Abstract
The distribution and composition of aquatic insect communities in streams at a local scale are considered to be primarily determined by environmental factors and interactive relationships within the system. Here, we evaluated the effects of forest fragmentation and forest cover changes on habitat characteristics of streamlets (igarapés) in Amazonian forests and on the aquatic insect communities found there. We also developed a habitat integrity index (HII) based on Petersen’s protocol (1992) to evaluate physical integrity of these streamlets and to determine its efficiency to interpret the environmental impacts on this system. We studied 20 small streams at the Biological Dynamics of Forest Fragments Project (BDFFP INPA/SI) study areas, Central Amazonia, 80 km north of Manaus, Amazonas State, Brazil. The vegetation cover was estimated by using LANDSAT images and classified in the following categories: exposed soil, pastures, secondary forests (capoeiras), and primary forests. Stream habitat features were evaluated by using a HII based on visual assessment of local characteristics. Aquatic insects were sampled in four major stream substrates: litter deposited in pools or backwaters, litter retained in riffles, sand, and marginal banks. Stream habitat characteristics were significantly correlated to land use and riparian forest condition. Overall aquatic insect richness and Ephemeroptera, Plecoptera, and Trichoptera (EPT) richness were significantly lower in pasture streams, and their taxonomic composition differed significantly from streams in forested areas. However, these metrics were not significantly correlated to the stream HII. Taxonomic composition of bank insect assemblages changed significantly between streams with low and high values of HII. There was no significant relationship between the proportion of primary forest cover and the faunal metrics. Only drastic changes in the vegetal cover seem to induce significant changes in the aquatic insect community. Matrix habitat heterogeneity, distance to forest fragments, the presence of areas of secondary forest, and the intrinsic capacity to disperse in many of the insect groups may have contributed to attenuate the effects of habitat disturbance on aquatic insect assemblages in streamlets.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human occupation in Amazonia and the associated loss of forest cover have resulted in the degradation of rivers and streams. Major impacts on Amazonian rivers include sediment filling and removal of substrate material in river beds, water draining, modification of shore areas, dam and reservoir construction, raw domestic sewage inputs, as well as agricultural, cattle, mining, and industrial effluents (McClain & Elsenbeer, 2001; Davidson et al., 2004; Melo et al., 2005). In addition, removal or substitution of riparian vegetation has a direct negative effect on the input of organic matter that constitutes the primary energy source of rivers trophic chains (De Long & Brusven, 1994; Pozo et al., 1997). These have resulted in changes in physical habitat, hydrology, and water quality in streams and rivers. All these factors lead to drastic changes in aquatic biota, causing loss of diversity in the system. The effects of human activity, especially deforestation in Central Amazonia, affect directly and negatively the small watercourses. Since there is a large amount of forest cover still intact, the impacts of forest loss and fragmentation are not readily perceived in higher order stretches (Smith et al., 1995; Davidson et al., 2004). Besides that, it is not always possible to detect the effects of these negative environmental impacts on the aquatic biota present in streamlets in a simple and fast way, because there is no protocol of environmental evaluation adapted to the Amazonian conditions.
Riparian forests are essential for the protection of fluvial systems as they prevent erosion, loss of nutrients, intake of sediments, and other pollutants, and they also contribute to the maintenance of the biota (Zweig & Rabeni, 2001; Sparovek et al., 2002). Modifications such as fragmentation or changes in the forest cover lead to alterations in the habitat structure, including litter fall (Sizer, 1992) and changes in the composition of allochthonous material carried to the streams, which determines changes in their structure and function (Benstead et al. 2003; Benstead & Pringle, 2004). In Brazil, Amazonian deforestation has happened at a fast rate, despite the efforts made by governmental and non-governmental agencies for the last years. In Manaus area, central Amazonia, studies about the effects of forest fragmentation on biota have been performed for more than 25 years (Bierregaard et al., 2001; Gascon et al., 2001). One of the central objectives of the Biological Dynamics of Forest Fragments Project (BDFFP) is to study the ecological effects of forest fragmentation on Tropical Forest areas (Lovejoy et al., 1983; Gascon et al. 2001). Nevertheless, almost all results obtained so far correspond to terrestrial systems. As part of the BDFFP, the Igarapés Project is devoted to the study of effects of forest fragmentation and changes of vegetation cover on the integrity of structure and function in small forest streams. This study aimed to evaluate the effects of landscape changes on the structure and functioning of small forest streams in the Brazilian central Amazon, as perceived by changes in aquatic insect communities. Furthermore, to accomplish this objective, we employed an index of habitat integrity adapted to Amazonian environmental conditions and compared the results with faunal characteristics of insect communities.
Study area
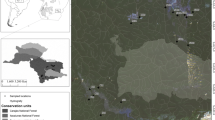
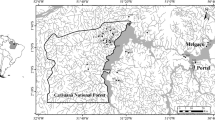
The BDFFP study site is composed of replicated series of forest preserves of 1, 10, and 100 ha areas experimentally isolated from the surrounding continuous primary forest matrix (for details on isolation procedures and characteristics of forest fragments, see Gascon & Bierregaard, 2001). It is situated 60–90 km north from the city of Manaus (Amazonas State, Brazil). The forest cover of BDFFP area was partially removed 30 years ago for establishing cattle farms. Some forest fragments were maintained; some of the cleared areas were abandoned; and the process of regeneration was naturally established. All the area is surrounded by primary forest which extends unbroken for hundreds of kilometers to the north, east, and west (Gascon & Bierregaard, 2001; Gascon et al. 2001) (Fig. 1).
