Abstract
Fishponds built across streams can greatly affect their functioning, especially through loss of ecological continuity but also changes in water availability and trophic resources. Yet, their consequences on communities and stream functioning remain largely understudied. We investigated effects of fishpond dams on the trophic ecology of macroinvertebrate communities in temporary low-order streams using C and N stable isotopes. Food resources and macroinvertebrates were sampled in one upstream and one downstream site of two temporary streams, one stream without (reference stream) versus one with a fishpond (impacted stream) and used for isotopic analyses. Results suggested moderate effects of fishponds on the upstream tributaries. In contrast, at the downstream impacted site, ten times higher macroinvertebrate biomass and modifications in the trophic niches were recorded, likely due to changes in resource availability/quality and dam-related hydrology. By modifying the food sources as well as water fluxes, fishpond dams tend to alter macroinvertebrate communities but also shift the trophic dynamics downstream. This assessment stresses the need for exploring their impacts on food webs and nutrient fluxes at larger downstream distances to better understand their effects before drawing conclusions in regard to their management.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fishponds, created by the construction of a dam on low-order streams and used for fish production, are very common agroecosystems across the Earth (Oertli & Frossard, 2013). These artificial ponds are usually considered factors causing stream alterations (Water Framework Directive: WFD, European Union, 2000). Therefore, they are highly criticised and their elimination is promoted by the WFD because they can lead to hydromorphological, chemical and ecological impairment in streams disturbing the natural physico-chemical and ecological continuum (e.g. Bunn & Arthington, 2002; Elosegi & Sabater, 2013; González et al., 2013; Four et al., 2017a, b). Yet, these alterations can be deeply influenced by the management of the fishponds. Among them, the degree of intensification of the system (e.g. the density of fish and/or the use of fertiliser/food) and/or the management of the dam are practices that can deeply influence the effect of the fishponds on the receiving streams (e.g. Banas et al., 2002; Gaillard et al., 2016a; Four et al., 2017b). These systems are known to provide multiple ecosystem services (Millenium Ecosystem Assessment (MEA), 2005; Aubin et al., 2014; Mathé & Rey-Valette, 2015), such as ensuring fish production and high levels of alpha diversity in and around the aquatic ecosystem (i.e. increase of plant and bird species richness; Pinet & Hélan, 2015; Ramsar convention, 1971). Additionally, when they are managed extensively (without using fertiliser and/or food) in agricultural landscape, some studies have shown that the presence of these agroecosystems along streams can also favour a decrease of suspended matter, pesticide and nutrient contents of the downstream water (Banas et al., 2002; Gaillard et al., 2016a, b). Since fishponds have been shown to have variable effects on low-order streams, it appears crucial to better evaluate the different potential alterations before drawing relevant conclusions in regard to their management. Surprisingly, only a few studies have investigated changes in organic matter (OM) caused by agroecosystems, especially by fishpond dams in low-order streams (Banas et al., 2002, 2008). Yet, that is now well known that environmental conditions such as the trophic niches (i.e. food origins, quality and quantity of the food sources) can deeply influence the food webs (i.e. the communities and the network of consumer-resource interactions) in a given ecosystem (Majdi et al., 2018). Therefore, analysing food webs allows more realistic representation of the consumption relationships between the diverse food sources and the different taxa of the community and consequently of energy flows in ecosystems that is required to better understand the effect of fishpond on ecosystem functioning (Perkins et al., 2014).
Stream food webs are mostly supported by three energy sources: terrestrially derived allochthonous material (such as leaf litter or dissolved organic matter derived from soils), in-stream autochthonous production (such as biofilms, macroalgae, bryophytes and/or macrophytes) and the transferring material (a mix of autochthonous production in upstream reaches and allochthonous material) (Vannote et al., 1980). Furthermore, in low-order streams without fishponds, most of the available energy supporting the food web originates from the surrounding river basin, their functioning is therefore highly dependent on allochthonous materials (Fisher & Likens, 1973). In contrast, fishponds strongly promote the production of autochthonous materials, such as phytoplankton and macrophytes (e.g.Wetzel, 2001; Banas & Masson, 2003; Torremorell et al., 2009; Nõges et al., 2010), that can be ultimately transferred downstream. Overall, compared to allochthonous material, autochthonous OM is generally composed of higher FPOM/CPOM (Fine Particulate Organic Matter/Coarse Particulate Organic Matter) ratios and exhibits higher potential degradability (e.g. lower C/N ratios, less refractory compounds) and more polyunsaturated fatty acids (Banas & Masson, 2003; Brett et al., 2009). Autochthonous material is thus generally thought to be a higher quality resource for consumers than allochthonous OM. Since resource quality has long been acknowledged as a major determinant of community structure and ecosystem functioning (e.g. Vannote et al., 1980; Sterner & Elser, 2002; Doucett et al, 2007), fishpond-induced changes in basal resource quality might greatly impact the functioning of downstream ecosystems.
To better understand the fishpond effects on forested temporary low-order stream functioning, we studied trophic niches in stream food webs (i.e. the invertebrate communities). We hypothesised that the presence of fishponds affecting the whole invertebrate communities i.e. increasing the generalist feeder (e.g. omnivorous and filter feeder) densities (Four et al. 2017a, b; Merritt & Cummins, 1996; Vannote et al., 1980), but also (i) changes the trophic niches of the whole communities by increasing the use of autochthonous resources (Guilpart et al., 2012) and, in turn, (ii) changes the trophic dynamics in forested low-order streams affecting the ecological integrity of those environments (e.g. Thorp & Delong, 1994). To test these hypotheses, we compared upstream and downstream sites of a reference stream (without fishpond) and an impacted stream (with a fishpond between the upstream and downstream site) by using C and N stable isotopes as biomarkers to characterise changes in isotopic niches (Bearhop et al., 2004) and in the relative contribution of the food resources to the diet of macroinvertebrates (e.g. Post, 2002; Boecklen et al., 2011; Layman et al., 2012). Bayesian isotope mixing models were used as they offer a quantitative approach for estimating the relative contribution of multiple food resources to the diet of an organism (e.g. Parnell et al., 2010). Finally, recently developed metrics based on the functional diversity and on the isotopic space allowed us to weigh these isotopic approaches with the structure of the community giving us the ability to evaluate global trophic dynamics changes in line with the presence of a fishpond dam (Cucherousset & Villéger, 2015).
