Abstract
Extensive fish production systems in continental areas are often created by damming headwater streams. However, these lentic systems favour autochthonous organic matter production. As headwater stream functioning is essentially based on allochthonous organic matter (OM) supply, the presence of barrage fishponds on headwater streams might change the main food source for benthic communities. The goal of this study was thus to identify the effects of barrage fishponds on the functioning of headwater streams. To this end, we compared leaf litter breakdown (a key ecosystem function in headwater streams), their associated invertebrate communities and fungal biomass at sites upstream and downstream of five barrage fishponds in two dominant land use systems (three in forested catchments and two in agricultural catchments). We observed significant structural and functional differences between headwater stream ecosystems in agricultural catchments and those in forested catchments. Leaf litter decay was more rapid in forest streams, with a moderate, but not significant, increase in breakdown rate downstream from the barrage fishponds. In agricultural catchments, the trend was opposite with a 2-fold lower leaf litter breakdown rate at downstream sites compared to upstream sites. Breakdown rates observed at all sites were closely correlated with fungal biomass and shredder biomass. No effect of barrage fishponds were observed in this study concerning invertebrate community structure or functional feeding groups especially in agricultural landscapes. In forest streams, we observed a decrease in organic pollution (OP)-intolerant taxa at downstream sites that was correlated with an increase in OP-tolerant taxa. These results highlighted that the influence of barrage fishponds on headwater stream functioning is complex and land use dependent. It is therefore necessary to clearly understand the various mechanisms (competition for food resources, complementarities between autochthonous and allochthonous OM) that control ecosystem functioning in different contexts in order to optimize barrage fishpond management.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Headwater streams, which may represent up to 80% of the total stream length (MacDonald and Coe 2007), are essential for ensuring high water quality and good status of downstream ecosystems (Alexander et al. 2007; Gomi et al. 2002). The functioning of streams, especially headwater streams, is closely related to their associated catchments (Fisher and Likens 1973; Wallace et al. 1997). Human activities in the catchments can significantly alter headwater streams (e.g. Bernot et al. 2010). Intensive agriculture, in particular, has been identified as a major stressor for freshwater ecosystems (Vörösmarty et al. 2010). As shown by several studies, conventional crop production markedly affects surface water with, for example, increases in nutrient concentrations (Hughes et al. 2008; White and Hammond 2009), sediment discharges (Collins and Anthony 2008) and pesticide concentrations (Kreuger 1998). Streams draining agricultural land are also often characterized by loss of hydromorphological features of natural streams (e.g. meanders) and loss of habitat heterogeneity due to dredging and channelization (Negishi et al. 2002; Pedersen 2009). These physical and chemical alterations may be a cause of biodiversity loss in streams (Liess et al. 2008; Rasmussen 2012) and affect fundamental ecological processes such as leaf litter decomposition and gross primary production (Peters et al. 2013; Rasmussen et al. 2012; Schäfer 2007; Robinson and Gessner 2000).
Beyond these quite well-known effects of pollution, far less attention has been paid to other aspects of river integrity. Yet, due to their small sizes, numerous headwater streams suffer strong hydromorphological impact effects (Elosegi and Sabater 2013). Yet, hydromorphology, defined as the complex interaction between water flow and channel form, is an essential status component for streams (Poole 2010). Hydromorphology can have considerable effects on water quality, community structure and stream ecosystem functioning (Elosegi et al. 2010). Among the various catchment uses that could affect river continuum and significantly alter water flow, barrage fishponds (i.e. drainable ponds used as extensive fish production systems), made by building a small dam on headwater streams, represent a common hydromorphological alteration. Fishponds account for a large proportion of all surface water bodies in France (1200 km2), the Czech Republic (410 km2) and Germany (420 km2) (Le Quéré and Marcel 1999; Pokorný and Hauser 2002). In France, it is estimated that there are over 251,000 ponds (Bartout and Touchart 2013), most of which are located on first-order streams. Although the ecosystem services provided by fishpond systems are increasingly recognized (Bekefi and Varadi 2007; Blayac et al. 2014; Mathé and Rey-Valette 2015), the presence of dams can strongly affect the biodiversity and functioning of streams (Bunn and Arthington 2002; Elosegi and Sabater 2013; González et al. 2013). Due to increases in water retention time, sediment retention and particulate organic matter production, to changes in water chemistry or to the partial or total reduction of the aquatic organism migration (Pringle 1997; Kunz et al. 2011; Gonzales et al. 2013; Colas et al. 2013), the effects of dams have most often been seen as negative for stream ecosystems. Yet, in the context of agricultural landscapes, some studies have shown that the presence of these fish production systems in streams can influence positively the flux of water, decreasing the suspended matter, and pesticide and nutrient contents of the downstream water (Banas 2001; Banas et al. 2002; Gaillard 2014; Gaillard et al. 2016a) except during the draining period when significant release of nutrients can be observed (Banas et al. 2008). More recently, Gaillard et al. (2016b) measured a significant reduction in pesticide peak concentrations downstream from barrage fishponds (from 49 to 99%, depending on the molecule). From this, it could be said that barrage fishponds improve water quality for streams in agricultural catchment areas. As a consequence, it could be expected that the influence of fishponds on the functioning of headwater streams might be highly dependent on the catchment land use.
To investigate headwater stream status, water or various biological indices mainly based on the sensitivity of some aquatic taxa (fish, diatoms, macroinvertebrates and macrophytes) to multiple stressors (Birk et al. 2012). However, these indices may be considered unsatisfying due to major inconsistencies with the Water Framework Directive (WFD, European Union 2000; Roche et al. 2005; Mondy et al. 2012). Recently, ecologists have emphasized the need to use both the structural and functional components of ecosystem communities to assess the ecological status of streams and the effects of various environmental stressors (Clarke et al. 2008; Graça 2001; Maltby and Hills 2008; Tachet et al. 2010). In order to understand in greater depth the influence that these stressors have on stream ecosystem functioning, leaf litter decomposition has been proposed as an integrative indicator of headwater stream functioning and developed as such by several authors (Gessner and Chauvet 2002; Graça 2001; Graça et al. 2007; Graça et al. 2015; Tank et al. 2010). This parameter is a key ecosystem level process that integrates the activity of both microbial decomposers (mostly aquatic hyphomycetes) and aquatic macroinvertebrates. It has been successfully applied to the ecological monitoring of streams (e.g. Woodward et al. 2012).
In order to investigate the influence of barrage fishponds on headwater streams along with the catchment land use, we selected five fishponds located on five headwater streams (two in agricultural landscapes and three in forested landscapes) and monitored (i) leaf litter processing (evaluated by the litter bag technique), (ii) fungal biomass and (iii) structural and functional metrics of macroinvertebrate communities associated with litter bags. The barrage fishponds were distributed along a gradient of land uses in the various catchments from extensive forest management to intensive cereal production. Since land use is well known to highly influence water quality of streams, we hypothesized that barrage fishponds could have differential effects on the functioning of headwater streams depending on the catchment land use.
Materials and methods
Study area
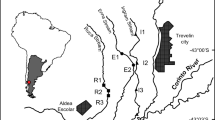
The study was conducted in the Lorraine region (north-eastern France) located at the extreme east end of the Paris sedimentary drainage basin near the Vosges mountains (Fig. 1). The climate is semi-continental with an average annual air temperature of 10.7 °C, an average minimum temperature of about 2.2 °C in January and an average maximum temperature of 19.7 °C in July (30-year average, Château-Salins, Meteo France 2011). The average annual precipitation is about 800 mm, and it is well distributed throughout the year. During the investigation period (21 January to 17 March 2014), the average air temperature was 5.1 °C, which falls within the temperature range of relatively warm years. The cumulated daily precipitation during the whole investigation period was 92.2 mm, which falls within the precipitation range of relatively dry years.
This region, with a total surface area of 23,547 km2, is characterized by a large cover of mixed deciduous forests (36%, where Quercus, Carpinus and Fagus are the dominant genera) and agricultural lands (27% arable lands and 20% pastures). Agricultural lands are often managed intensively using pesticides and chemical fertilizers (Joulin 2006). Moreover, the Lorraine region, with a fish production of 1100 t (CRAL 2005), ranks third in freshwater fish production in France thanks to a high density of fishponds covering an area of about 7000 ha (Le Quéré and Marcel 1999). Most of these fishponds were created along a headwater stream continuum in the Middle Ages and are commonly exploited extensively for fish production. Numerous fishponds are often exposed to pesticide pression as a result of agricultural land use (Lazartigues et al. 2012, 2013a; Lazartigues et al. 2013b).