The study area is classified as tropical moist forest with a mean annual rainfall of about 2,200 mm, ranging 1,900–2,500 mm. There is a pronounced dry season from June to October, with less than 100 mm of monthly rainfall. The forest canopy reaches up to 30–37 m tall, with emergent trees as high as 55 m. This is one of the most diverse forest communities in the world, with at least 280 tree species/ha (Oliveira & Mori, 1999). About 1,300 tree species occur in the project area (Lawrence, 2001). The landscape is located in Pleistocene terraces of interglacial origin (RADAM Brasil, 1978) at 80–100 m above sea level and altitudinal differences of 40–50 m between plateaus and stream valleys (Gascon & Bierregaard, 2001).
The first to third order streamlets (1:50,000 scale) we studied belong to catchments of three different rivers (Urubu, Cuieiras, and Preto da Eva Rivers), with similar general characteristics such as geomorphology and distance from the Negro and Amazonas Rivers. The streams have black acidic waters (pH 4.5–5.4) with low conductivity (7.9–16.7 μS cm−1), and the mean water temperature is of approximately 24°C (Mortati, 2004). Their streambeds are typically comprised of sand patches and litter.
Forest streams under natural conditions (primary forest areas) are highly shaded due to the reduced canopy openness, and they present distinct channel morphology, depending on the terrain characteristics. In wide, flat-bottomed stream valleys (“baixios”), there is more connectivity between the shallow riverbed and the marginal ponds or flooded adjacent areas. Banks, when formed, are firmly sustained by roots and vegetation, with cuttings only under roots and especially in narrow-angled meanders. The height of banks depends on the terrain declivity, stream valley width, and stream size. Banks are sometimes incipient in small order streams. There is a large amount of leaf and wood detritus deposited in pools as well as in meanders, and logs and twigs constitute the main structures that retain this material. These obstacles to the stream flow produce pools and small riffles that increase the habitat heterogeneity in the system. In streams that cross open areas such as pastures, light incidence is very high; the streams get progressively shaded in old secondary forests, where herbaceous plants grow on the margins or on the streambed. Although qualitative changes are expected in the allochthonous matter deposited in the streams, there are no differences in litter availability in secondary forest streams when compared to primary forests (Webster et al., 1990; Mortati, 2004). In pastures, there are no retention mechanisms (such as logs and twigs) on the streambeds, which are frequently silted, and the water spreads widely over the ground. Stands of the riparian vegetation (herbs, shrubs, and trees) may wither away and eventually die. Furthermore, the marginal banks are fragile and may crumble, as they are protected only by grass.
Methods
Experimental design
We established 150 m reaches of 20 streams for sampling (Fig. 1). The sampling design intended to produce independent samples and to maximize their distribution into the BDFFP area. We had two sample reaches of one stream, but these had different landscape conditions (a stream that drains a 10-ha forest fragment and subsequently crosses a secondary forest area).
Catchments exhibited a range in land use from primary forest to secondary forest or pasture (Table 1). The secondary forest landscape included three vegetation types: (1) areas dominated by Vismia Vand., 1788 (Clusiaceae), (2) areas dominated by Cecropia Loefl., 1758 (Cecropiaceae), or (3) mixed conditions where both species were present. These landscape differences result from the managing practices employed during the process of forest fragment isolation: Vismia secondary forests grew where the original vegetation was burned, whereas Cecropia dominated where the original vegetation was only logged (Williamson et al., 1998). According to Moreira (2002), the ages of secondary forests in the study area varied between 2 and 20 years old and were distributed in three age classes (Table 1). Older secondary forests may grow up to 25 m tall, but with lower tree density than in primary forests. The studied areas in primary forest landscapes have different sizes (10, 100 ha, and continuous forest areas). The maximum distance between the sampling sites and the nearby continuous forest was of 1 km (Fig. 1).
Landscape analysis
We used Landsat TM 5 (Thematic Mapper) images, (path 232 row 62—2001) RGB, bands 3 (0.63–0.69 μm), 4 (0.76–0.90 μm), 5 (1.55–1.75 μm) with 30 m resolution for the landscape analysis in this study. We produced a classified image by using Maximum Likelihood algorithm in supervised classification with ERDAS 8.7 Software. We generated a linear buffer zone 150 m wide around each stream stretch by using ArcView 3.2. For each buffer zone, we calculated the proportion of the area covered by primary forest, secondary forest, pasture, and exposed soil. Some studies researched the classification of these categories and mapped the extent and temporal dynamics of secondary vegetation at local level in Amazon (e.g., Adams et al. 1995; Alves & Skole 1996; Steininger 1996).
Stream habitat evaluation
We measured 12 habitat characteristics (items) to describe the environmental conditions in the studied reaches based on the protocols of Petersen (1992) for visual assessment relative to land use, riparian zone, streambed characteristics, and stream channel morphology, to produce a habitat integrity index (HII). Each item was composed of four to six alternatives ordered in relation to perceived aspects of habitat integrity. To assure that each item had the same weight in the analysis, the observed values (a o) were standardized in relation to the maximum value for each item (a m, Eq. 1). The final index is the mean value for the total sampled habitat characteristics (n, Eq. 2). These transformations produce an index that vary between 0 and 1 and that is directly related to the integrity of habitat conditions (Table 2).
Biological variables
We took one subsample of macroinvertebrates composed by three sweeps from each of the four main substrates, randomly spread in a stretch of 50 m at each stream with an aquatic sweep net of 1 mm mesh size and 30 cm of diameter. The substrates sampled were leaf litter in pool and backwater areas, leaf litter in riffle areas, sand patches, and root/vegetation at stream banks. Subsamples from the two sampling events (March and October 2001) were combined into one sample from each stream. The total sampled area for each stream was 1.7 m2. The samples were washed and preliminarily sorted in the field and fixed at 80% ethanol. Later, we sorted the specimens into morphospecies and identified them up to species or higher taxonomic level using identification keys (e.g., Belle, 1992; Angrisano, 1995; Merritt & Cummins, 1996; Wiggins, 1996; Nieser & Melo, 1997; Carvalho & Calil, 2000; Da Silva et al., 2003; Olifiers et al., 2004; Manzo, 2005; Pes et al., 2005) and aid of specialists. All insects in the samples were enumerated and identified. Three different metrics were used to represent aquatic insect communities: taxonomic richness, Ephemeroptera, Plecoptera and Trichoptera (EPT) richness, and aquatic insect taxonomic composition (Benstead et al., 2003; Benstead & Pringle, 2004). These metrics are considered sensitive to habitat changes in the aquatic environment (Barbour et al. 1996, Silveira et al., 2005).