Materials and methods
Study sites
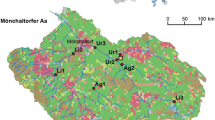
The study was carried out in two first-order temporary streams located on the Sarre river basin (Great East Region, North-eastern France; Fig. S1). The climate is temperate with an average annual air temperature of around 10.7°C and mean annual precipitation of 800 mm (30-year average, Château-Salins, Meteo France 2011). To investigate the effect of fishponds on the low-order stream trophic functioning, we selected two hydrosystems: one stream hampered by a fishpond dam (noted as impacted stream) and one stream without fishpond (considered as a reference stream). These two hydrosystems are located in a very closed geographical area (adjacent catchments in the same forest) and were representatives of the forest pond-stream ecosystems previously studied in this area (see Four et al., 2017a, b). Care was taken to have similar environmental characteristics in terms of lotic system (regardless of the fishpond presence), soil occupation and forest management. Both catchments were covered with deciduous forest (mainly Carpinus betulus L., Fagus sylvatica L. and Quercus spp.) of homogeneous development stage suggesting similar allochthonous inputs in streams. The dominant riparian vegetation at each sampling site is given in Table 1. The two streams also had similar water characteristics (at least at upstream sites; Table 1). At downstream fishpond site the temperature was significantly higher of 1.7°C on average during the period under investigation i.e. January to April 2015 (Four et al., 2017b). However, all sampling sites were at good ecological status according to the French “water guide” SEQ-eau (MEED-French Water Agency, 2003).
For each of the two studied streams, we selected two sampling sites along an upstream—downstream gradient with similar distance from the head of the catchment and similar catchment size. On the impacted stream, the fishpond is located in between the upstream and downstream sampling sites. The four sampling sites corresponded to upstream from the fishpond (named UF), downstream from the fishpond (DF), upstream reference (UR) and downstream reference (DR).
The selected streams showed strong seasonal drying patterns, with a drying period from late spring to autumn, and then a flowing period from autumn to spring. At the DF site, water flow was maintained during the summer season due to leaks from the dam that is a common alteration with fishpond dams (Four et al., 2017a, b). The two lotic systems were inhabited by a few aquatic plant species dominated by bryophytes (mainly Fontinalis antipyretica Hedw.), except at the DF site that was characterised by an absence of aquatic vegetation (BF pers. obs.). At the four sampling sites, substrates were dominated by clay, silt and gravels.
The fishpond area is 4.7 ha. It was created in the Middle Ages and it is extensively managed for fish polyculture (mainly Cyprinus carpio L. 1758, Rutilus rutilus L. 1758, Perca fluviatilis L. 1758 and Esox lucius L. 1758). Management operation corresponds to a three-year cycle production with three steps. The first corresponds to the filling of the pond, due to water inputs from small tributaries and precipitation events. The second step corresponds to a pseudo-balance phase during which fish are stocked and grown in fishponds (3 years for this fishpond), without any artificial input (no fertiliser and no food added). Finally, during the third and last step, ponds are emptied to allow fish harvest. Our study was conducted during the second step, 2 years after the last emptying.
Sample collection and processing
Due to the dry periods that characterise first-order streams in this region (observed in all studied points except downstream from the pond) invertebrate taxa were expected to exhibit annual cycles with both aquatic and terrestrial stages and/or desiccation-resistant stages to survive during the dry periods buried in the streambed sediments. Thus, to have a maximum number of taxa, samples were taken at the end of March 2015. This period corresponds to the end of the macroinvertebrate aquatic stages, before most insect emergence (in the Great East region). At each site, various habitats were identified and their proportions were evaluated. Then, ten samplings were performed by Surber net (20*25 cm aperture, 500 µm mesh) for each different habitat identified with respect to their proportion in stream. The objective of this sampling was to obtain the best representation of the whole structure and composition of the macroinvertebrate communities. At the same time, we collected samples of each potential food resource for the four studied sampling sites: leaf litter (in stream), biofilm (on gravel, for UF, UR and DR, and on wood for DF) and mud (the first 5th cm deep). Furthermore, additional food resources recorded in parts of all sites were also collected (i.e. bryophytes, collected at UF, UR and DR, and filamentous green algae at DF). Water samples were collected at each site 0.5, 1 and 1.5 months before the invertebrate sampling date, in order to sample fine Suspended Organic Matter (SOM) in the water column. Leaf litter was considered as allochthonous resource, biofilm and bryophytes as autochthonous resources, SOM as transferring resource and mud as a pool of decaying detritus resources.
At the laboratory, macroinvertebrates were identified to the lowest practicable taxonomic level (i.e. species, if not genus or family depending on the invertebrate, Tachet et al., 2010). They were then counted and assigned to a Functional Feeding Group (FFG): scrapers, shredders, collector-gatherers, collector-filterers and predators and others according to Merrit & Cummins (1996; Table S1; “others” taxa correspond to pooled rare taxa for which each taxa represented alone less than one percent of the whole community at a given sampling site). Shredders were divided into insect-shredders (Insecta) and shrimp-shredders (Crustacea) because shrimp-shredders may have a generalist omnivore behaviour, feeding on multiple resources (Cogo et al., 2014; de Castro et al., 2016).
Prior to analysis, molluscs were removed from their shells to avoid contamination from non- dietary carbonates (Bunn et al., 1999). Then, organisms were dried (48 h at 55°C), and dried biomass and proportion of each taxon in all samples were measured for each site. Stable Isotope Analysis (SIA) was performed for all taxa that made up at least 1% of the community on at least one site. Concerning predators, all the samples presenting enough material for analysis were also kept for SIA in order to have an overview of the isotopic signature of the macroinvertebrate communities’ top predators. Using this approach, taxa used for SIA represented at least 93.4% of the whole community (in biomass) when we consider the four studied sites. Large macroinvertebrates were used individually for SIA; small macroinvertebrates were pooled by family prior to analysis.
Concerning food resources, at each sampling site, three water samples were taken (as described above), carried to the laboratory and then filtered on SOM Whatman GF/F filter to recover the SOM. Leaf litter and bryophytes were rinsed with distilled water. For all food resources, care was taken to remove macroinvertebrates or other organic material mixed with a given food resource. Prior to analysis, subsamples of each food resource were acidified using the method described by Jacob et al. (2005); as effervescence was apparent only for some SOM subsamples (1 N HCl), acid washing was performed only for SOM samples. Then, food resources were dried (48 h at 55°C).
All dried organic samples were ground into fine and homogeneous powder with a mixer mill (RETSCH MM400) and stored in Eppendorf tubes until processing. Approximately 0.8–2 mg of dried animal tissue and 2–5 mg of food resources were used for the SIA.
The δ13C and δ15N values of all samples were determined at the INRA Technical Platform for Functional Ecology using an elemental analyser (vario ISOTOPE cube, Elementar, Hanau, Germany) interfaced in line with an isotope ratio mass spectrometer (IsoPrime 100, Isoprime Ltd, Cheadle, UK). Results were expressed in standard delta notation (δX = [(Rsample/Rstandard)−1] × 103), in part per thousand (‰), relative to standard international references (i.e. Vienna PeeDeBelemnite (V-PDB) for C and atmospheric air for N), where X is the element under investigation and R is the ratio: 13C/12C or 15N/14N. The analytical precision values of the working standard of δ13C and δ15N were ± 0.05‰.