Study sites
To investigate the influence of fishponds on headwater stream functioning, we selected five barrage fishponds located on first-order headwater streams (according to the classification of Strahler 1957) in a limited geographical area (60 km2) with homogenous geology (Triassic sedimentary deposits). We studied potential differences in leaf litter processing, fungal biomass and structural and functional metrics of macroinvertebrate communities between sites located upstream (Up) and downstream (Down) from the fishponds. At each sampling point (i.e., Up and Down of the five fishponds), a 30-m-long reach was selected at approximately 50 m from the ponds to avoid the drawdown zone upstream as well as the direct effect of water fall from dams downstream.
Selection of the fishponds was based on the following criteria: (i) major water inflows brought by one main tributary to the pond and (ii) similar land uses between upstream and downstream sampling points in each catchment area. In order to carry this field study, we selected sites representative of the field context where ponds are located either on agricultural catchments (but always with few percent of other land cover) or in forest (but always with few percent of agricultural area). Classification of our sites into two land use categories was done with an accurate protocol, on the basis of three different criteria. To define a catchment in the category ‘Forest’, it is necessary to meet the three following criteria:
-
1.
River basin must be dominated by native deciduous forest.
-
2.
Stream running through a significant distance of forest
-
3.
Main land cover surrounding the sampling points must be also dominated by native tree species. In this case, organic matter (OM) available as food source for trophic webs is dominated by tree litters (i.e. leaves and branches, as observed for the three forested sites).
If these three criteria are met, the catchment was ranged in the category Forest. If not, the catchment was considered in the category ‘Agriculture’. By applying these criteria, we categorized there three fishponds into ‘Forest sites’ (noted F1, F2 and F3) and two into ‘Agriculture sites’ (noted A1 and A2). In the forest catchments, the dominant tree species were Carpinus betulus, Fagus sylvatica and Quercus sp. Among arable lands, the cultivated surface areas and the proportion of crop variety varied annually as follows. The farmers mainly grew rapeseed (Brassica napus), wheat (Triticum aestivum) and barley (Hordeum vulgare) in a 3-year cultural rotation as commonly practised in the region (Xiao et al. 2014) (Table 1).
Fishpond areas were 4.4, 31.6, 4.9, 1.5 and 7.2 ha for A1, A2, F1, F2 and F3, respectively (Table 1). The volumes of these ponds were estimated at 44000, 568,000, 49,000, 16,000 and 108,000 m3 (Banas 2001; Gaillard 2014; Lazartigues 2013). The ratios between the pond and catchment surface areas, which can serve as a proxy for hydraulic retention time (HRT), were 1:20, 1:11, 1:20, 1:42 and 1:21 for A1, A2, F1, F2 and F3, respectively (Table 1). Highest HRT was expected for A2 and lowest HRT was expected for F2. In the Lorraine region (n = 105 sites), the median pond to surface ratio is 1:31 which means the studied sites were typical of ponds in the region (unpublished data).
All the fishponds under investigation are extensively managed for polyculture, including species with different diets and behaviour (Cyprinus carpio, Rutilus rutilus, Tinca tinca, Perca fluviatilis, Esox lucius and/or Sander lucioperca). Management operations include a 2-year cycle production with three steps. The first corresponds to filling of the ponds due to water inputs from small streams, surface water runoff and precipitations. The following step corresponds to a pseudo-balance phase during which fish are stocked and grown in ponds. It must be emphasized that additional feeding and direct use of fertilizers or pharmaceutical compounds are not added in the five studied fishponds, as is usually the case. Finally, the third step is drainage in late autumn or early spring. All of the water is discharged downstream to allow fish harvest every 1 or 2 years.
The riparian vegetation, basin surface area and land use characteristics of the ten sampling points are given in Table 1.
Water physical and chemical parameters
The experiment was carried out over 8 weeks (21 January 2014 corresponding to day 0 to 17 March 2014 corresponding to day 55). Water quality was monitored at all sampling points (upstream and downstream from the five fishponds). Water temperature was measured throughout the entire experimental period with temperature loggers at a 15-min time step (HOBO Pendant data logger UA-001-64). Turbidity, pH, dissolved oxygen, conductivity and oxidation-reduction potential were measured (n = 6–8) throughout the decomposition experiment (PONSEL ODEON X line tools). Water samples were collected at each litter sampling date (day 0, 14, 34 and 55). Samples were taken to the laboratory in a cool box for nitrate, nitrite, ammonium and soluble reactive phosphorus (SRP) analyses. Within 24 h, water samples were filtered (Whatman GF/F) and nutrient measurements were conducted on the filtered water. Nitrate concentrations were determined using the hydrazine reduction method (NF ISO 15923-1, 2014), nitrite concentrations using the sulphanilamide method (NF ISO 15923-1, 2014), ammonium concentrations using the indophenol blue spectrophotometric method (NF T 90-015-2, 2000) and SRP concentrations using the molybdate method (NF ISO 15923-1, 2014). All the water physical and chemical results are reported in Table 2.
Leaf decomposition
In autumn 2013, leaves were collected just after abscission from the same stand of maple trees (Acer pseudoplatanus) in the Vosges Mountains using a net hung between the trees 1 m above the ground. Maple leaves were chosen because they are supposed to have a medium to fast litter breakdown rate (Lecerf 2005; Petersen and Cummins 1974), an important property for our study because study sites can be temporarily dry out. Thus, the whole experiment must be conducted between the end of autumn and before summer drying. Like in these small streams a large part of the shredder communities implicated in the litter decay have annual cycles with larvae aquatic stage during the winter and adult terrestrial stage during the summer, we have chosen to conduct the study at the end of their aquatic stage and just before emergencies (i.e. from January to March 2014).
Leaf litter was air-dried in a room and stored in the dark under dry conditions before being used. Leaf petioles were removed, and then leaves were weighed in batches of 3 ± 0.02 g, moistened with deionized water, placed in 40 fine and 120 coarse mesh bags (0.5 and 10 mm mesh size, respectively, following the method described by Gessner and Chauvet 2002) and deployed at the ten sampling points on day 0. Coarse mesh bags allowed for shredder colonization whereas fine mesh bags excluded them and therefore only reflecting microbial activity (largely by microfungi) and leaching. Coarse mesh bags were used to determine the total leaf breakdown rate at each site, and fine mesh bags were used to determine fungal biomass.
Four coarse mesh bags were collected at each sampling point after 14, 34 and 55 days of exposure, and the fine mesh bags were collected after 34 days of exposure. All leaf bags were removed from the streams using 0.5 mm (mesh size) sieves to avoid invertebrate loss, stored individually in zip-lock bags and returned to the laboratory in a cool box for processing. In the laboratory, leaves were carefully washed on a 0.5 mm sieve to remove sediment and exogenous organic matter and to collect invertebrates. Then, samples from coarse mesh bags were oven-dried at 105 °C for 48 h and weighed to the nearest 0.01 g and ground. In order to minimize the bias from sediment contamination, results were expressed in ash free dry mass (AFDM). Leaf AFDM was determined on 500 mg sub-samples of ground leaves ashed in a muffle furnace (550 °C for 4 h). As described by Abelho (2001), leaching of soluble compounds can account for more than 40% of initial dry mass loss. In order to minimize the effect of this initial leaching, four unexposed samples were placed in drinking water for 2 days, weighed and ashed in a muffle furnace to determine initial leaf AFDM. The leaf mass remaining in the bags exposed in streams was expressed as a ratio between samples and initial leached litter expressed in AFDM.
Fungal biomass
The role of fungal biomass was demonstrated to highly influence the microbial conditioning of leaves, an important driver of leaf decay into the streams (Gessner and Chauvet 1994). Fungal biomass was measured after 34 days of exposure (2nd sampling date) corresponding to the maximum values of fungal biomass expected for this type of leaves in low-order streams (Gessner and Chauvet 1994). A set of five 12-mm-diameter discs were cut from five random leaves of each fine mesh bag cleaned sample, avoiding the central veins, and frozen at −18 °C until processing for ergosterol content as a measure of fungal biomass (Gessner and Chauvet 1993).