Data analysis
We performed Spearman’s rank correlation tests among the HII values and the values of each measured protocol variable and vegetation cover as well as with insect community metrics. For calculations, taxa composition of each stream reach area (total or by substrate type) was represented by the coordinates of the first axis of a NMDS analysis based on species presence–absence data (one dimension; distance measure: Euclidian distance) The percentage of variation of the Euclidian distance matrix captured by the first axis is expressed by r 2. The ANOSIM was used to compare and determine differences between stream communities of primary forest, forest fragments, secondary forest, and pasture. Taxa richness comparisons between streams were made using a rarefaction method (Gotelli & Colwell, 2001). We performed individual-based rarefactions, because we only had one composite sample per stream. The ANOVA and the Tukey HSD test were used to compare and to determine differences between the resultant richness values of stream communities of primary forest, forest fragments, secondary forest, and pasture.
Due to the use of multiple Spearman’s correlation tests, we performed the false discovery rate (FDR) approach (Benjamini & Hochberg, 1995) to control for type I errors (n = 223; α = 0.05). In our analysis, after application of FDR, we accepted P-values <0.006. For details and discussion on this method, see García (2004). The species indicator analysis (Dufrêne & Legendre, 1997) was used to relate taxa and landscapes. The statistical programs Past 1.4 (Hammer et al., 2001), PC-ORD 4 (McCune & Mefford, 1999), and STATISTICA 6.0 (StatSoft, 2001) were used. For all analyses, taxa with only one specimen were not considered. We adopted a significance level of α = 0.05 in all tests.
Results
The aquatic insect fauna
We observed 151 taxa distributed in 10 orders in the samples, which held 5,746 individuals. The numbers of taxa recorded in each stream reach area ranged 25–70 (Appendix in Electronic supplementary material). The average numbers of insect taxa in stream reaches of primary and secondary forests were very similar to one another (51.8 ± 9.5 and 50.0 ± 5.6, respectively) and larger than in pasture areas (34 ± 10.2). EPT was represented by 68 taxa, of which 5–35 were collected from each stream reach area. Trichoptera was composed of 44 taxa, Ephemeroptera of 20, and Plecoptera of only 4. The average numbers of EPT taxa in stream reaches of primary forest, secondary forest, and pasture were 28.2 ± 5.1, 26.2 ± 2.8 and 15.3 ± 10.0 respectively.
Regarding to secondary forests, older forests (>14 years old) showed higher number of taxa than 2–7 year-old forests.
Comparing rarefaction-based species richness, pasture streams showed lower values of insect taxa richness (F = 10.217; P = 0.000) and EPT richness (F = 6.934; P = 0.003) (Figs. 2 and 3) than streams in primary forests, forest fragments, and secondary forests. Regarding substrate diversity, pasture streams showed lower insect taxa richness in sand (F = 7.052; P = 0.003) and riffle litter (F = 16.558; P = 0.000) and lower values of EPT richness in riffle litter (F = 12.080; P = 0.000) and pool litter (F = 4.770; P = 0.0137). Banks showed no differences between vegetation covers.
The results of ANOSIM showed that pasture streams have different communities from primary and secondary forest streams, but not of forest fragment streams (Table 3). The first axis of a NMDS (final stress = 36.06; r 2 = 0.46) also provided a contrast of pasture, secondary forest, and primary forest areas (Fig. 4). Primary forest streams had more exclusive taxa (14 insect taxa; 5 EPT), whereas secondary forest and pasture streams presented seven exclusive taxa each (1 EPT). Comparing primary forest plus secondary forest streams with secondary forest plus pasture streams, more taxa were exclusive of the first group (46 insect taxa; 24 EPT) than of the second one (6; 3). Continuous forest, fragment (both primary forests), and secondary forest streams showed very similar fauna, but few species were considered characteristic of primary forest streams by the indicator species analysis and none for secondary forest streams (Fig. 4 and Appendix in Electronic supplementary material).
Forest cover relationships
Stream reaches with larger percentages of canopy cover showed higher HII values (r = 0.77; P < 0.001). Among the 12 measured habitat variables present in HII composition, the following seven showed significant correlations with vegetation cover: (1) land use pattern beyond the riparian zone (r = 0.760; P < 0.001), (2) width of riparian forest (r = 0.606; P = 0.004), (3) completeness of riparian forest (r = 0.596; P = 0.004), (4) vegetation of riparian zone within 10 m of channel (r = 0.762; P < 0.001), (5) retention devices (r = 0.713; P < 0.001), (6) channel sediments (r = 0.708; P < 0.001), and (7) aquatic vegetation (r = 0.662; P < 0.001). These results indicate a close relationship between the physical structure of the streams and the integrity of the riparian forest (Fig. 5). However, there was no significant correlation between forest cover and the faunal metrics insect richness, EPT richness, and insect taxa composition (except for the stream bank fauna).