Data analysis
Differences in the abundance, biomass, relative biomass but also in the isotopic signature of food resources and isotopic signature of FFGs among the four sampling sites (i.e. UF, DF, UR, DR) were tested using generalised mixed models to adequately deal with the non-normal distribution of the data (R glm package; Hastie & Pregibon, 1992). Furthermore, when a significant level was reached, post hoc tests (R emmeans package,Searle et al., 1980) were applied to identify the various groups. Taxonomic composition of the communities was examined among the four sites using clustering analyses (Ward method) and non-metric multidimensional scaling (NMDS; Clarke, 1993) followed by PerMANOVA analyses (McArdle & Anderson, 2001). When a significant level was reached, we also performed pairwise contrast analyses (Martinez Arbizu, 2017) to identify the significant different groups. The similarity matrix was calculated using Bray–Curtis ecological distance on log-transformed densities (abundance or biomass).
In the present study, some metrics (isotopic position, divergence and dispersion) recently proposed by Cucherousset & Villéger (2015) were also used to investigate the trophic structure of macroinvertebrate communities among the four sampling sites. These metrics are based on the isotopic diversity of the macroinvertebrate communities and integrate weighing factors of the relative biomass of each organism in the community. In this respect, this approach offers two advantages: (i) it takes into account the density (relative biomass in our study) of each taxon in the communities (for each sampling site) and (ii) it is mathematically independent of the sample size of each taxon. Those metrics are computed on an isotopic two-dimensional (2-D) standardised space, where each axis was scaled to have the same range (e.g. 0–1) for each stable isotope investigated (Figs. 2–S4). Standardisation was done to avoid isotopic diversity quantification bias due to range differences that exist between δ13C and δ15N in freshwater communities (Cucherousset and Villéger, 2015). In our study, the standardisation of the isotopic 2-D space was performed considering all invertebrate community isotopic signatures obtained at the four sampling sites (i.e. for a given site, the range of the invertebrate community for a stable isotope may be lower than 1). Those metrics use the position in the stable isotope scaled space of the different organisms (mean values and associated standard deviations) represented in the food web and the relative biomass of organisms in the community. The isotopic position metric allows measuring the isotopic centre of gravity of the community in the standardised 2-D space based on the relative biomass of each taxon and their positions in the scaled space. The isotopic divergence (IDiv) allows measuring the distribution of organisms (as a function of their relative biomass) within the convex hull. The isotopic dispersion (IDis) allows measuring the weighted-mean distance to the centre of gravity of all organisms (as a function of the relative biomass of each organism in the community).
Then, indices of similarity based on the isotopic niche of the invertebrate community were used to investigate the effect of fishponds on the trophic niches of macroinvertebrate communities as also proposed by Cucherousset and Villéger (2015). Convex hulls allow integration of organisms positioned at the edge of UF, DF, UR or DR isotopic niches in the scaled isotopic space. These indices of similarity allow comparing the community between two given sites (calculations were performed in order to compare all pair combinations).They represent the ratio between the niche volume shared and the volume of the union of the two convex hulls. They range between 0 and 1. The value ‘zero’ indicates no overlap between two trophic niches and the value ‘one’ indicates a perfect overlap. A complete description of these different indices and metrics can be found in Cucherousset and Villéger (2015).
We used Bayesian stable isotope mixing models (Parnell et al., 2010) to calculate the contribution of the food resources for the consumer FFGs. This analysis was used to finally assess the fishpond effect on stream trophic pathways. Mixing models were performed with stream food resources for all consumers (predators included). In the partition analysis, the food resources of the sites were considered separately to determine the contribution of each resource for the consumer FFGs. As mixing models may give erroneous results when the isotope ratios of food resources overlap and/or when the isotopic signatures of consumers fall outside the range of sources (Fry, 2013; Brett, 2014), we chose to pool close food resources and to remove food resources with unrealistic values before analysis. Consequently, filamentous algae and biofilm food resources were pooled. Because of the high δ15N values of copepods in comparison to the consumer FFG signatures (δ15N of 7.1–8‰ on average; Table 3), copepods were removed. Due to the low δ13C values recorded for the bryophytes in comparison to the consumer FFG signatures (except for scrapers) and to be homogeneous in the number of food resources with the DF site, bryophytes were not considered in the mixing model for the dietary reconstructions of collectors, shredders and predators. The fractionation values used in the mixing model were 0.3‰ ± 2.0‰ for C and 2.5‰ ± 2.2‰ for N (mean (± SD) values of fractionation recorded for n = 90 and n = 77 for C and N, respectively; Caut et al., 2009).
Results
Differences in the invertebrate communities among sites
Overall, in the 40 Surber samples (4 sampling sites × 10 replicates), 8,077 individuals were found. Regardless of the sampling site, among 56 identified taxa, Gammarus pulex L. 1758 accounted for more than 55% of the total biomass, whereas Glyphotaelius, Nemoura and Asellus accounted for 15.8, 4.2 and 4.2%, respectively. Siphlonuridae, Ameletidae, Stenophylax, Oligostomis, Hydropsychidae, Oligochaeta, Chironominae and Sphaeridae each accounted for 1–3%. All other taxa accounted for less than 1% of the total biomass.
Cluster analyses and NMDS ordination of communities showed clear and significant community differences among the four sites (Fig. 2; PerMANOVA results: P < 0.001 for both abundance and biomass). Also, according to pairwise comparison results, only DF is significantly different from the three others (i.e. UF, UR and DR) in abundance and biomass (Table S2). The community at that site was characterised by the absence of all the scrapers (i.e. the Siphlonuridae and Ameletidae) while those taxa represented between 7 and 12% of the total abundances and between 9 and 22% of the total biomasses at the three other sites (Fig. 2; Table 2). Abundance, total and relative biomass values for the different FFGs recorded at each measuring station and significance results are given in Table 2. Abundances recorded at the UF site was in the same range as that recorded at both reference sites (UR, DR) with on average 3302 versus 2174 and 2426 individuals m−2 for UF, UR and DR, respectively. In contrast, at the downstream fishpond site, abundance was almost three times higher with 8 198 individuals m−2 and showed a significant difference with the other three groups (stream:location: F = 9.17, df = 1, P = 0.005; for significant differences between groups see Table 2). Total biomass recorded at the UF site was in the same range as that recorded at both reference sites with on average 2.4 versus 1.8 and 1.8 g of dried invertebrates m−2 for UF, UR and DR, respectively. In contrast, at the downstream fishpond site, total biomass was almost ten times greater with 20.7 g of dried invertebrates m−2 and showed a significant difference with the other three groups (stream:location: F = 6.65, df = 1, P = 0.01). Concerning relative biomass, invertebrate communities were also similar between UF, UR and DR (no statistical differences were found, except between UF and UR and DR sites for ‘other’ and ‘gatherer’ FFGs; Table 2). According to the results from the multiple GLMs performed on the different FFGs, differences between sites were always due to at least significant differences of shrimp-shredder, scraper and gatherer densities between DF and the three other sites (UF, UR and DR). Finally, at these three sites, communities were dominated by insect–shredders, while at the DF site communities were dominated by shrimp-shredders.