Frozen discs were freeze-dried and weighed before estimation of ergosterol content. Ergosterol extraction and quantification were performed following a method based on solid-phase extraction (Waters, Oasis HLB, 60 mg, 3 cm3, Milford, MA, USA) and high-performance liquid chromatography (Gessner and Chauvet 1993). Fungal biomass in leaves was expressed as mycelium mass per gram of dry leaf litter.
Benthic invertebrates
Invertebrates from the 34-day exposure coarse mesh bags retained on 0.5-mm sieves (in the field and in the laboratory after leaf wash) were preserved in 70% ethanol until being identified, counted and measured (Tachet et al. 2010). Identification and count were performed under a stereo-microscope (Nikon SMZ-800N) to genus or species when possible or to family, sub-family or tribe for some Diptera. Body length was measured to the nearest millimetres (from the first 50 individuals of each taxon per leaf bag). Biomass of taxa was evaluated by length-mass relationship (Benke et al. 1999; Méthot et al. 2012). A number of biotic metrics were calculated for each leaf bag (abundance, richness, Shannon’s and Simpson’s diversity (indices), Pielou’s evenness (index) of benthic macroinvertebrates, richness and densities of Ephemeroptera, Plecoptera and Trichoptera (EPT) and Crustaceans). Organic pollution (OP) tolerance of taxa was also evaluated using saprobic value trait modalities from Tachet et al. (2010) and was expressed as the relative abundance of intolerant or tolerant taxa (i.e. xeno-saprobic and oligo-saprobic taxa or alpha meso-saprobic and poly-saprobic taxa, respectively) in a leaf bag. Benthic macroinvertebrates were also assigned into functional feeding groups (FFGs: shredders, collector-gatherers, collector-filterers, scrapers, predators, parasite-piercers) according to their affinity score (i.e. 0–5) fixed by feeding habits as described by Tachet et al. (2010). Since invertebrate biomass is known as one of the most important drivers of leaf litter breakdown in headwater streams, benthic macroinvertebrate communities assigned to each FFG (e.g. shredder) were expressed as relative biomass of FFG and as total biomass of FFG per gram of leaf litter AFDM remaining, in a leaf bag. It should be noted that benthic macroinvertebrate communities involved in the leaf decomposition process were only a part of the whole communities leaving in the river bed, and so, interpretations could just underline differences of this part of the whole communities.
Data analysis
Leaf litter breakdown rates were determined by fitting mass-loss data into negative exponential decay models (%AFDMt = e−kt, where %AFDMt is the percentage of leaf litter AFDM remaining at time t (day) corrected by the initial AFDM (after leaching) and k (g day−1) is the breakdown rate constant) as described by Benfield (2006). The best-fit model was identified by comparing the Akaike information criteria (AIC) (Akaike 1973) of five leaf litter breakdown models: (i) one parameter: common model with all the data; (ii) two parameters: location of the sites (upstream vs. downstream); (iii) two parameters: dominant land use in the catchment (agricultural vs. forested); (iv) four parameters: agriculture upstream, agriculture downstream, forest upstream and forest downstream or (v) ten parameters: the ten sampling points. According to this method, the best model is defined by the lowest AIC. Then, differences between fitted models were compared with nested model testing. Furthermore, in order to have an overview of the data distribution, the best regression model, the common model and coarse mesh bag leaf litter AFDM were plotted and analysed graphically. Comparison of the k values (corresponding to the various breakdown rates) among identified treatments of the best model was done using their 95% confidence intervals.
Fungal biomass and invertebrate metrics were compared by Kruskal-Wallis test or two-way ANOVA followed by Turkey’s HSD tests (Zar 1996) to identify structural differences of communities among site locations (upstream vs. downstream from fishponds) and dominant land use (DLU) in the catchments (forested vs. agricultural). The Bray-Curtis dissimilarity matrix was computed (Bray and Curtis 1957) on the log-transformed abundance taxa data and then tested by a permutational multivariate analysis of variance (PERMANOVA) (McArdle and Anderson 2001) using location, DLU and their interaction to evaluate differences in the composition of benthic macroinvertebrate communities. Non-metric multidimensional scaling (NMDS) ordination was then used to ordinate Bray-Curtis dissimilarities between samples, and results were graphically represented. Ellipses of each significantly different group identified with the PERMANOVA were overlaid on the graph representing the 95% confidence interval standard error from the centroid of each group.
The relative FFG biomass (expressed as percentage of total macroinvertebrate biomass) was used to investigate the macroinvertebrate distribution among functional groups. Besides, the mean FFG biomass per gram of leaf litter (g−1 AFDM) was determined in an attempt to reflect the macroinvertebrate feeding activity potential from the communities.
Overall differences among groups (Location, DLU, Loc*DLU) were analysed with PERMANOVA (Bray-Curtis dissimilarity matrix on the log-transformed FFG data).
All data analyses were performed with R software (R development Core Team 2008) using ‘vegan’, ‘ggplot2’, ‘nlstools’ and ‘lattice’ packages. The significance level for all statistical analyses was set at 0.05.
Results
Water physical and chemical parameters
The physical and chemical parameters (Table 2) showed that water was of very good quality according to the French reference document SEQ-Eau (MEDD and French water agency 2003).
Mean daily temperature during the experimental period was <8 °C. Temporal trends in the temperature raw data were identified by applying a locally weighted scatterpoint smoothing (lowess) (Figure in SI). Temperature slowly increased during the study period. There was no difference in temperature among sites, but differences were observed between upstream and downstream locations. Significant differences among physico-chemical parameters between upstream and downstream locations of each pond were detected by means of non-parametric paired Wilcoxon signed-rank test. In the forested sites, temperature appeared to be significantly lower upstream compared to downstream. In the agricultural sites, temperature was higher upstream compared to downstream at the beginning of the experiment. This tendency was inversed in the middle of the experiment. There was also a difference in temperature variation between upstream and downstream locations. In the forested sites, temperature variations was higher upstream (coefficient of variation = 41.5%) than downstream (CV = 30.5%). In the agricultural streams, temperature variation was lower upstream (CV = 26.7%) compared to downstream (CV = 41.8%).
Dissolved oxygen concentrations were generally above 10 mg/L. No temporal trend was observed during the study period. There was no difference in dissolved oxygen among sites, but differences were observed between upstream and downstream locations for A2 only. In this agricultural pond, lower dissolved oxygen concentrations were measured upstream compared to downstream. pH varied between 6.1 and 8.3 among sites. Slightly lower pH values were measured at the forest sites compared to agricultural sites. Significantly lower pH values were measured at the upstream location compared to the downstream location for F2 and A2. Turbidity measurements were in the range of 4 and 244 NTU. Turbidity was significantly higher upstream compared to downstream for A1, A2 and F2.
Conductivities were in the range of 83 and 1202 μS/cm, a subject to large temporal and spatial variation. Similarly, nitrate concentrations were in the range of 0.5 and 35.1 mg/L. Higher conductivities were associated with agricultural sites, presumably as a result of fertilizer application (beginning of March) or desorption from soil. In the agricultural sites, nitrate concentrations were significantly higher upstream compared to downstream.
Leaf litter breakdown
Comparison of the five AIC (corresponding to the five leaf litter breakdown models) highlighted two best-fit models (Table in SI). These models considered either four parameters (model (iv): upstream forested sites: Up F, downstream forested sites: Down F, upstream agricultural sites: Up A and downstream agricultural sites: Down A) or ten parameters (model (v): five streams × two locations) (AIC = 192.78 and 192.77, respectively). The two models did not show any significant statistical differences (ANOVA, p = 0.08, Table in SI). We therefore chose to retain the more parsimonious one, i.e. the model requiring the smallest number of parameters (four parameters, namely Up F, Down F, Up A and Down A).