Habitat integrity and faunal metrics
There was not significant correlation between HII values and aquatic insect metrics (total insect richness r = 0.223; P = 0.330; EPT richness r = 0.252; P = 0.290; insect taxa composition r = −0.552; P = 0.010). None of the habitat variables was significantly correlated with total insect richness and only one, aquatic vegetation, was correlated with EPT richness (r = 0.620; P = 0.003). Six variables were significantly correlated to insect taxa composition: (1) land use pattern beyond the riparian zone (r = 0.590; P = 0.005), (2) width of riparian forest (r = 0.800; P < 0.001), (3) completeness of riparian forest (r = 0.675; P = 0.001), (4) retention devices (r = 0.607; P = 0.004), (5) channel sediments (r = 0.702; P = 0.001), and (6) aquatic vegetation (r = 0.810; P < 0.001). The variables vegetation of riparian zone within 10 m of channel, bank structure, bank undercutting, stream bottom and pool-riffle or meander distances, and detritus showed no significant relationships with the faunal metrics. These contrasting results, i.e., the strong correlation between forest cover and stream habitat integrity, the weak correlation between forest cover and the faunal metrics, and the significant relation between some stream habitat variables and faunal metrics, point out an indirect relation between forest cover and faunal variables (Fig 6a–c).
Some taxa were positively correlated with HII values: Calcopteryx (Polythoridae), Anacroneuria (Perlidae), Protosialis (Sialidae), Gyrelmis, Heterelmis, Macrelmis (Elmidae), Helicopsyche (Helicopsychidae), and Macrostemum (Hydropsychidae). Conversely, Callibaetis (Baetidae), Belostoma (Belostomatidae), Tenagobia (Corixidae), Smicridea (Hydropsychidae), and Pyralidae were negatively correlated and occurred only in deforested areas.
Separate analyses by substrate type showed significant correlation only for insects dwelling in banks and HII concerning taxa composition (r = −0.591; P = 0.005) (Fig. 6d). Regarding habitat variables, taxa composition showed higher values and more significant correlations than others: (1) land use pattern beyond the riparian zone (r = −0.614; P = 0.003 for banks), (2) width of riparian forest (r = −0.612; P < 0.003 for riffle litter), (3) completeness of riparian forest (r = 0.628; P = 0.002 for sand substrate), (4) vegetation of riparian zone within 10 m of channel (r = −0.594; P = 0.005 for banks), (5) retention devices (r = −0.690; P = 0.001 for banks), and (6) aquatic vegetation (r = 0.685; P < 0.001 for riffle litter). The latter variable was the only significantly correlated with other metrics (r = 0.678; P = 0.001 for insect richness in pool litter and r = 0.580; P = 0.006 for insect richness in riffle litter).
Discussion
Forest cover condition
Stream habitat attributes closely matched the vegetation cover condition, as initially expected. However, there were no significant relationships between modifications in the vegetation cover and variables such as bank structure and undercutting, stream substrate and pool-riffle or meander distances. Some of these variables are also dependent on the stream size, slope, and geological features of the sampling areas (Gordon et al., 1992; Wood & Armitage, 1997; Church, 2002). Moreover, depending on vegetation cover type or land use (e.g., cattle raising, different agricultural practices, forestry), changes in vegetation cover may lead to few or discrete physical changes in the streams (Allan et al., 1997; Harding et al., 1999; Price & Leigh, 2006). The environmental quality and proportion of the secondary forests at the buffer zone around the sampled stream reaches may have influenced our results. The streams included in the present study are characteristic of the region; they have no rocky substrates and streambeds are mainly constituted of sand and detritus (Zuanon & Sazima, 2004; Mendonça et al., 2005). In addition, few differences were indicated in HII between substrates with different proportions of clay and silt, although significant increase was expected in sediment input (Allan et al., 1997; Wood & Armitage, 1997; Nerbonne & Vondracek, 2001; Church, 2002). Sediment inputs induce siltation within and on the surface of the substrate, leading to habitat modifications, disturbance of trophic resources and in feeding mechanisms of stream fauna (Fossati et al. 2001; Mol & Ouboter, 2004). In the present study, only two pasture streams showed this condition.
There was no significant relationship between the amount of detritus (litter) and vegetation cover. Davies et al. (2005) compared streams with different logging intensity in Tasmania and showed a decrease in the input and retention of organic matter. De Long & Brusven (1994) suggested that lower rates of allochthonous input may result in a system with detrital dynamics which bears macroinvertebrate communities different from those found in comparable undisturbed streams. According to Webster et al. (1990), differences in litter input between disturbed and undisturbed catchments seem to be due to the replacement of the original mature vegetation by successional species. Successional forests, which consist of herbaceous species and small shrubs, typically contribute less litter to a stream than mature forests. Furthermore, the absence of large limbs and fallen trees reduces stream capacity to retain and process organic material after entering the system (Webster et al., 1990). Besides, sedimentation may diminish the availability of detritus by burying leaf packs (Fossati et al., 2001; Mol & Ouboter, 2004). Nevertheless, Mortati (2004) did not find significant differences in detritus availability in the streams included in the present study, although a reduction of detritus was expected in open areas. The 20-year-old second growth forest that comprises the riparian vegetation along one of these streams reaches up to 20 m tall and shows a complex, highly structured environment that may have contributed to restore and maintain a detritus source and processing.
Faunal metrics
Despite the fact that streamlets in primary forest areas had largest number of insect taxa, there was no significant relationship between the proportion of primary forest around the stream reaches and insect richness. Fidelis da Silva (2006) showed that the decrease of macroinvertebrate taxa richness in these same streams was related to proportion of pasture and gaps in canopy cover. Roque & Trivinho-Strixino (2000) and Roque et al. (2003) compared forested and non-forested sites in Atlantic Forest streams in the State of São Paulo, Brazil, and found highest values for taxonomic richness in stream sections with intact riparian zones. On the other hand, in a study of Atlantic Forest streams in the State of Rio de Janeiro, Brazil, Egler (2002) found significant differences between species richness from forested and cultivated areas, but not between forested and deforested or second growth areas.