Differences in the isotopic signatures of resources and consumers among sites
The isotopic signature of the studied food resources varied widely within and among the studied streams (Fig. 1a; Table 3). The lowest δ13C were recorded for bryophyte and biofilm/filamentous algae (i.e. autochthonous) resources. The highest δ13C were recorded for mud and SOM food resources. As underlined by our results, the signatures of the food resources found at the UF site and at both reference sites (UR and DR) were in the same range. In contrast, at the DF site, autochthonous resources (i.e. only biofilm/filamentous algae, given the absence of bryophytes) appeared to be 13C depleted in comparison to all other sites (Fig. 1a; Table 3). Pooled biofilm/filamentous algae and SOM were significantly 13C depleted (stream:location: F = 21.0, df = 1, P < 0.001 and F = 22.2, df = 1, P = 0.001, respectively; Table 3) with on average 8.7‰ and 4.7‰ lower value at the DF site in comparison to the other three sites. Due to the low number of samples measured for copepods, no statistical analysis was performed, but samples were on average 10.2‰ 13C depleted at this site (DF) in comparison to the downstream reference site (DR).
Representation of the δ13C and δ15N of food resources (mean ± SD) and invertebrate communities (small coloured circles) (a) and the invertebrate functional feeding groups (b) in the four sampling sites (orange = upstream fishpond = UF; red = downstream fishpond = DF; green = upstream reference = UR; dark green = downstream reference = DR)
Overall, concerning δ15N, the lowest values were recorded for the mud and the highest values were recorded for the copepods. Our results did not show significant differences between sites for this isotopic ratio.
The macroinvertebrates also exhibited wide variations in isotopic composition among the study sites and the FFGs (Figs. 1b, S3; Table 3). Globally, the isotopic signature of all the macroinvertebrate samples appeared to be 13C depleted by about 2.5‰ and slightly 15N enriched at the DF site in comparison to the other three sites (Table 3). However, we should note that the lowest δ13C values were recorded for the scrapers which were absent at the DF site. The highest δ13C values were recorded for different FFGs depending on the considered sampling site (i.e. on average: predators for UF; gatherers and shrimp-shredders for DF; filterers and shrimp-shredders for both reference sites). Concerning δ15N, values were more homogeneous among the four sites as the lowest values were globally recorded for the insect-shredders and the highest values were recorded for the predators (Table 3). As for the food resources, the UF site and both reference sites recorded similar δ13C and δ15N isotopic composition among all the FFGs (no significant differences were shown with the post hoc tests). In contrast, significant 13C-depleted values were shown for the gatherers, the shredders (shrimps and insects) and the predators when we consider the DF site. Even if no statistical analysis was performed on the filter-feeders (due to the low number/absence of replicates in several sites), they also showed important 13C-depleted values at the DF site in comparison to the other three sites. Concerning δ15N results, values were more variable among and between sites and generally did not allow us to identify significant differences among sites except for the insect-shredders for which we found significantly higher values at the DF site in comparison to both reference sites.
Variation in functional diversity and trophic niches of the macroinvertebrate communities among sites
Changes in functional diversity and trophic niches of the macroinvertebrate communities showed that the DF site differed from the other three sites (Fig. 2). At this site, the scaled isotopic position was on average 16% (± 3%) 13C depleted and 25% (± 6%) 15N enriched in comparison to the other three sites. Likewise, IDiv and IDis metrics were 1.6 (± 0.1) and 1.3 (± 0.1) times lower at the DF site than at the other three sites, respectively.
Stable isotope values of the invertebrate taxa at upstream fishpond site, downstream fishpond site, upstream reference site, downstream reference site (in orange, red, light green, dark green, respectively) in a scaled two-dimensional isotopic space (δ13C and δ15N) based on the whole community at the four sites and illustration of the isotopic diversity metrics. a isotopic position measures the biomass-weighted isotopic position of the macroinvertebrate community at each sampling site, b isotopic divergence (IDiv) measures the distribution of biomass-taxa importance within the border of the convex hull (coloured polygon; It ranges between 0 and 1. It is minimal when most of the relative biomass of the community (represented by different organisms) is close to the centre of gravity of the convex hull. IDiv is maximal when a great part of the biomass of the community is located on the edge of the convex hull, c isotopic dispersion is the biomass-weighted mean distance to the centre of gravity of all taxa at each site (It ranges between 0 and 1. It is minimal when all the organisms have close isotopic position and it is maximal when most of the relative biomass of the community is far from its centre of gravity)
Results concerning the indices of similarity based on the shared isotopic niche of the invertebrate communities among the four sampling sites showed high degree of similarity for all combinations with UF, UR and DR sites (range from 60 to 70% of similarity) while they showed marked differences for all combinations including the DF site (range from 17 to 25% of similarity; Fig. S4).
According to the similarity of the isotopic signature of the food resources and the consumers found at upstream fishpond site and both reference sites (see GLMs results in Table 3) and also according to the similarity of the communities (see PerMANOVA and pairwise comparison results in Fig. S2 and Table S2), we chose to pool the data from these three sites before applying the mixing models (Fig. 3). Consequently, only two groups were presented in the analyses hereafter (i.e. the downstream fishpond site, noted DF and pooled sites, named Pooled UF + UR + DR).
Relative contribution of the different food resources sampled to each functional feeding group [A scrapers, B collector-gatherers, C collector-filterers, D shrimp-shredders, E insect-shredder and (F) predators] at pooled “similar” sites (i.e. pooled data from upstream fishpond site and both reference sites) (left panel) and at downstream fishpond site (right panel). SOM suspended organic matter
At Pooled UF + UR + DR, scrapers assimilated more bryophytes (40% of their diet), biofilms and SOM than leaf litter or mud (Fig. 3; Table S3). As outlined above, bryophytes had very low δ13C values in comparison to the other FFGs at Pooled UF + UR + DR (i.e. gatherers, filterers, shredders and predators) and, taking into account all collected food sources, the mixing models indicated that bryophytes represented less than 5% of the diet for all these FFGs at Pooled UF + UR + DR. Furthermore, this food resource was absent at the DF site. For these reasons, we chose to remove it from the mixing models. Consequently, for these FFGs, mixing models were performed with similar food resources between the two groups (Pooled UF + UR + DR and DF). Results were presented in Fig. 3 and Table S3. Overall, mud appeared to be a low assimilated resource (2%-16% of the diets of all macroinvertebrates) regardless of the considered group. Regardless of the FFGs, leaf litter was on average more assimilated at DF than at Pooled UF + UR + DR (38% vs. 10% of the diets, respectively). In contrast, SOM was on average less assimilated at DF than at Pooled UF + UR + DR (40% vs. 66% of the diets, respectively). Similar assimilation was recorded for biofilm (19% vs 16% of the diets) and mud (5% of the diets in both cases) at Pooled UF + UR + DR and DF, respectively.