The values of remaining leaf litter were well distributed around the common model values (model (i)) for both upstream sites, while they were always above the mean model values for downstream agricultural sites and below the mean model values at the last date for downstream forested sites (Fig. 2). The four leaf litter breakdown rates (g day−1) obtained were 0.027 (Up F), 0.021 (Up A), 0.032 (Down F) and 0.011 (Down A) (Fig. 3). The breakdown rate 95% interval confidences analysed revealed significant differences between downstream forested sites and agricultural sites (greater decomposition at forested sites). Significant differences between downstream agricultural sites and all other sites were measured (with two to three times lower breakdown rates for downstream agricultural sites).
Leaf litter ash free dry mass (AFDM) remaining of alder leaves in litter bags after 14, 34 and 55 days of exposure at the ten sampling points. Results are presented considering upstream agricultural sites (A Up), downstream agricultural sites (A Down), upstream forested sites (F Up) and downstream forest sites (F Down). Open circles are leaf litter AFDM remaining values for each studied group (A Up, A Down, F Up and F Down). Solid lines represent the regression lines computed for each group, and dashed lines represent the regression lines computed with all the data
Effect of fishponds on the stream daily rates of total leaf litter decomposition (k) considering the dominant land use in the catchment. Data represent the four parameters of the best-fit model that is upstream in forested sites, downstream in forested sites, upstream in agricultural sites and downstream in agricultural sites (studied groups). Vertical bars indicate the 95% confidence interval of the group mean values, and the various letters indicate significantly different groups (based on 95% CI)
Decomposition rates were also expressed in terms of grams (degree day)−1 in place of per day so as to correct differences among sites. The four leaf litter breakdown rates (grams (degree day)−1) obtained were 0.006 (Up F), 0.004 (Up A), 0.007 (Down F) and 0.002 (Down A). The breakdown rate 95% interval confidences analysed revealed significant differences between forested and agricultural sites (greater decomposition at forested sites) as well as between downstream and upstream agricultural sites.
Fungal biomass
Fungal biomass results are shown in Fig. 4. After 34 days of exposure in the streams, fungal biomass on maple leaves varied between 15.4 (Down A) and 79.3 (Down F) milligrams of mycelium gram of leaf dry mass among the studied groups. Fungal biomass was markedly affected by location (upstream vs. downstream) and dominant land use in the catchments (agricultural vs. forested) (two-way ANOVA, p = 0.03, p < 0.001 and interaction p < 0.001, respectively). Fungal biomass was similar at upstream forested and agricultural sites. Much lower values of fungal biomass were observed only at downstream agricultural sites. No significant difference was measured in forested sites between upstream (Up F) and downstream (Down F) locations (Fig. 4).
Macroinvertebrate communities
A total of 6792 benthic macroinvertebrates were sampled in coarse mesh bags after 34 days of experiment, resulting in 44 taxa distributed in 32 families. The structure of the communities was not significantly different among the four studied groups (Up F, Up A, Down F, Down A) for individual abundances, taxon richness, Shannon and Simpson diversities and Pielou evenness (p = 0.13, p = 0.86, p = 0.46, p = 0.47, p = 0.39, respectively) (Table 3). Significant differences in relative EPT abundances were found between both dominant land uses in the catchments (forest vs. agriculture) with around 30-fold more EPT in the forest communities than in the agricultural communities (p < 0.001), but no significant difference was observed between upstream and downstream locations. Significant differences in EPT richness were found between three groups (Up F, Down F, Up/Down A) with greater values at the upstream forested sites (p < 0.001) (Table 3). By contrast, significant differences in crustacean abundances were found between the studied groups with 15-fold lower relative abundances at the upstream forested sites than at the upstream agricultural sites (p < 0.001) but no differences were observed for the downstream sites (intermediate values). Furthermore, significant differences in the percentages of intolerant taxa (xeno- and oligo-saprobic taxa) and tolerant taxa (alpha meso- and poly-saprobic taxa) with regard to organic matter pollution only discriminated the upstream forested sites characterized by the highest relative abundance of intolerant taxa and the lowest relative abundance of tolerant taxa (p < 0.001, p = 0.003).
Results of the PERMANOVA on pairwise Bray-Curtis dissimilarity matrices of benthic macroinvertebrate abundances (named taxa composition) showed significant effects of land use (p = 0.001), location (p = 0.001) and their interaction (p = 0.002) (Table 4). The ordination of the studied groups’ communities by NMDS showed four different community compositions from the overlap of confidence interval ellipses (Fig. 5).
The main feeding habits (FFG) of the benthic macroinvertebrate communities were not significantly different between the upstream and downstream sites (from barrage fishponds) as underlined by the results of PERMANOVA analyses on the composition and total biomass of functional feeding groups (Table 4). Moreover, these results highlighted functional differences according to the dominant land uses in the catchments. Our findings showed that shredders dominated the functional composition of all the communities with at least 50% of the biomass represented by shredder taxa (Fig. 6a). Furthermore, gatherers, filterers and parasites always represented less than 8% of the biomass of the different communities whatever the studied group (Fig. 6a). The percentage of shredder biomass decreased from forested sites (66–65%) to agricultural sites (58–50%) and scraper biomass displayed the inverse trend with values ranging from 11 to 13% in forested sites and from 22 to 18% in agricultural sites. The relative biomass of predators varied between 8 and 14% among the groups (Up F, Up A, Down F, Down A). Concerning the total biomass of communities spread in the FFGs, the results highlighted higher biomass for forested sites (Fig. 6b). This greater biomass in forested sites was well divided among the FFG with a (quasi) systematic higher biomass obtained in forested sites. The greatest difference was observed in the total biomass of shredders. The latter was almost 10-fold higher for forested sites than it was for agricultural sites. Similarly, the biomass of shredders showed an average value 2-fold higher for downstream than for upstream forested sites (Fig. 6b). Nevertheless, at downstream site F3, the shredder biomass was almost eight times lower than at both other downstream sites (F1 and F2) and subsequently associated with EPT density decrease (data not shown).
Discussion
In order to evaluate the effects of barrage fishponds on headwater streams, we will first discuss the need to take into account the land uses within the studied catchments. On these grounds, we will then discuss the results we obtained for invertebrate communities, fungal biomass and leaf litter decomposition for a better understanding of fishpond effects on headwater stream functioning.
Effect of land use on headwater stream functioning
Looking only at the upstream sampling points of the five studied sites, we did not record any differences in benthic macroinvertebrate abundance, taxon richness, diversity or evenness between agricultural and forested sites. Similarly, shredders were dominant for both land uses suggesting that shredding activity is an important ecosystem process in running waters, especially in headwater streams where allochthonous leaf litter is abundant. However, we recorded significant differences in benthic macroinvertebrate community compositions depending on the catchment land use (forest vs. agriculture). We noted only 45% of common species, as well as 30-fold more EPT, 15-fold less crustaceans and almost 14% more taxa intolerant to organic pollution at upstream sites located in forested catchments compared to agricultural catchments. The quasi-systematic disappearance of the most sensitive taxa in agricultural catchments (Capnia sp., Nemoura sp., Habrophlebia sp., Oligostomis reticulata) indicated that land use considerably influences the stream community. Several studies have already shown the effects of agriculture on terrestrial (Mazzia et al. 2015; Frampton and Dorne 2007) and aquatic (Castela et al. 2008; Schäfer et al. 2007) macroinvertebrate communities. These communities are often affected by pesticide inputs especially for the most sensitive taxa (e.g. Ippolito et al. 2012; Liess and Van der Ohe 2005; Schäfer et al. 2007). During our study, Gaillard et al. (2016a, b) followed pesticides in three out of our five studied sites (i.e. F2, A1 and A2). They measured very low concentrations of pesticides upstream from F2 (a forested site, with maximum concentrations of tritosulfuron, 0.1 μg.L−1). Forested sites F1 and F3 were not investigated in this study for pesticides. However, since F2 was by far the most exposed forested site to agricultural inputs (among the ponds under forest cover, F2 had the catchment with the highest arable land percentage and high nitrate concentrations confirmed agricultural inputs; Tables 1 and 2), we could expect that pesticide levels in F1 and F3 would be lower than in F2. On the other hand, agricultural sites were characterized by high pesticide concentrations (with maximum MCPA concentrations of 26.5 μg L−1 measured upstream from A1 and 8.26 μg L−1 measured upstream from A2). As such, we cannot exclude that pesticides associated with conventional crop production could have an effect on the macroinvertebrate communities.