Although pasture streams showed significantly lower values of richness, none of the habitat variables, nor HII or percentage of forest cover, were good predictors to taxa richness. We expected some significant relationships, because the width and structure of riparian forest and aquatic vegetation are associated features related to environmental integrity in regard to canopy openness and light (Petersen, 1992). As mentioned above, the lack of riparian forest directly contributes to an increase of sediment and decrease of organic matter inputs, influencing the structure of the macroinvertebrate community by reducing habitat availability or by making the habitat unsuitable for survival (Vannote et al., 1980; Sizer, 1992; Wood & Armitage, 1997; Nerbone & Vondraek, 2001; Zweig & Rabeni, 2001; Sparovek et al., 2002; Davies et al., 2005). However, relationships between aquatic insect and EPT richness with sediment and organic matter inputs were not significant in this study. These two insect metrics only showed differences across sites in relation to HII values and percentage of vegetation cover. Aquatic insect assemblages were strongly altered and impoverished in highly disturbed sites with very low vegetation cover.
The results related to the taxonomic composition suggest a replacement of faunal components associated with characteristics of the physical environment and habitat integrity but a single marginally significant relationship was obtained with HII. Some factors seem to have stronger impact on the taxonomic changes in aquatic insect communities, such as riparian forest width and structure, canopy openness, retention devices, aquatic vegetation and sediments. Retention devices are important in keeping high habitat heterogeneity (Barbour et al., 1999). Many insect taxa were absent or represented by smaller numbers of individuals under low HII values, while others such as grazers and algal piercers showed increasing number of individuals under the same conditions. These results corroborate several studies that pointed out changes in insect taxonomic composition and function related to deforestation (e.g. De Long & Brusven, 1994; Barbour et al., 1996; Naiman & Decamps, 1997; Benstead et al., 2003; Benstead & Pringle, 2004; Silveira et al., 2005).
The absence of significant correlations between habitat variables, HII scores, and insect taxonomic composition in pool and riffle litter (except width of riparian forest and aquatic vegetation for riffle litter) suggests that assemblage composition is more homogeneous in these substrates. Alternatively, these assemblages may only be affected in terms of composition by impacts that drastically modify the habitat. However, there were significant relationships between habitat integrity and insect composition in banks. Similar results were found by Roy et al. (2003) and were attributed to banks acting as refuges for the fauna from other substrates in streams under perturbation.
We observed conspicuous gaps in the distribution of values for biological variables and HII values between areas with no forest and those presenting small percentages of vegetation cover. Even limited riparian vegetation seems to maintain the distinctive habitat characteristics in streams that are essential for the occurrence of many insect taxa.
Our results highlight the importance of riparian vegetation in the maintenance of the biota of streams and its function as ecological corridors (e.g., Naiman & Decamps, 1997; De Lima & Gascon, 1999; Anbumozhi et al. 2005; Nakamura & Yamada, 2005). Four factors related to the present study are worth further discussion: the importance of secondary forests, the extensive area of primary forest around the pasture matrix of the study area, that some streamlets cross areas with different vegetation, and the potential capacity of dispersion in aquatic insects.
Both forest fragments and secondary forests present faunal characteristics similar to the continuous forest areas in most cases. Older secondary forests behave like forests, with reduced light incidence, recovery of retention devices on streambeds, and less loss of sediments to streamlets. Younger secondary forests are more open, dominated by shrubs, herbs, and grasses. They present higher light incidence and less capacity to keep sediments from entering the streamlets. Abandoned pasture areas may present variable growth of secondary forests. In some settings, a field abandoned for 2 years can have 4-m-tall woody vegetation, while elsewhere a similarly aged plot could still be dominated by grasses and sedges (Walker et al., 1999). Thus, the habitat matrix presents great heterogeneity, and the secondary forest may play an important role in the connectivity among the forest areas, which facilitates the dispersal of aquatic insects as well as the colonization of streams.
Depending on the area or their position, streams in forest fragments suffer more or less edge effects, which allows the entrance of habitat matrix specimens. On the other hand, parts of streams that cross pasture areas or secondary forests are influenced by upstream forests. The isolation of forest fragments depends on the distance of continuous forest areas or other fragments and on the connectivity with secondary forests. In the study area, the distance from fragments and secondary forests to primary forest areas is not longer than 1 km. Another feature to be taken into account is the occurrence of at least one narrow riparian corridor in most studied streamlets.
The dispersal of aquatic insects may occur longitudinally (in the same stream) or transversally, among watersheds (Malmqvist, 2002; Elliott, 2003; Macneale et al., 2005). Some species have great capacity of dispersal (further than 1 km), as in Ephemeroptera, Odonata, and Coleoptera (Bilton et al., 2001; Petersen et al., 2004; Macneale et al., 2005). Since one of the main determinant colonization factors is adequate substrate (Sanderson et al., 2005), its occurrence, even in small proportion, may favor the presence of a determined taxon. Distance is an important factor for dispersal. Petersen et al. (2004), during studies in the UK, observed that the number of Plecoptera, Ephemeroptera, and Trichoptera adults captured decreases as the distance of the river increases. However, they did not find differences between forests and deforestation areas in relation to the occurrence of dispersal. Sanderson et al. (2005) compared macroinvertebrate assemblages at 188 running-water sites in the catchment of the River Rede, northeast England, and concluded that there was a significant influence of the species composition of neighboring sites on determining local species assemblages.
These previously published results support our findings in some way. They might explain that the low faunal metrics response in relation to changes in vegetation cover and to fragmentation can be due to the effect of the heterogeneous matrix, as well as, the presence and proximity of a wide area of surrounding forests in the present study.
References
Adams, J. B., D. E. Sabol, V. Kapos, D. A. Roberts, M. O. Smith & A. R. Gillespie, 1995. Classification of multispectral images based on fractions of end members: Application to land-cover change in the Brazilian Amazon. Remote Sensing of Environment 52: 137–154.