At DF, based on 95% credibility limits given by the model (provided in Table S3), gatherers, shrimp-shredders and insect-shredders assimilated more leaf litter than their homologous FFGs at Pooled UF + UR + DR (predators also exhibited similar results). In contrast, at the DF site, shrimp-shredders and insect-shredders showed on average two times lower assimilation of SOM and differences with Pooled UF + UR + DR were almost significant [mean (95% credibility limits): 34% (10–53%) vs. 79% (52–98%) for shrimp-shredders and 44% (18–65%) vs. 82% (64–97%) for insect-shredders at DF and Pooled UF + UR + DR, respectively].
Discussion
Macroinvertebrate counting, identification, community abundances and biomass showed important change of the communities downstream the fishpond as well as stable isotopes of C and N measurements. These results were used to understand how the presence of a fishpond affected macroinvertebrate communities. The calculation of isotopic diversity metrics and mixing model approaches also showed that mainly downstream the fishpond, trophic niches of invertebrates’ communities and so trophic dynamics in streams could be profoundly affected by this type of agroecosystem. Such information is critical for understanding the consequences of fishponds on stream functioning, both at the upstream and downstream sections. Indeed, the impacts of fishpond dams on temporary low-order streams are particularly understudied, despite increasing criticisms of these agroecosystems as the WFD largely promotes their elimination.
According to changes in the isotope ratios of some available food sources downstream from the fishpond, our result showed modifications of the nature of food resources at this site. That was especially the case for the autotrophic sources (i.e. pooled biofilm and algae), for the transferring matter (i.e. SOM) and for the copepods that were mainly 13C depleted relative to the other three sites (Table 3, Fig. 1). In comparison, the isotopic variations in leaf litter and mud among the four sites were smaller. Our results concerning the transferring matter and the copepods at the downstream fishpond site suggest that the nature of OM coming from the pond differs from those found in the other three sites and this may be in line with an increasing of the ratio autochthonous OM/allochthonous OM. At the UF, UR and DR sites, the differences between the 13C isotopic signatures of SOM and those of leaf litter (Fig. 1) suggest that SOM was mainly derived from decaying allochthonous material. The increase in the δ15N values recorded for SOM compared to leaf litter could be due to their colonisation by fungi and bacteria that might increase the 15N content of these resources (Costantini et al., 2014). In contrast, considering the close isotopic signature of plankton (bulk of phyto- and zooplankton) recorded into the fishpond (on average -33.9‰ and 3.6‰ for δ13C and δ15N, respectively; unpublished data) and those of the SOM from the downstream fishpond site, it confirms substantial contribution from autochthonous OM production in fishponds to the SOM at the downstream fishpond site. Furthermore, two mechanisms could explain the depletion in 13C observed for the biofilm-algae at the downstream fishpond sites. First, biofilms are known to contain bacteria, fungi and detritus (that may at least partly come from transferring matter) that could explain isotopic signature variations with the proportion of allochthonous versus autochthonous OM in the system (Rasmussen, 2010). Second, we can suggest that addition of labile food resources coming from the fishpond enhanced the heterotrophic loop downstream from the fishpond (Guenet et al, 2010; Kuehn et al., 2014). Several authors have already mentioned that this mechanism may cause 13C depletion in the isotopic signature of the autotrophic biofilm and algae due to an increase in the proportion of mineralised dissolved inorganic carbon (DIC) relative to the atmospheric DIC in the system (McCarthy et al., 1984; Perga, 2004; Finlay & Kendall, 2007; Fry, 2008).
We hypothesised a related shift in the macroinvertebrate communities and/or in their assimilated resources in the impacted stream due to labile organic matter addition and/or migration of invertebrates from the fishpond. Considering all of the results for UF, UR and DR (macroinvertebrate community structure and composition, isotopic signatures of FFGs and isotopic metrics), our work showed a limited effect of the fishponds on the macroinvertebrate communities at the upstream tributaries. This is probably due to a weak colonisation of this environment by the organisms living in the pond. This result could be explained by the much contrasted functioning between lentic and lotic ecosystems (Wetzel, 2001; Dodds & Whiles, 2010) and also by the temporary nature of these streams. As these temporary streams showed drying periods during summer, we propose that it may strongly constrain the macroinvertebrate communities living in these ecosystems (Acuña et al., 2005), limiting colonisation by the macroinvertebrate communities of the fishponds. In contrast, at the downstream fishpond site, macroinvertebrate community structure and composition were very different from the other three sites showing a stronger effect of the fishpond dam. At this site, flow rate change and thermal modification were recorded and it is now well known that these physical alterations caused by small dams can affect the downstream communities in streams (Menéndez et al., 2012; Martínez et al., 2013; Four et al., 2017a, b). Among them, fish communities could be favoured by permanent flows that could affect the macroinvertebrates communities and trophic cascades in streams. However, even if their abundance was not specifically followed in this study, their presence is probably very unlikely due to the environmental conditions in these streams. Furthermore, dam-induced hydrological alterations (shift from temporary to permanent flow downstream from the dam) due to leaks from the dam could be directly involved in the observed macroinvertebrate community change (Four et al., 2017b). This hypothesis is supported by our observations since, at this site, the community was dominated by Gammarus pulex, a taxon characterised by an essentially aquatic life cycle, while the other three sites were dominated by insects known to exacerbate terrestrial phases during dry periods (Tachet et al., 2010). Nevertheless, this previous study conducted on these streams and other similar ones in this area showed that Gammarus pulex was not dominant in permanent streams without fishpond, suggesting that flow discontinuity was not the only parameter that influenced the abundance of this taxon (Four et al., 2017b).
While dam-induced changes in stream macroinvertebrate communities have long been associated with physico-chemical changes and habitat alterations (e.g. Menéndez et al., 2012; Martínez et al., 2013), changes in the nature of food sources could constitute another non-exclusive hypothesis (e.g. Merritt & Cummins, 1996; Doucett et al., 2007). Our results showed that all the macroinvertebrate trophic groups taken individually had lower δ13C values at the downstream fishpond site compared to all other sites. This observation was corroborated by the 13C depletion recorded downstream from the fishpond also suggesting a modification of the isotopic niches downstream from the fishpond underlying a modification of the food resource—taxon interactions (Doucett et al., 2007). Similarly, the lower values of isotopic divergence and dispersion recorded at the downstream site from the fishpond could indicate a simplification of the macroinvertebrate communities or at least a decrease in functional diversity, especially with the loss of specific taxa (Siphlonuridae and Ameletidae) and/or the increase in the relative abundance of a few taxa (Cucherousset & Villéger, 2015). This result was confirmed by the loss of one trophic guild (i.e. scrapers) and by the large dominance of Gammaridae in relative abundance at the downstream fishpond site. Another noteworthy result was the greater total invertebrate community biomass which was mainly due to the high abundance of Gammaridae at the downstream fishpond site. Gammaridae are known to exhibit generalist and omnivore behaviour (Rosemond et al., 1998; Cogo et al., 2014; Crenier et al., 2017) and consequently to have the capacity to feed and grow well on different and alternative food resources (Merritt & Cummins, 1996). In this particular environment, due to their omnivore behaviour, the complementarities between allochthonous OM (i.e. leaf litter) and autochthonous OM produced in the fishpond (in terms of density and quality) as well as the permanent nature of water flow, this taxon could have been largely favoured in comparison to more specialised taxa.