Beyond EPT, the absence of a relationship between macroinvertebrate taxon richness, diversity, evenness and land use was in agreement with other studies whose authors did not establish any correlation between pesticides (associated with agricultural land use) and those metrics (Brock and Budde 1994; Maltby and Hills 2008). In addition, Morrissey et al. (2015) showed that crustacean taxa are often less sensitive than EPT taxa to acute or chronic insecticide exposition, which could corroborate our results concerning the high crustacean abundance in streams from agricultural catchments. Thus, the aquatic macroinvertebrates of agricultural sites are probably less sensitive taxa to diverse agricultural perturbations, as also suggested by the results of relative abundance of intolerant organisms.
In the literature, community modifications related to anthropogenic environmental perturbations, which are themselves linked to conventional agricultural practices, were also often associated with changes in their functional feeding habits (Flores et al. 2014; Rasmussen et al. 2012; Schäfer et al. 2007). Rasmussen et al. (2012) reported a decrease in leaf litter breakdown with increasing pesticide inputs. However, in our study, the results were not as straightforward. Indeed, the leaf litter breakdown rate did not vary significantly between forested and agricultural upstream sites when it was expressed in grams per day and was only lower when corrected in grams (degree day)−1. Moreover, the higher temperatures observed in agricultural sites could have been due to riparian management (i.e. streams with little riparian shading in agricultural sites). Besides, shredder biomass showed significant differences between forested and agricultural sites suggesting potential feeding habit differences. We observed lower shredder biomass in the agricultural catchments, but most of it came from Gammaridae (Gammarus pulex and G. roeseli) compared to the forested sites where EPT dominated. Several studies showed that high leaf litter breakdown rates can be correlated with shredder communities dominated by Gammaridae (Dangles and Malmqvist 2004; Piscart et al. 2009). As already outlined, Gammaridae appeared to be better shredders than some EPT taxa. Such information is noteworthy given the fact that leaf litter fungal conditioning was similar for the various upstream sites whatever the land use. Despite the known effects of agriculture on microbial activity measured in several studies (Bundschuh et al. 2011; Robinson and Gessner 2000; Rasmussen et al. 2012; Schäfer et al. 2011), our results did not indicate any direct effects of agriculture on fungal biomass. However, nutrient losses in freshwater associated with soil fertilization by farmers were investigated in several laboratory and field studies showing that moderate increase in nutrients favoured microbial biomass and activity (Danger et al. 2013; Ferreira et al. 2015; Robinson and Gessner 2000; Suberkropp 1998). This is consistent with the nitrate concentrations measured at our sampling points which were correlated with land uses, with 2- to 16-fold higher values at upstream agricultural sites (Fig. 1 and Table 2).
Effect of barrage fishponds on the ecosystem functioning of headwater streams
Numerous studies have investigated the effects that dam-induced water flow changes have on the fish and macroinvertebrate communities of downstream ecosystems (Bunn and Arthington 2002; Bredenhand and Samways 2009; Casas et al. 2000; Martínez et al. 2013). Nevertheless, only few of them conjointly considered community modifications and leaf litter breakdown, a fundamental ecosystem function of headwater streams (Casas et al. 2000; Martínez et al. 2013). In any case, to our knowledge, no research had been conducted yet on leaf litter breakdown and their associated communities when extensive fish production in pond dams was coupled with land uses in the river basins. Land uses can affect habitat quality, which is an important parameter for communities and their functional activities in streams. On these grounds, we hypothesized that barrage fishponds could have differential effects on the functioning of headwater streams depending on the catchment land use.
For example, as in forested catchments, ponds induce large aperture in the canopy and consequently change the access to solar energy, in this study, we observed an increase in downstream temperatures only in the forested catchments. This observation is consistent with another study whose authors showed an increase in water temperatures (1 °C, on average) between upstream and downstream sites (Touchart and Bartout 2011). Concerning water quality (monitored during the step of growing fish, see “Study sites” section), our results indicated a decrease in nutrients and turbidity (used as an indicator of suspended matter concentration) from upstream to downstream sites (Table 2). The differences were even more significant when concentrations were at their highest at upstream sites (e.g. in agricultural catchments and F2). This is in agreement with observations made by several authors who reported suspended matter and nutrient retention in ponds (Banas 2001; Passy et al. 2012). For example, Passy et al. (2012) measured 27 to 56% nitrogen retention in ponds depending on the studied year. Gaillard et al. (2016a, b) observed the same trend for pesticide concentrations suggesting that fishponds can improve downstream water quality, an important parameter for macroinvertebrate and fungal communities.
The results of our investigation into the structure and composition of the benthic macroinvertebrate communities showed that upstream/downstream differences were more pronounced for fishponds in forested catchments than for those in agricultural catchments. For example, EPT richness and abundance exhibited greater decreases at forested sites (between Up F and Down F) than at agricultural sites (differences of 15.3 and 14.5%, respectively, for forested sites vs. 0.4 and 7.5% for agricultural sites; Table 3). The results on the organic tolerance status of our communities showed the same trend. The percentages of tolerant and intolerant individuals observed at downstream forested sites were close to those recorded at upstream and downstream agricultural sites. These results were in accordance with a decrease in or disappearance of the most sensitive taxa (Capnia sp., Habrophlebia sp. and O. reticulata) between upstream and downstream forested sites, whereas they were already absent in agricultural sites. Indeed, we observed more common species at the downstream sites (i.e. at both agricultural and forested downstream sites) than at the upstream sites (57.5 and 31%, respectively). A hypothesis is that alteration to the flow regimes of waters downstream from the fishponds could favour the homogenization of the benthic habitat, which in turn could have an effect on the whole benthic macroinvertebrate community structure and composition (Tachet et al. 2010). However, no substratum homogenization was visually observed at our downstream sites as compared with upstream sites (personal observation). This phenomenon might be related to substratum composition because fishponds in the Lorraine region are already located on homogenous and impermeable clay substratum (CRAL 1988). By contrast, the higher macroinvertebrate community similarity and the higher densities of organic intolerant taxa observed for upstream and downstream agricultural sites are consistent with the results of other authors who reported non-cumulative effects of multiple stressors on co-tolerant species (Christensen et al. 2006; Kneitel and Chase 2004; Vinebrooke et al. 2004). As an example, Kneitel and Chase (2004) suggested that ecological trade-offs can have synergetic interactions on the taxa since the exposure to one stressor can select for species or individuals tolerant to that stressor (e.g. in this study, pesticides or nutrients in agricultural catchments) but potentially to an additional stressor (e.g. in this study, fishpond effects due to increasing temperatures or autochthonous organic matter in the downstream rivers). Furthermore, as evidenced by Gaillard et al. (2016a, b), fishponds can have positive effects on pesticide concentrations in streams. Indeed, fishponds cause disruptions of stream continuum (i.e. of fine particulate organic matter (FPOM)/coarse particulate organic matter (CPOM) ratio, temperature and ecological continuity), which could have antagonist effects on the stream water quality that could diminish their impact on macroinvertebrate communities especially when the stream ecosystems are already disrupted by toxicants and nutrient runoff.
Ward and Stanford (1983) showed that headwater dams can increase the FPOM/CPOM ratio due to preferential sedimentation of the largest and heaviest particulates in lentic systems. Indeed, while determining the FFGs of these communities, we expected a shift in the main feeding habits of the downstream communities with more collector feeders and fewer shredders. Surprisingly, according to the PERMANOVA results, we did not measure any significant differences in the functional feeding composition and biomass of the communities between upstream and downstream sites. Shredders dominated the benthic macroinvertebrate communities regardless of the group studied (Up A, Up F, Down A or Down F). This finding suggests that coarse particulate organic matter remains the main food source from all our headwater stream ecosystems (despite land use modification and fishpond addition) and that community modifications have no direct effects on FFGs.