Allan, D., D. L. Erickson & J. Fay, 1997. The influence of catchment land use on stream integrity across multiple spatial scales. Freshwater Biology 37: 149–161.
Alves, S. D. & D. Skole, 1996. Characterizing land cover dynamics using multi-temporal imagery. International Journal of Remote Sensing 17: 835–839.
Anbumozhi, V., J. Radhakrishnan & E. Yamaji, 2005. Impact of riparian buffer zones on water quality and associated management considerations. Ecological Engineering 24: 517–523.
Angrisano E. B., 1995. Insecta Trichoptera. In Lopretto, E. C. & G. Tell (eds), Ecosistemas de Águas Continentales. Ed. SUR, La Plata: 1199–1238.
Barbour, M. T., J. Gerritsen, G. E. Griffith, R. Frydenborg, E. McCarron, J. S. White & M. L. Bastian, 1996. A framework for biological criteria for Florida streams using benthic macroinvertebrates. Journal of the North American Benthological Society 15: 185–211.
Barbour, M. T., J. Gerritsen, B. D. Snyder & J. B. Stribling, 1999. Rapid Bioassessment Protocols for Use in Streams & Wadeable Rivers: Periphyton, Benthic Macroinvertebrates & Fish, 2nd edn. United States Environmental Protection Agency, EPA, 841-B-99-002, Washington DC.
Belle, J., 1992. Studies on ultimate instar larvae of Neotropical Gomphidae, with description of Tibiagomphus gen. nov. (Anisoptera). Odonatologica 2: 1–25.
Benstead, J. P. & C. M. Pringle, 2004. Deforestation alters the resource base and biomass of endemic stream insects in eastern Madagascar. Freshwater Biology 49: 490–501.
Benstead, J. P., M. M. Douglas & C. M. Pringle, 2003. Relationships of stream invertebrate communities to deforestation in eastern Madagascar. Ecological Applications 13: 1473–1490.
Benjamini, Y. & Y. Hochberg, 1995. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society B 57: 289–300.
Bierregaard, R. O., Jr., C. Gascon, T. E. Lovejoy & R. Mesquita (eds), 2001. Lessons from Amazonia: The Ecology and Conservation of a Fragmented Forest. Yale University Press, New Haven.
Bilton, D. T., J. R. Freeland & B. Okamura, 2001. Dispersal in freshwater invertebrates. Annual Review of Ecology and Systematics 32: 159–181.
Carvalho, A. L. & E. R. Calil, 2000. Chaves de identificação para as famílias de Odonata (Insecta) ocorrentes no Brasil, adultos e larvas. Papéis Avulsos de Zoologia 41: 223–241.
Church, M., 2002. Geomorphic thresholds in riverine landscapes. Freshwater Biology 47: 541–557.
Gotelli, N. & R. K. Colwell, 2001. Quantifying biodiversity: Procedures and pitfalls in the measurement and comparison of species richness. Ecology Letters 4: 379–391.
Da Silva, E. R., F. F. Salles, J. L. Nessimian & L. B. N. Coelho, 2003. A identificação das famílias de Ephemeroptera (Insecta) ocorrentes no Estado do Rio de Janeiro: Chave pictórica para as ninfas. Boletim do Museu Nacional 508: 1–6.
Davidson, E. A., C. Neill, A. V. Krusche, V. V. R. Ballester, D. Markewitz & R. O. Figueiredo, 2004. Loss of nutrients from terrestrial ecosystems to streams and the atmosphere following land use change in Amazonia. In Ecosystems and Land Use Change. Geophysical Monograph Series: 147–158.
Davies, P. E., L. S. J. Cook, P. D. McIntosh & S. A. Munks, 2005. Changes in stream biota along a gradient of logging disturbance, 15 years after logging at Ben Nevis, Tasmania. Forest Ecology and Management 219: 132–148.
De Long, M. D. & M. A. Brusven, 1994. Allochthonous imput of organic matter from different riparian habitats of an agriculturally impacted stream. Environmental Management 18: 59–71.
Egler, M., 2002. Utilizando a comunidade de macroinvertebrados bentônicos na avaliação da degradação de ecossistemas de rios em áreas agrícolas. Master’s Thesis, ENSP, FIOCRUZ, Rio de Janeiro.
Elliott, J. M., 2003. A comparative study of the dispersal of 10 species of stream invertebrates. Freshwater Biology 48: 1652–1668.
Fossati, O., J. G. Wasson, C. Hery, R. Marin & G. Salinas, 2001. Impact of sediment releases on water chemistry and macroinvertebrate communities in clear water Andean streams (Bolivia). Archiv für Hydrobiologie 151: 33–50.
De Lima, M. G. & C. Gascon, 1999. The conservation value of linear forest remnants in Central Amazonia. Biological Conservation 91: 241–247.
Dufrêne, M. & P. Legendre, 1997. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecological Monographs 67: 345–366.
Fidelis da Silva, L. 2006. Estrutura da comunidade de insetos aquáticos em igarapés na Amazônia Central, com diferentes graus de preservação da cobertura vegetal e apresentação de chave de identificação para gêneros de larvas da ordem Odonata. Master’s Thesis, UFAM/INPA, Manaus.
García, L. V., 2004. Escaping the Bonferroni iron claw in ecological studies. Oikos 105: 657–663.
Gascon, C. & R. O. Bierregaard Jr., 2001. The Biological dynamics of forest fragments project—The study site, experimental design and research activity. In Bierregaard, R. O., Jr., C. Gascon, T. E. Lovejoy & R. Mesquita (eds), Lessons from Amazonia: The Ecology and Conservation of a Fragmented Forest. Yale University Press, New Haven: 31–45.