Furthermore, when we consider the priori categorised FFGs, i.e. gatherers, shrimp-shredders and insect-shredders, and predators, our results from the mixing model show that they have more generalist feeding habits downstream from the fishpond compared to other sites. At pooled sites (corresponding to samples from both reference sites and upstream fishpond site) the basal trophic food webs were mainly supported firstly by SOM (or bryophytes for scrapers) and secondly by biofilm. In this group (pooled sites), low contribution of leaf litter to the assimilated resources was shown to be even, especially for shredders. This result could suggest that due to the low nutritional value of leaf litter (Brett et al., 2009), macroinvertebrates need to complete their diet by other better quality resources (with higher nutrient and energetic contents) to fulfil their nutritional requirements (Nelson, 2011; Danger et al., 2013). In contrast, at the downstream fishpond site, the basal trophic food webs were supported by both leaf litter and SOM (except for collector-filterers). The difference of assimilated resources between the downstream fishpond site and the pooled sites could be explained by the change in the energetic and mineral content of the basal food resources with the addition of labile and nutrient rich food resources coming from the fishpond at the downstream fishpond site (Banas & Masson, 2003). We suggest that downstream from the fishpond, macroinvertebrates could better assimilate low-quality food (i.e. leaf litter) due to energetic and nutrient compensations by the autochthonous OM produced in the fishpond. However, such changes in OM quality were not investigated in this study and should certainly deserve further attention. But, the higher leaf litter assimilation found could be part of the explanation of the higher rates of litter decomposition recorded downstream from the fishpond by Four et al. (2017b).
To conclude we showed that studies of this kind can increase the knowledge of fishpond impacts on stream functioning. Certainly, fishponds are known to impair the ecological continuity of streams but, when established in temporary streams, their impact may not be as significant in the upstream temporary tributaries. In fact, we showed that fishponds might not drastically change upstream macroinvertebrate communities underlining that in temporary lotic ecosystems, ecological stream continuity appears to have a limited significance with regard to basal food web recovering (i.e. macroinvertebrate communities) because of the aerial dispersion patterns of the dominant taxa (Acuña et al., 2005), or the limited colonisation of organisms from the fishpond. This study highlighted that the quality of these ecosystems (to favour colonisation and survival of these adapted taxa) and the density of temporary streams in a same river basin (to facilitate insect cross-colonisation) are more important than the presence of fishponds to maintain temporary upstream functioning. On the other hand, our study showed that fishponds caused substantial modifications of the trophic dynamics in the downstream reaches. Since fishponds are generally found in river basins altered by human activity such as agricultural practices (Four et al., 2017a) and that they promote macroinvertebrate densities (especially that of Gammarus pulex) in streams, presence of fishponds on small streams could enhance allochthonous and autochthonous OM consumption in streams. As a consequence, that could facilitate the immobilisation and degradation of at least part of the OM surplus produced in fishponds and/or in the river basin integrating them into the food webs. Moreover, it could also favour the in-stream detoxification of agricultural pollutants with their adsorption on the transferred OM and a global intensified metabolism at the ecosystem level (Gan et al., 2004; Hameed et al., 2011). This study adds to earlier results showing that fishponds can favour stream pesticide reduction by increasing pesticide adsorption and degradation (Gaillard et al., 2016a, b). According to the complexity of the effects of anthropogenic activities (i.e. fishpond dams, agriculture and related practices in the river basins) on stream ecological processes and water quality at a larger scale, it would be relevant to integrate the spectrum of ecosystem services (Tibi & Therond, 2017) provided by fishponds in management decisions, especially in areas of intensive agricultural activities. For that, generalisation of this kind of study that integrate community change (e.g. biomass change of the different functional feeding groups of the community), trophic niche change (i.e. assimilations) and functional diversity change (based on metrics ponderated by the densities of the communities) are crucial and may help to better understand our findings and therefore help policy makers to better consider the complexity of human activities in river basins and to finally promote ecosystem services from aquatic ecosystems.
References
Acuña, V., I. Muñoz, A. Giorgi, M. Omella, F. Sabater & S. Sabater, 2005. Drought and postdrought recovery cycles in an intermittent Mediterranean stream: structural and functional aspects. Journal of the North American Benthological Society 24: 919–933.
Aubin J., H. Rey-Valette S. Mathé M. Legendre J. Slembrouck, E. Chia, G. Masson, M. Callier, J.-P. Blancheton, A. Tocqueville, D. Caruso & P. Fontaine, 2014. Guide de mise en œuvre de l’intensification écologique pour les systèmes aquacoles. Inra-Rennes. 131 p.
Banas, D. & G. Masson, 2003. New plate sediment traps for lentic systems. Archiv für Hydrobiologie 158: 283–288.
Banas, D., G. Masson, L. Leglize & J. C. Pihan, 2002. Discharge of sediments, nitrogen (N) and phosphorus (P) during the emptying of extensive fishponds: effect of rain-fall and management practices. Hydrobiologia 472: 29–38.
Banas, D., G. Masson, L. Leglize, P. Usseglio-Polatera & C. E. Boyd, 2008. Assessment of sediment concentration and nutrients loads in effluents drained from extensively-managed fishponds in France. Environmental Pollution 152: 679–685.
Bearhop, S., C. E. Adams, S. Waldron, R. A. Fuller & H. MacLeod, 2004. Determining trophic niche width: a novel approach using stable isotope analysis. Journal of Animal Ecology 73: 1007–1012.
Boecklen, W. J., C. T. Yarnes, B. A. Cook & A. C. James, 2011. On the Use of Stable Isotopes in Trophic Ecology. Annual Review of Ecology, Evolution, and Systematics 42: 411–440.
Brett, M., 2014. Resource polygon geometry predicts Bayesian stable isotope mixing model bias. Marine Ecology Progress Series 514: 1–12.
Brett, M. T., M. J. Kainz, S. J. Taipale & H. Seshan, 2009. Phytoplankton, not allochthonous carbon, sustains herbivorous zooplankton production. Proceedings of the National Academy of Sciences 106: 21197–21201.