From our results on the influence of fishponds on the main feeding habits of macroinvertebrate communities estimated by calculating the percentage of each FFG in biomass (Fig. 6b), little effect was expected on the leaf litter breakdown rates downstream from the fishponds. However, we measured that leaf litter breakdown remained stable at downstream forested sites (in g day−1 or in grams (degree day)−1), but dropped at downstream agricultural sites (in g day−1 or in grams (degree day)−1) despite stability of the shredder biomass. Applying fungal biomass as an indicator of leaf litter microbial conditioning (Gessner and Chauvet 1997), our results have evidenced significant differences for downstream agricultural sites with three to four times less fungal biomass than in all other studied groups. Numerous studies reported close correlation between leaf conditioning by hyphomycetes and an increase in palatability or nutritional value of leaf litter (Gessner and Chauvet 1997, 2002; Gulis and Suberkropp 2003). Furthermore, authors have shown that shredders preferentially choose and feed on conditioned leaf litter with high palatability and high nutritional value (Chung and Suberkropp 2009; Graça 2001; Graça et al. 2015; Nelson 2011). Thus, even if we have no significant difference in the biomass (relative and total) of shredders between upstream and downstream sites for both catchment types, the difference in shredding activity could be explained by lower palatability of leaf litter at downstream agricultural sites.
The decrease in fungal biomass observed at downstream agricultural sites suggests that fishponds affect relatively more the fungal community growth in agricultural sites than in forested sites. As highlighted in the first part of this discussion, we hypothesized that fungal community growth was sustained by high nutrient content in upstream agricultural sites. However, at downstream agricultural sites, values were 6- to 8-fold lower (for nitrates) than at upstream sites. These results could provide some explanation for such low fungal biomass at downstream agricultural sites. Moreover, based on the passive and short-distance dispersal of hyphomycetes (Bärlocher 2009; Thomas et al. 1991) and the low availability of growing substrate (leaf litter) especially in fishponds from agricultural sites (lack of riparian trees when compared to forested sites; Fig. 1), we therefore suggest that fishponds in agricultural landscapes could cause larger gaps for hyphomycete ecological continuity than in forested landscapes.
Conclusion
The barrage fishponds under investigation showed that they affect ecosystem functioning in headwater streams, but the effects depend on the dominant land use in the catchment. In forested landscapes, we observed that fishponds have significant effects on the structure and composition of benthic macroinvertebrates and moderate effects on ecosystem functioning. In agricultural landscapes, we found that the structure and composition of benthic macroinvertebrates are less affected but, on the other hand, that the ecosystem functioning is strongly affected by fishponds. Most studies dealing with the influence of water flow and quality alteration associated with dams or barrage fishponds are based essentially on physical and chemical water parameters or on community structure (mostly macroinvertebrates and fish). Our study indicates that those parameters are not sufficient to estimate freshwater status especially when it concerns the effects of complex stressors such as dams or fishpond dams in this study.
References
Abelho M (2001) From Litterfall to breakdown in streams: a review. Sci World J 1:656–680. doi:10.1100/tsw.2001.103
Akaike H (1973) Information theory and an extension of the maximum likelihood principle. In: Petrov BN, Csaki F (eds) 2nd International Symposium on Information Theory. Akademiai Kiado, Budapest, pp. 267–281
Alexander RB, Boyer EW, Smith RA, Schwarz GE, Moore RB (2007) The role of headwater streams in downstream water quality. J Am Water Resour Assoc 43(1):41–59
Banas D (2001) Flux de matière en étangs piscicoles extensifs : Rétention, Sédimentation, Exportation. PhD report. Metz University
Banas D, Masson G, Leglize L, Pihan JC (2002) Discharge of sediments, nitrogen (N) and phosphorus (P) during the emptying of extensive fishponds: effect of rain-fall and management practices. Hydrobiologia 472:29–38
Banas D, Masson G, Leglize L, Usseglio-Polatera P, Boyd CE (2008) Assessment of sediment concentration and nutrients loads in effluents drained from extensively-managed fishponds in France. Environ Pollut 152(3):679–685
Bärlocher F (2009) Reproduction and dispersal in aquatic hyphomycetes. Mycoscience 50:3–8. doi:10.1007/S10267-008-0449-X
Bartout P, Touchart L (2013) L’inventaire des plans d’eau français : outil d’une meilleure gestion des eaux de surface. Annales de Géographie 122(691):266–289
Bekefi E, Varadi L (2007) Multifunctional pond fish farmers in Hungary. Aquac Int 15:227–233
Benke AC, Huryn AD, Smock LA, Wallace JB (1999) Length-mass relationships for freshwater macroinvertebrates in North America with particular reference to the southeastern United States. J N Am Benthol Soc 18(3):308–343
Bernot MJ, Sobota DJ, Hall RO, Mulholland PJ, Dodds WK, Webster JR, Tank JL, Ashkenas LR, Cooper LW, Dahm CN, Gregory SV, Grimm NB, Hamilton SK, Johnson SL, McDowell WH, Meyer JL, Peterson B, Poole GC, Valett HM, Arango C, Beaulieu JJ, Burgin AJ, Crenshaw C, Helton AM, Johnson L, Merriam J, Niederlehner BR, O’Brien JM, Potter JD, Sheibley RW, Thomas SM, Wilson K (2010) Inter-regional comparison of land-use effects on stream metabolism. Freshw Biol 55:1874–1890
Birk S, Bonne W, Borja A, Brucet S, Courrat A, Poikane S, Solimini A, Van de Bund W, Zampoukas N, Hering D (2012) Three hundred ways to assess Europe’s surface waters: an almost complete overview of biological methods to implement the Water Framework Directive. Ecol Indic 18:31–41
Blayac T, Mathe S, Rey-Valette H, Fontaine P (2014) Perceptions of the services provided by pond fish farming in Lorraine (France). Ecol Econ 108:115–123
Bray JR, Curtis JT (1957) An ordination of the upland forest communities of southern Wisconsin. Ecol Monogr 27:325–349
Bredenhand E, Samways MJ (2009) Impact of a dam on benthic macroinvertebrates in a small river in a biodiversity hotspot: Cape Floristic Region, South Africa. J Insect Conserv 13:297–307. doi:10.1007/s10841-008-9173-2
Brock TCM, Budde BJ (1994) On the choice of structural parameters and endpoints to indicate responses of freshwater ecosystems to pesticide stress. In: Hill IA, Heimbach F, Leeuwangh P, Matthiesen P (eds) Freshwater field tests for hazard assessment of chemicals. Lewis Publishers, Boca Raton, FL, pp. 19–56
Bundschuh M, Zubrod JP, Kosol S, Maltby L, Stang C, Duester L, Schulz R (2011) Fungal composition on leaves explains pollutant-mediated indirect effects on amphipod feeding. Aquat Toxicol 104:32–37. doi:10.1016/j.aquatox.2011.03.010
Bunn SE, Arthington AH (2002) Basic principles and ecological consequences of altered flow regimes for aquatic biodiversity. Environ Manag 30:492–507. doi:10.1007/s00267-002-2737-0
Casas JJ, Zamora-Munoz C, Archila F, Alba-Tercedor J (2000) The effect of a headwater dam on the use of leaf bags by invertebrate communities. Regulated Rivers Research and Management 16:577–591
Castela J, Ferreira V, Graça MAS (2008) Evaluation of stream ecological integrity using litter decomposition and benthic invertebrates. Environ Pollut 153:440–449. doi:10.1016/j.envpol.2007.08.005
Chung N, Suberkropp K (2009) Contribution of fungal biomass to the growth of the shredder, Pycnopsyche gentilis (Trichoptera: Limnephilidae). Freshw Biol 54:2212–2224. doi:10.1111/j.1365-2427.2009.02260.x