Gascon, C., W. F. Lawrence & T. E. Lovejoy, 2001. Fragmentação florestal e biodiversidade na Amazônia Central. In Garay, I. & B. F. S. Dias (orgs), Conservação da biodiversidade em ecossistemas tropicais: avanços conceituais e revisão de novas metotologias de avaliação e monitoramento. Editora Vozes, Petrópolis: 112–127.
Gordon, N. D., T. A. McMahon & B. L. Finlayson, 1992. Stream hydrology. An introduction for ecologists. John Wiley & Sons, Chichester.
Hammer, O., D. A. T. Harper & P. D. Ryan, 2001. Past. Paleontological statistic software for education and data analysis. Paleontologia Eletronica 4(1): 9 pp.
Harding, J. S., R. G. Young, J. W. Hayes, K. A. Shearer & J. D. Stark, 1999 Changes in agricultural intensity and river health along a river continuum. Freshwater Biology 42: 345–357.
Lawrence, W. F., 2001. The Hyper-Diverse Flora of the Central Amazon: An Overview. In Bierregaard, R. O. Jr., C. Gascon, T. E. Lovejoy & R. Mesquita (eds), Lessons from Amazonia: The Ecology and Conservation of a fragmented forest. Yale University Press, New Haven and London: 47–53.
Lovejoy, T. E., R. O. Bierregaard, J. M. Rankin & H. O. R. Schubart, 1983. Ecological dynamics of tropical forest fragments. In Sutton, S. L., T. C. Whitmore & A. C. Chadwick (eds) Tropical Rain Forest: Ecology and Management. Blackwell Scientific Publication, Oxford: 377–384.
Macneale, K. H., B. L. Peckarsky & G. E. Likens, 2005. Stable isotopes identify dispersal patterns of stonefly populations living along stream corridors. Freshwater Biology 50: 1117–1130.
Malmqvist, B., 2002. Aquatic invertebrates in riverine landscapes. Freshwater Biology 47: 679–694.
Manzo, V., 2005. Key to the South America of Elmidae (Insecta: Coleoptera) with distributional data. Studies of Neotropical Fauna and Environment 40: 201–208.
McClain, M. E. & H. Elsenbeer, 2001. Terrestrial inputs to Amazon streams and internal biogeochemical processing. In McClain, M. E., E. Victoria & J. Rishey (eds), The Biogeochemistry of the Amazon Basin. Oxford University Press, Oxford: 185–207.
McCune, B. & M. J. Mefford, 1999. Multivariate Analysis of Ecological Data. Version 4.14 MjM Software, Gleneden Beach, Oregon.
Melo, E. G. F., M. S. R. Silva & S. A. F. Miranda, 2005. Influência antrópica sobre águas de igarapés na cidade de Manaus – Amazonas. Caminhos de Geografia 5: 40–47.
Mendonça, F. P., W. E. Magnusson & J. Zuanon, 2005. Relationships between habitat characteristics and fish assemblages in small streams of Central Amazonia. Copeia 4: 750–763.
Merritt, R. W. & K. W. Cummins (eds), 1996. An Introduction to the Aquatic Insects of North America. Kendall/Hunt Publishing Company, Dubuque.
Mol, J. H. & P. E. Ouboter, 2004. Downstream effects of erosion from small-scale gold mining on the instream habitat and fish community of a small neotropical rainforest stream. Conservation Biology 18: 201–214.
Moreira, M. P., 2002. O uso de sensoriamento remoto para avaliar a dinâmica de sucessão secundária na Amazônia Central. Master’s Thesis, INPA/UFAM, Manaus.
Mortati, A. F., 2004. Colonização por peixes no folhiço submerso: implicações das mudanças na cobertura florestal sobre a dinâmica da ictiofauna de igarapés na Amazônia Central. Master’s Thesis, INPA/UFAM, Manaus.
Naiman, R. J. & H. Decamps, 1997. The ecology of interfaces: Riparian zones. Annual Review of Ecology and Systematics 28: 621–658.
Nakamura, F. & H. Yamada, 2005. Effects of pasture development on the ecological functions of riparian forests in Hokkaido in northern Japan. Ecological Engineering 24: 539–550.
Nerbone, B. A. & B. Vondracek, 2001 Effects of local land use on physical habitat, benthic macoinvertebrates and fish in the Whitewater River, Minesota, USA. Environmental Management 28: 87–99.
Nieser, N. & A. L. Melo, 1997. Os heterópteros aquáticos de Minas Gerais. Editora UFMG, Belo Horizonte.
Olifiers, M. H., L F. M. Dorvillé, J. L. Nessimian & N. Hamada, 2004. A key to Brazilian genera of Plecoptera (Insecta) based on nymphs. Zootaxa 651: 1–15.
Oliveira, A. A. & S. Mori, 1999. A central Amazonian terra firme forest. I. High tree species richness on poor soils. Biodiversity and Conservation 8: 1219–1244.
Pes, A. M. O., N. Hamada & J. L. Nessimian, 2005. Chaves de identificação de larvas para famílias e gêneros de Trichoptera (Insecta) da Amazônia Central, Brasil. Revista Brasileira de Entomologia 49: 181–204.
Petersen, R. C., Jr., 1992. The RCE: A riparian, channel, and environmental inventory for small streams in agricultural landscape. Freshwater Biology 27: 295–306.
Petersen, I., Z. Masters, A. G. Hildrew & S. J. Ormerod, 2004. Dispersal of adult aquatic insects in catchments of differing land use. Journal of Applied Ecology 41: 934–950.
Price, P. & D. S. Leigh, 2006. Morphological and sedimentological responses of streams to human impact in the southern Blue Ridge Mountains, USA. Geomorphology 78: 142–160.
Pozo, J., E. González, J. R. Díez, J. Molinero & A. Elósegui, 1997. Inputs of particulate organic matter to streams with different riparian vegetation. Journal of the North American Benthological Society 16: 602–611.