Bunn, S. E. & A. H. Arthington, 2002. Basic principles and ecological consequences of altered flow regimes for aquatic biodiversity. Environmental Management 30: 492–507.
Bunn, S. E., P. M. Davies & T. D. Mosisch, 1999. Ecosystem measures of river health and their response to riparian and catchment degradation. Freshwater Biology 41: 333–345.
Caut, S., E. Angulo & F. Courchamp, 2009. Variation in discrimination factors (Δ 15 N and Δ 13 C): the effect of diet isotopic values and applications for diet reconstruction. Journal of Applied Ecology 46: 443–453.
Clarke, K. R., 1993. Non-parametric multivariate analyses of changes in community structure. Austral Ecology 18: 117–143.
Cogo, G., C. Biasi & S. Santos, 2014. The effect of the macroconsumer Aegla longirostri (Crustacea, Decapoda) on the invertebrate community in a subtropical stream. Acta Limnologica Brasiliensia 26: 143–153.
Convention on Wetlands of International Importance especially as Waterfowl Habitat, 1971. Ramsar (Iran). UN Treaty Series No. 14583.
Costantini, M. L., E. Calizza & L. Rossi, 2014. Stable isotope variation during fungal colonisation of leaf detritus in aquatic environments. Fungal Ecology 11: 154–163.
Crenier, C., J. Arce Funck, A. Bec, F. Perrière, E. Billoir, J. Leflaive, F. Guérold, V. Felten & M. Danger, 2017. Minor food sources can play a major role in secondary production in detritus-based ecosystems. Freshwater Biology. 62(7): 1155–1167. https://doi.org/10.1111/fwb.12933.
Cucherousset, J. & S. Villéger, 2015. Quantifying the multiple facets of isotopic diversity: new metrics for stable isotope ecology. Ecological Indicators 56: 152–160.
de Castro, D. M. P., D. R. de Carvalho, P. dos Santos Pompeu, M. Z. Moreira, G. B. Nardoto & M. Callisto, 2016. Land use influences niche size and the assimilation of resources by benthic macroinvertebrates in tropical headwater streams. PLoS ONE 11: e0150527.
Danger, M., J. Cornut, E. Chauvet, P. Chavez, A. Elger & A. Lecerf, 2013. Benthic algae stimulate leaf litter decomposition in detritus-based headwater streams: a case of aquatic priming effect? Ecology 94: 1604–1613.
Dodds, W. K. & M. R. Whiles, 2010. Freshwater Ecology: Concepts and Environmental Applications of Limnology, 2nd ed. Elsevier Science Publishing Co Inc, Amsterdam.
Doucett, R. R., J. C. Marks, D. W. Blinn, M. Caron & B. A. Hungate, 2007. Measuring terrestrial subsidies to aquatic food webs using stable isotopes of hydrogen. Ecology 88: 1587–1592.
Elosegi, A. & S. Sabater, 2013. Effects of hydromorphological impacts on river ecosystem functioning: a review and suggestions for assessing ecological impacts. Hydrobiologia 712: 129–143.
European Union, 2000. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community action in the field of water policy. Official Journal of the European Union, Brussels, Belgium. http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32000 L0060
Finlay, J. C. & C. Kendall, 2007. Stable isotope tracing of temporal and spatial variability in organic matter sources to freshwater ecosystems. Stable isotopes in ecology and environmental science 2: 283–333.
Fisher, S. G. & G. E. Likens, 1973. Energy flow in bear brook, new hampshire: an integrative approach to stream ecosystem metabolism. Ecological Monographs 43: 421–439.
Four, B., E. Arce, M. Danger, J. Gaillard, M. Thomas & D. Banas, 2017a. Catchment land use-dependent effects of barrage fishponds on the functioning of head water streams. Environmental Science and Pollution Research 24: 5452–5468.
Four, B., M. Thomas, E. Arce, A. Cébron, M. Danger & D. Banas, 2017b. Fishpond dams affect leaf-litter processing and associated detritivore communities along intermittent low-order streams. Freshwater Biology 62: 1741–1755. https://doi.org/10.1111/fwb.12984.
Fry, B., 2008. Stable Isotope Ecology, 3rd ed. Springer, New York.
Fry, B., 2013. Alternative approaches for solving underdetermined isotope mixing problems. Marine Ecology Progress Series 472: 1–13.
Gaillard, J., M. Thomas, A. Iuretig, C. Pallez, C. Feidt, X. Dauchy & D. Banas, 2016a. Barrage fishponds: reduction of pesticide concentration peaks and associated risk of adverse ecological effects in headwater streams. Journal of Environmental Management 169: 261–271.
Gaillard, J., M. Thomas, A. Lazartigues, B. Bonnefille, C. Pallez, X. Dauchy, C. Feidt & D. Banas, 2016b. Potential of barrage fish ponds for the mitigation of pesticide pollution in streams. Environmental Science and Pollution Research 23(1): 23–35.
Gan, J. J., P. C. Zhu, S. D. Aust & A. T. Lemley, 2004. Pesticide Decontamination and Detoxification. ACS Symposium Series; American Chemical Society: Washington, DC, United States of America.
González, J. M., S. Molla, N. Roblas, E. Descals, O. Moya & C. Casado, 2013. Small dams decrease leaf litter breakdown rates in Mediterranean mountain streams. Hydrobiologia 712: 117–128.
Guenet, B., M. Danger, L. Abbadie & G. Lacroix, 2010. Priming effect: bridging the gap between terrestrial and aquatic ecology. Ecology 91: 2850–2861.
Guilpart, A., J.-M. Roussel, J. Aubin, T. Caquet, M. Marle & H. Le Bris, 2012. The use of benthic invertebrate community and water quality analyses to assess ecological consequences of fish farm effluents in rivers. Ecological Indicators 23: 356–365.
Hameed, A., S. Ahmad, R. T. Ahmad & H. Karar, 2011. Pesticide detoxification in invertebrates, plants and microbes. Life Sciences International Journal 5: 2186–2194.
Hastie, T. J. & D. Pregibon, 1992. Generalized linear models. Chapter 6 of Statistical Models in S. eds J. M. Chambers and T. J. Hastie, Wadsworth & Brooks/Cole.
Jacob, U., K. Mintenbeck, T. Brey, R. Knust & K. Beyer, 2005. Stable isotope food web studies: a case for standardized sample treatment. Marine Ecology Progress Series 287: 251–253.
Kuehn, K. A., S. N. Francoeur, R. H. Findlay & R. K. Neely, 2014. Priming in the microbial landscape: periphytic algal stimulation of litter-associated microbial decomposers. Ecology 95: 749–762.
Layman, C. A., M. S. Araujo, R. Boucek, C. M. Hammerschlag-Peyer, E. Harrison, Z. R. Jud, P. Matich, A. E. Rosenblatt, J. J. Vaudo, L. A. Yeager, D. M. Post & S. Bearhop, 2012. Applying stable isotopes to examine food-web structure: an overview of analytical tools. Biological Reviews 87: 545–562.