Christensen MR, Graham MD, Vinebrooke RD, Findlay DL, Paterson MJ, Turner MA (2006) Multiple anthropogenic stressors cause ecological surprises in boreal lakes. Glob Chang Biol 12:2316–2322. doi:10.1111/j.1365-2486.2006.01257.x
Clarke A, Mac Nally R, Bond N, Lake PS (2008) Macroinvertebrate diversity in headwater streams: a review. Freshw Biol 53:1707–1721. doi:10.1111/j.1365-2427.2008.02041.x
Colas F, Baudoin J, Danger M, Usseglio-Polatera P, Wagner P, Devin S (2013) Synergistic impacts of sediment contamination and dam presence on river functioning. Freshw Biol 58(2):320–336
Collins AL, Anthony SG (2008) Assessing the likelihood of catchments across England and Wales meeting “good ecological status” due to sediment contributions from agricultural sources. Environmental Science and Policy 11:163–170
CRAL (Chambre Régionale d’Agriculture de Lorraine) (2005) Référentiel diversification 2005. Available at: <http://www.cra-lorraine.fr/fichiers/div-pisciculture-etang.pdf>, (accessed December 2015)
CRAL (Chambre Régionale d’Agriculture de Lorraine) (1988) Pédologie lorraine
Danger M, Cornut J, Chauvet E, Chavez P, Elger A, Lecerf A (2013) Benthic algae stimulate leaf litter decomposition in detritus-based headwater streams: a case of aquatic priming effect? Ecology 94:1604–1613
Dangles O, Malmqvist B (2004) Species richness-decomposition relationships depend on species dominance: biodiversity and dominance in ecosystems. Ecol Lett 7:395–402. doi:10.1111/j.1461-0248.2004.00591.x
Elosegi A, Sabater S (2013) Effects of hydromorphological impacts on river ecosystem functioning: a review and suggestions for assessing ecological impacts. Hydrobiologia 712:129–143. doi:10.1007/s10750-012-1226-6
Elosegi A, Díez J, Mutz M (2010) Effects of hydromorphological integrity on biodiversity and functioning of river ecosystems. Hydrobiologia 657:199–215. doi:10.1007/s10750-009-0083-4
European Union (2000) Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community action in the field of water policy. Official Journal of the European Union, Brussels, Belgium. http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32000L0060
Ferreira V, Castagneyrol B, Koricheva J, Gulis V, Chauvet E, Graça MAS (2015) A meta-analysis of the effects of nutrient enrichment on litter decomposition in streams: nutrient enrichment and litter decomposition. Biol Rev 90:669–688. doi:10.1111/brv.12125
Fisher SG, Likens GE (1973) Energy flow in bear brook, New Hampshire: an integrative approach to stream ecosystem metabolism. Ecol Monogr 43:421. doi:10.2307/1942301
Flores L, Banjac Z, Farré M, Larrañaga A, Mas-Martí E, Muñoz I, Barceló D, Elosegi A (2014) Effects of a fungicide (imazalil) and an insecticide (diazinon) on stream fungi and invertebrates associated with litter breakdown. Sci Total Environ 476-477:532–541. doi:10.1016/j.scitotenv.2014.01.059
Frampton GK, Dorne JLCM (2007) The effects on terrestrial invertebrates of reducing pesticide inputs in arable crop edges: a meta-analysis. J Appl Ecol 44:362–373. doi:10.1111/j.1365-2664.2007.01277.x
Kneitel JM, Chase JM (2004) Trade-offs in community ecology: linking spatial scales and species coexistence. Ecol Lett 7:69–80. doi:10.1046/j.1461-0248.2003.00551.x
Gaillard J (2014) Rôle des étangs de barrage à vocation piscicole dans la dynamique des micropolluants en têtes de bassins versants. PhD report. Lorraine University
Gaillard J, Thomas M, Iuretig A, Pallez C, Feidt C, Dauchy X, Banas D (2016a) Barrage fishponds: reduction of pesticide concentration peaks and associated risk of adverse ecological effects in headwater streams. J Environ Manag 169:261–271
Gaillard J, Thomas M, Lazartigues A, Bonnefille B, Pallez C, Dauchy X, Feidt C, Banas D (2016b) Potential of barrage fish ponds for the mitigation of pesticide pollution in streams. Environ Sci Pollut Res:1–13. doi:10.1007/s11356-015-5378-6
Gessner MO, Chauvet E (2002) A case for using litter breakdown to assess functional stream integrity. Ecol Appl 12:498. doi:10.2307/3060958
Gessner MO, Chauvet E (1997) Growth and production of aquatic hyphomycetes in decomposing leaf litter. Limnol Oceanogr 42:493–505. doi:10.4319/lo.1997.42.3.0496
Gessner MO, Chauvet E (1994) Importance of stream microfungi in controlling breakdown rates of leaf litter. Ecology 75:1807–1817. doi:10.2307/1939639
Gessner MO, Chauvet E (1993) Ergosterol-to-biomass conversion factors for aquatic hyphomycetes. Appl Environ Microbiol 59:502–507
Gomi T, Sidle RC, Richardson JS (2002) Understanding processes and downstream linkages of headwater systems. Bioscience 52(10):905–916
González JM, Mollá S, Roblas N, Descals E, Moya Ó, Casado C (2013) Small dams decrease leaf litter breakdown rates in Mediterranean mountain streams. Hydrobiologia 712:117–128. doi:10.1007/s10750-012-1144-7
Graça MAS (2001) The role of invertebrates on leaf litter decomposition in streams—a review. Int Rev Hydrobiol 86:383–393
Graça MAS, Bärlocher F, Gessner MO (2007) Methods to study litter decomposition: a practical guide, 2nd ed. Springer editions, pp 329. doi:10.1007/1-4020-3466-0
Graça MAS, Ferreira V, Canhoto C, Encalada AC, Guerrero-Bolaño F, Wantzen KM, Boyero L (2015) A conceptual model of litter breakdown in low order streams: litter breakdown in low order streams. Int Rev Hydrobiol 100:1–12. doi:10.1002/iroh.201401757
Gulis V, Suberkropp K (2003) Interactions between stream fungi and bacteria associated with decomposing leaf litter at different levels of nutrient availability. Aquat Microb Ecol 30:149–157
Hughes G, Lord E, Wilson L, Gooday R, Anthony S (2008) Updating previous estimates of the load and source apportionment of nitrogen to waters in the UK. Defra Project: WQ0111 104 p
Ippolito A, Todeschini R, Vighi M (2012) Sensitivity assessment of freshwater macroinvertebrates to pesticides using biological traits. Ecotoxicology 21:336–352. doi:10.1007/s10646-011-0795-x
Joulin A (2006) Les produits phytosanitaires utilisés par l’agriculture lorraine. Résultats de l’enquête réalisée auprès des distributeurs lorrains sur la campagne 2004/2005. Available at: <http://draaf.lorraine.agriculture.gouv.fr/IMG/pdf/06_55_Les_produits_phyto_utilises_par_l_agriculture_lorraine_2004_2005_cle0b11a1.pdf>, (accessed August 2014)
Kunz MJ, Wuest A, Wehrli B, Landert J, Senn DB (2011) Impact of a large tropical reservoir on riverine transport of sediment, carbon, and nutrients to downstream wetlands. Water Resour Res 47:W12531. doi:10.1029/2011WR010996
Kreuger J (1998) Pesticides in stream water within an agricultural catchment in southern Swenden, 1990-1996. Sci Total Environ 216:227–251
Lazartigues A (2013) Pesticides et polyculture d’étang: de l’épandage sur le bassin versant aux résidus dans la chair de poisson. PhD report. Lorraine University
Lazartigues A, Banas D, Feidt C, Brun-Bellut J, Thomas M (2012) Pesticide pressure and fish farming in barrage pond in Northeastern France part I: site characterization and water quality. Environ Sci Pollut Res 19:2802–2812. doi:10.1007/s11356-012-0784-5
Lazartigues A, Thomas M, Cren-Olivé C, Brun-Bellut J, Le Roux Y, Banas D, Feidt C (2013a) Pesticide pressure and fish farming in barrage pond in Northeastern France. Part II: residues of 13 pesticides in water, sediments, edible fish and their relationships. Environ Sci Pollut Res 20:117–125. doi:10.1007/s11356-012-1167-7
Lazartigues A, Banas D, Feidt C, Brun-Bellut J, Gardeur JN, Le Roux Y, Thomas M (2013b) Pesticide pressure and fish farming in barrage pond in northeastern France. Part III: how management can affect pesticide profiles in edible fish? Environ Sci Pollut Res 20:126–135. doi:10.1007/s11356-012-0824-1