Roque, F. O. & S. Trivinho-Strixino, 2000. Fragmentação de habitats nos córregos do Parque Estadual do Jaraguá (SP): Possíveis impactos na riqueza de macroinvertebrados e considerações para conservação in situ. In Anais do II Congresso Brasileiro de Unidades de Conservação, Campo Grande: 752–760.
Roque, F. O., S. Trivinho-Strixino, G. Strixino, R.C. Agostinho & J. C. Fogo, 2003. Benthic macroinvertebrates in streams of Jaragua State Park (Southeast of Brazil) considering multiple spatial scale. Journal of Insect Conservation 7: 63–72.
Roy, A. H., A. D. Rosemond, D.S. Leigh, M. J. Paul & B. Wallace, 2003. Habitat-specific responses of stream insects to land cover disturbance: Biological consequences and monitoring implications. Journal of North American Benthological Society 22: 292–307.
Sanderson, R. A., M. D. Eyre & S. P. Rushton, 2005. The influence of stream invertebrate composition at neighbouring sites on local assemblage composition. Freshwater Biology 50: 221–231.
Silveira, M. P., D. F. Baptista, D. F. Buss, J. L. Nessimian & M. Egler, 2005. Application of biological measures for stream integrity assessment in south-east Brazil. Environmental Monitoring and Assessment 101: 117–128.
Sizer, N. C., 1992. The impact of edge formation on regeneration and litterfall in a tropical rain forest fragment in Amazonia. Ph.D. Thesis, University of Cambridge, Cambridge.
Sparovek, G., S. B. L. Ranieri, A. Gassner, I. C. De Maria, E. Schnug, R. F. Santos & A. Joubert, 2002. A conceptual framework for definition of the optimal width of riparian forests. Agriculture Ecosystems and Environment 90: 169–175.
Smith, N. J. H., E. A. S. Serrão, P. T. Alvim & I. C. Falesi, 1995. Amazônia: Resiliency and dynamism of the land and its people. United Nations University Press, Tokyo.
StatSoft, Inc., (2001). STATISTICA (data analysis software system), version 6.
Steininger, M. K., 1996. Tropical secondary forest regrowth in the Amazon: Age, area, and change estimation with Thematic Mapper data. International Journal of Remote Sensing 17: 9–27.
Vannote, R. L., G. W. Minshall, K.W. Cummins, J. R. Sedell & C. Cushing, 1980. The river continuum concept. Canadian Journal of Fisheries and Aquatic Sciences 37: 130–137.
Walker, R., W. Salas, G. Urquhart, M. Keller, D. Skole & M. Pedlowski (orgs), 1999. Secondary vegetation: ecological, social, and remote sensing issues. Report on the workshop. “Measurement and Modeling of the Inter-Annual Dynamics of Deforestation and Regrowth in the Brazilian Amazon”. Florida State University.
Webster, J. R., S. W. Golladay, E. F. Benfield, D. J. D’Angelo & G. T. Peters, 1990. Effects of forest disturbance on particulate organic matter budgets of small streams. Journal of North American Benthological Society 9: 120–140.
Wiggins, G. B., 1996. Larvae of North American caddisfly genera (Trichoptera), 2nd edn. University of Toronto Press, Toronto.
Williamson, G. B., R. C. G. Mesquita, K. Ickes & G. Ganade, 1998. Estratégias de árvores pioneiras nos Neotrópicos. In Gascon, C. & P. Moutinho (eds), Floresta Amazônica: Dinâmica, Regeneração e Manejo. Instituto Nacional de Pesquisas da Amazônia (INPA), Manaus, Amazonas, Brazil: 131–144.
Wood, P. J. & P. D. Armitage, 1997. Biological effects of fine sediment in the lotic environment. Environmental Management 21: 203–207.
Zuanon, J. & I. Sazima. 2004. Natural history of Stauroglanis gouldingi (Siluriformes: Trichomycteridae), a miniature sand-dwelling candiru from central Amazonia streamlets. Ichthyological Exploration of Freshwaters 15: 201–208.
Zweig, L. D. & C. H. Rabeni, 2001. Biomonitoring for deposited sediment using benthic invertebrates: A test on 4 Missouri streams. Journal of the North American Benthological Society 20: 643–657.
Acknowledgments
This work was supported by the BDFFP, FAPEAM PIPT 2004–2006, Fundação O Boticário de Proteção à Natureza (0630_20041), and CNPq (Edital Universal 2005–2007). We thank Ocírio Pereira and José Ribamar Marques de Oliveira for invaluable field assistance. Ana Maria Oliveira Pes (DCEN, INPA), Alcimar do Lago Carvalho (Museu Nacional, UFRJ), Elidiomar Ribeiro da Silva (UNI-RIO), and Maria Inês da Silva dos Passos (IB, UFRJ) helped with the identification of the insects. Darcílio Fernandes Baptista (FIOCRUZ) provided valuable comments and suggestions on this manuscript. Daniela Maeda Takiya (UFPR) revised the final English text. The manuscript was greatly improved by comments and critical reviews from Dr. Joel Trexler and two anonymous referees. This is contribution number 09 of Igarapés Project and contribution number 515 of the BDFFP Technical Series.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publication number 515 of the PDBFF Technical Series. PDBFF-INPA/STRI (Biological Dynamics of Forest Fragments Project, Instituto Nacional de Pesquisas da Amazonia and Smithsonian Tropical Research Institute) and number 09 of the Igarapés Project.
Handling editor: J. Trexler
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nessimian, J.L., Venticinque, E.M., Zuanon, J. et al. Land use, habitat integrity, and aquatic insect assemblages in Central Amazonian streams. Hydrobiologia 614, 117–131 (2008). https://doi.org/10.1007/s10750-008-9441-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-008-9441-x