Majdi, N., N. Hette-Tronquart, E. Auclair, A. Bec, T. Chouvelon, B. Cognie, M. Danger, P. Decottignies, A. Dessier, C. Desvilettes, S. Dubois, C. Dupuy, C. Fritsch, C. Gaucherel, M. Hedde, F. Jabot, S. Lefebvre, M. P. Marzloff, B. Pey, N. Peyrard, T. Powolny, R. Sabbadin, E. Thébault & M.-E. Perga, 2018. There’s no harm in having too much: a comprehensive toolbox of methods in trophic ecology. Food webs 16: e00100.
Martinez Arbizu, P., 2017. pairwiseAdonis: Pairwise multilevel comparison using adonis. R package version 0.0.1.
Martínez, A., A. Larranaga, A. Basaguren, J. Pérez, C. Mendoza-Lera & J. Pozo, 2013. Stream regulation by small dams affects benthic macroinvertebrate communities: from structural changes to functional implications. Hydrobiologia 711: 31–42.
Mathé, S. & H. Rey-Valette, 2015. Local knowledge of pond fish-farming ecosystem services: management implications of stakeholders’ perceptions in three different contexts (Brazil, France and Indonesia). Sustainability 7: 7644–7666.
McArdle, B. H. & M. J. Anderson, 2001. Fitting multivariate models to community data: a comment on distance-based redundancy analysis. Ecology 82: 290–297.
McCarthy, J., W. Taylor & J. Taft, 1984. Geothermal and cold springs faunas: inorganic carbon sources affect isotope values. Marine Biology 65: 49–60.
Menendez, M., E. Descals, T. Riera & O. Moya, 2012. Effect of small reservoirs on leaf litter decomposition in Mediterranean headwater streams. Hydrobiologia 691: 135–146.
Merritt, R. W. & K. W. Cummins, 1996. An introduction to the aquatic insects of North America, 3rd ed. Kendall/Hunt, Duduque, Iowa.
Millennium Ecosystem Assessment (Program) ed. 2005. Ecosystems and human well-being: synthesis. Island Press, Washington, DC.
Nelson, D., 2011. Gammarus-microbial interactions: a review. International Journal of Zoology 2011: 6.
Nõges, T., H. Luup & T. Feldmann, 2010. Primary production of aquatic macrophytes and their epiphytes in two shallow lakes (Peipsi and Võrtsjärv) in Estonia. Aquatic Ecology 44: 83–92.
Oertli, B. & P.-A. Frossard, 2013. Mares et étangs—Ecologie, gestion, aménagement et valorisation. Presses Polytechniques et universitaires romandes.
Parnell, A. C., R. Inger, S. Bearhop & A. L. Jackson, 2010. Source partitioning using stable isotopes: coping with too much variation. PLoS ONE 5: E9672.
Perga, M.-E, 2004. Etude de l’origine du carbone des réseaux trophiques lacustres à partir des compositions isotopiques du carbone et de l’azote des poissons et du zooplancton. Université de Savoie.
Perkins, M. J., R. A. McDonald, F. J. F. van Veen, S. D. Kelly, G. Rees & S. Bearhop, 2014. Application of nitrogen and carbon stable isotopes (δ15 N and δ13C) to quantify food chain length and trophic structure. PLoS ONE 9: e93281.
Pinet, F. & C. Hélan, 2015. La Caldésie à feuilles de parnassie. Une plante d’importance européenne dans les étangs de la Brenne (Indre - France); témoin possible d’une histoire des étangs. In: Mieux comprendre les étangs. Expériences nationales et internationales. Du Berry Limousin à l’Europe Orientale. Les Monédières. pp. 171–179. eds Touchart, L., P. Bartout & O. Motchalova.
Post, D. M., 2002. Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83: 703–718.
Rasmussen, J. B., 2010. Estimating terrestrial contribution to stream invertebrates and periphyton using a gradient-based mixing model for δ 13 C. Journal of Animal Ecology 79: 393–402.
Rosemond, A. D., C. M. Pringle & A. Ramirez, 1998. Macroconsumer effects on insect detritivores and detritus processing in a tropical stream. Freshwater Biology 39: 515–523.
Searle, S. R., F. M. Speed & G. A. Milliken, 1980. Population marginal means in the linear model: an alternative to least squares means. The American Statistician 34: 216–221.
Sterner, R. W. & J. J. Elser, 2002. Ecological Stoichiometry. Princeton University Press, Princeton, USA.
Tachet, H., F. Richoux, M. Bournaud & P. Usseglio-Polatera, 2010. Invertébrés d’eau douce : systématique, biologie, écologie. CNRS, Paris (FR).
Thorp, J. H. & M. D. Delong, 1994. The riverine productivity model: an heuristic view of carbon sources and organic processing in large river ecosystems. Oikos 70: 305.
Tibi, A. & O. Therond, 2017. Evaluation des services écosystémiques rendus par les écosystèmes agricoles. Une contribution au programme EFESE. Synthèse du rapport d’étude, Inra (France): 118.
Torremorell, A., M. E. Llames, G. L. Pérez, R. Escaray, J. Bustingorry & H. Zagarese, 2009. Annual patterns of phytoplankton density and primary production in a large, shallow lake: the central role of light. Freshwater Biology 54: 437–449.
Vannote, R. L., G. W. Minshall, K. W. Cummins, J. R. Sedell & C. E. Cushing, 1980. The river continuum concept. Canadian Journal of Fisheries and Aquatic Sciences 37: 130–137.
Wetzel, R. G., 2001. Limnology: Lake and River Ecosystems, 3rd ed. Elsevier Science Publishing Co Inc., Amsterdam.
Acknowledgements
The authors gratefully acknowledge the financial support for this project by the ‘Agence de l’Eau Rhin-Meuse’ and the ‘Zone Atelier Moselle’. We sincerely thank E. Arce, P. Chaud, B. Le Carrer and Y. Namokel for their field and laboratory work, as well as the fish farmers, the ‘Office National des Forêts’ for providing us with access permits for sampling. We are also extremely grateful to the INRA of Champenoux for allowing us to use the laboratory facilities and conduct the stable isotope analysis at PTEF OC 081 from the UMR 1137 and UR 1138. The PTEF facility is supported by the French National Research Agency through the Laboratory of Excellence ARBRE (ANR-11-LABX-0002-01). We also want to thank the three anonymous reviewers and the associate editor, M. M. Sánchez-Montoya, who help to improve this final manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: María del Mar Sánchez-Montoya
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Four, B., Thomas, M., Danger, M. et al. Using stable isotope approach to quantify pond dam impacts on isotopic niches and assimilation of resources by invertebrates in temporary streams: a case study. Hydrobiologia 834, 163–181 (2019). https://doi.org/10.1007/s10750-019-3920-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-019-3920-0