana.
Shete, B. S. & N. P. Shinkar, 2013. Dairy industry wastewater sources, characteristics & its effects on environment. International Journal of Engineering Research & Technology 3: 1611–1615.
Singh, N. B., R. Singh & M. M. Imam, 2014. Wastewater management in dairy industry: pollution abatement and preventive attitudes. International Journal of Environmental Science and Technology 3: 672–683.
Sliva, L. & D. D. Williams, 2001. Buffer zone versus whole catchment approaches to studying land use impact on river water quality. Water Research 35: 3462–3472.
Smale, M. A. & C. F. Rabeni, 1995. Hypoxia and hyperthermia tolerances of headwater stream fishes. Transactions of the American Fisheries Society 124: 698–710.
Small, K., R. K. Kopf, R. J. Watts & J. Howitt, 2014. Hypoxia, blackwater and fish kills: experimental lethal oxygen thresholds in juvenile predatory lowland river fishes. PLoS ONE 9: e94524.
Soininen, J., 2014. A quantitative analysis of species sorting across organisms and ecosystems. Ecology 95: 3284–3292.
Soriano, A., R. León, O. Sala, R. Lavado, V. Deregibus, M. Cauhepe, R. Coupland & J. H. Lemcoff, 1991. Río de la Plata grasslands. In Coupland, R. T. (ed.), Natural Grasslands. Introduction and Western Hemisphere. Elsevier, Amsterdam: 367–407.
STAT-SOFT INC, 2009. Statistica: User guides. Tulsa, USA.
Steedman, R. J., 1988. Modification and assessment of an index of biotic integrity to quantify stream quality in Southern Ontario. Canadian Journal of Fisheries and Aquatic Sciences 45: 492–501.
Strayer, D. L. & D. Dudgeon, 2010. Freshwater biodiversity conservation: recent progress and future challenges. Journal of the North American Benthological Society 29: 344–358.
Ter Braak, C. J. F. & P. Smilauer, 1998. Canoco Reference Manual and User’s Guide to Canoco for Windows: Software for Canonical Community Ordination (Version 4). Microcomputer Power, Ithaca.
Terán, G. E., M. F. Benitez & J. M. Mirande, 2020. Opening the Trojan horse: phylogeny of Astyanax, two new genera and resurrection of Psalidodon (Teleostei: Characidae). Zoological Journal of the Linnean Society 20: 1–18.
Walsh, C. J., A. H. Roy, J. W. Feminella, P. D. Cottingham, P. M. Groffman & R. P. Morgan II, 2005. The urban stream syndrome: current knowledge and the search for a cure. Journal of North American Benthology Society 24: 706–723.
Wang, L., J. Lyons, P. Kanehi, R. Bannerman & E. Emmons, 2000. Watershed urbanization and changes in fish communities in southeastern Wisconsin streams. Journal of the American Water Resourses Association 36: 1173–1189.
Wilcock, R. J., J. W. Nagels, G. B. McBride, K. J. Collier, B. T. Wilson & B. A. Huser, 1998. Characterisation of lowland streams using a single-station diurnal curve analysis model with continuous monitoring data for dissolved oxygen and temperature. New Zealand Journal of Marine and Freshwater Research 32: 67–79.
Yi, Y., Z. Yang & S. Zhang, 2011. Ecological risk assessment of heavy metals in sediment and human health risk assessment of heavy metals in fishes in the middle and lower reaches of the Yangtze River basin. Environmental Pollution 159: 2575–2585.
Young, K. D. & E. L. Thackston, 1999. Housing density and bacterial loading in urban streams. Journal of Environmental Engineering 125: 1177–1180.
Acknowledgements
This research was jointly supported by the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), the Universidad Nacional de La Plata (UNLP), and the Autoridad de la Cuenca Matanza-Riachuelo (ACUMAR). We wholeheartedly thank Eugenio Coconier and Roberto Jensen for their collaboration during the field studies. We also are grateful to Sergio Bogan for his support in the identification of certain specimens, and the Fundación de Historia Natural Félix de Azara for housing the fish vouchers in the ichthyological collection. This document represents the Scientific Contribution N° 1174 of the Instituto de Limnología Dr. Raúl A. Ringuelet (CONICET-UNLP). Dr. Donald F. Haggerty, a retired academic career investigator and native English speaker, edited the final version of the manuscript.
Funding
This research received the joint support of the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), the Universidad Nacional de La Plata (UNLP), and Autoridad de la Cuenca Matanza-Riachuelo (ACUMAR)
Author information
Authors and Affiliations
Contributions
JMPdP: investigation, conceptualization, methodology, formal analysis, resources, data curation, writing of the original draft, writing and editing of the revised version, visualization. AP: investigation, conceptualization, methodology, formal analysis, resources, data processing, writing of the original draft, writing and editing of the revised version, visualization. IG: resources, data processing, writing and editing of the revised version. TM: resources, data processing, writing and editing of the revised version. JGdS: resources, data processing, writing and editing of the revised version. MM: resources, data processing, writing and editing of the revised version. DC: investigation, conceptualization, methodology, formal analysis, resources, data processing, writing and editing of the revised version, visualization, supervision, project administration, funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethics approval
Care during the collection and handling of fish for this study complied with the Buenos Aires Province (Argentina) Wildlife and Fisheries Authority guidelines and policies (Law 11,477). The collections for this study were not part of faunal surveys and did not employ any type of experimental procedure, surgery, or chemical agents that would induce neuromuscular blockage or injury on the organisms collected. All the fish collected were euthanized as humanely as possible by anesthesia to prevent unnecessary suffering.
Additional information
Handling editor: Fernando M. Pelicice.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Paredes del Puerto, J.M., Paracampo, A.H., García, I.D. et al. Fish assemblages and water quality in pampean streams (Argentina) along an urbanization gradient. Hydrobiologia 848, 4493–4510 (2021). https://doi.org/10.1007/s10750-021-04657-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-021-04657-z