Lecerf A (2005) Perturbations anthropiques et fonctionnement écologique des cours d’eau de tête de bassin : étude du processus de décomposition des litières. Thèse de doctorat. Université de Toulouse
Liess M, Van der Ohe PC (2005) Analyzing effects of pesticides on invertebrate communities in streams. Environ Toxicol Chem 24:954–965
Liess M, Schäfer RB, Schriever CA (2008) The footprint of pesticide stress in communities—species traits reveal community effects of toxicants. Sci Total Environ 406:484–490. doi:10.1016/j.scitotenv.2008.05.054
Le Quéré G, Marcel J (1999) La pisciculture d’étangs française. Rapport de l’Institut Technique de l’Aviculture (ITAVI), Unsaaeb, Ofival, ministère de l'Agriculture et de la pêche Paris, p 57
MacDonald LH, Coe D (2007) Influence of headwater streams on downstream reaches in forested areas. For Sci 53(2):148–168
Maltby L, Hills L (2008) Spray drift of pesticides and stream macroinvertebrates: experimental evidence of impacts and effectiveness of mitigation measures. Environ Pollut 156:1112–1120. doi:10.1016/j.envpol.2008.04.013
Martínez A, Larrañaga A, Basaguren A, Pérez J, Mendoza-Lera C, Pozo J (2013) Stream regulation by small dams affects benthic macroinvertebrate communities: from structural changes to functional implications. Hydrobiologia 711:31–42. doi:10.1007/s10750-013-1459-z
Mathé S, Rey-Valette H (2015) Local knowledge of pond fish-farming ecosystem services: management implications of stakeholders’ perceptions in three different contexts (Brazil, France and Indonesia). Sustainability 7:7644–7666. doi:10.3390/su7067644
Mazzia C, Pasquet A, Caro G, Thénard J, Cornic JF, Hedde M, Capowiez Y (2015) The impact of management strategies in apple orchards on the structural and functional diversity of epigeal spiders. Ecotoxicology 24:616–625. doi:10.1007/s10646-014-1409-1
McArdle BH, Anderson MJ (2001) Fitting multivariate models to community data: a comment on distance-based redundancy analysis. Ecology 82:290–297
Meteo France (2011) Fiche climatologique: statistiques 1981–2010 et records. Château Salins
Méthot G, Hudon C, Gagnon P, Pinel-Alloul B, Armellin A, Poirier AMT (2012) Macroinvertebrate size–mass relationships: how specific should they be? Freshwater. Science 31:750–764. doi:10.1899/11-120.1
Ministère de l’environnement et du développement durable et Agences de l’eau (2003) Système d’évaluation de la qualité de l’eau des cours d’eau. Rapport de présentation SEQ-Eau (V2)
Mondy CP, Villeneuve B, Archaimbault V, Usseglio-Polatera P (2012) A new macroinvertebrate-based multimetric index (I2M2) to evaluate ecological quality of French wadeable streams fulfilling the WFD demands: a taxonomical and trait approach. Ecol Indic 18:452–467. doi:10.1016/j.ecolind.2011.12.013
Morrissey CA, Mineau P, Devries JH, Sanchez-Bayo F, Liess M, Cavallaro MC, Liber K (2015) Neonicotinoid contamination of global surface waters and associated risk to aquatic invertebrates: a review. Environ Int 74:291–303. doi:10.1016/j.envint.2014.10.024
Negishi JN, Inoue M, Nunokawa M (2002) Effects of channelisation on stream habitat in relation to a spate and flow refugia for macroinvertebrates in northern Japan. Freshw Biol 47:1515–1529. doi:10.1046/j.1365-2427.2002.00877.x
Nelson D (2011) Gammarus-microbial interactions: a review. International Journal of Zoology :6. doi:10.1155/2011/295026Article ID 295026
Passy P, Garnier J, Billen G, Fesneau C, Tournebize J (2012) Restoration of ponds in rural landscapes: modelling the effect on nitrate contamination of surface water (the Seine River Basin, France). Sci Total Environ 430:280–290. doi:10.1016/j.scitotenv.2012.04.035
Pedersen ML (2009) Effects of channelisation, riparian structure and catchment area on physical habitats in small lowland streams. Fundamental and Applied Limnology/Archiv für Hydrobiologie 174:89–99
Peters K, Bundschuh M, Schäfer RB (2013) Review on the effects of toxicants on freshwater ecosystem functions. Environ Pollut 180:324–329
Petersen RC, Cummins KW (1974) Leaf processing in a woodland stream. Freshw Biol 4:343–368
Piscart C, Genoel R, Doledec S, Chauvet E, Marmonier P (2009) Effects of intense agricultural practices on heterotrophic processes in streams. Environ Pollut 157:1011–1018. doi:10.1016/j.envpol.2008.10.010
Pokorný J, Hauser V (2002) The restoration of fish ponds in agricultural landscapes. Ecol Eng 18:555–574
Poole GC (2010) Stream hydrogeomorphology as a physical science basis for advances in stream ecology. J N Am Benthol Soc 29:12–25
Pringle CM (1997) Exploring how disturbance is transmitted upstream: going against the flow. J N Am Benthol Soc 16:425–438
Rasmussen JJ (2012) Pesticide effects on the structure and function of stream ecosystems. PhD report. Aarhus University
Rasmussen JJ, Wiberg-Larsen P, Baattrup-Pedersen A, Monberg RJ, Kronvang B (2012) Impacts of pesticides and natural stressors on leaf litter decomposition in agricultural streams. Sci Total Environ 416:148–155. doi:10.1016/j.scitotenv.2011.11.057
Robinson CT, Gessner MO (2000) Nutrient addition accelerates leaf breakdown in an alpine Springbrook. Oecologia 122:258–263
Roche PA, Billen G, Bravard JP, Décamps H, Pennequin D, Vindimian E, Wasson JG (2005) Les enjeux de recherche liés à la directive-cadre européenne sur l’eau. Comptes Rendus Géoscience 337:243–267. doi:10.1016/j.crte.2004.10.012
Schäfer RB, Caquet T, Siimes K, Mueller R, Lagadic L, Liess M (2007) Effects of pesticides on community structure and ecosystem functions in agricultural streams of three biogeographical regions in Europe. Sci Total Environ 382:272–285. doi:10.1016/j.scitotenv.2007.04.040
Schäfer R, Pettigrove V, Rose G, Allinson G, Wightwick A, Von der Ohe PC et al (2011) Effects of pesticides monitored with three sampling methods in 24 sites on macroinvertebrates and microorganisms. Environ Sci Technol 45:1665–1672
Strahler AN (1957) Quantitative analysis of watershed geomorphology. Eos, Transactions, American Geophysical Union 38(6):913–920
Suberkropp K (1998) Effect of dissolved nutrients on two aquatic hyphomycetes growing on leaf litter. Mycology Research 102:998–1002
Tachet H, Richoux P, Bournaud M, Usseglio-Polatera P (2010) Invertébrés d’eau douce. Systématique, biologie, écologie. CNRS éditions. 607 pp. ISBN: 978-2-271-06945-0
Tank JL, Rosi-Marshall EJ, Griffiths NA, Entrekin SA, Stephen ML (2010) A review of allochthonous organic matter dynamics and metabolism in streams. J N Am Benthol Soc 29:118–146. doi:10.1899/08-170.1
Team RDC (2008) R a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Thomas K, Chilvers GA, Norris RH (1991) Changes in concentration of aquatic hyphomycete spores in Lees Creek, ACT, Australia. Mycol Res 95(Z):178–183
Touchart L, Bartout P (2011) La gestion du risque thermique en étang. Le cas de la dérivation / Riscuri si catastrofe / Cluj-Napoca / p : 149–161. (Revue indexée dans Index Copernicus International et dans Directory of Open Access Journals)
Vinebrooke RD, Cottingham KL, Norberg J, Scheffer M, Dodson SI, Maberly SC, Sommer U (2004) Impacts of multiple stressors on biodiversity and ecosystem functioning: the role of species co-tolerance. Oikos 104:451–457
Vörösmarty CJ, McIntyre PB, Gessner MO, Dudgeon D, Prusevich A, Green P, Glidden S, Bunn SE, Sullivan CA, Liermann CR, Davies PM (2010) Global threats to human water security and river biodiversity. Nature 467:555–561
Wallace JB, Eggert SL, Meyer JL, Webster JR (1997) Multiple trophic levels of a forest stream linked to terrestrial litter inputs. Science 277:102–104
Ward JV, Stanford JA (1983) The serial discontinuity concept of lotic ecosystems. In: T.D. Fontaine, S.M. Bartell (eds) Dynamics of lotic ecosytems. Ann Arbor Sciences, Ann Arbor, pp 29–42
White PJ, Hammond JP (2009) The sources of phosphorus in the waters of Great Britain. Journal of Environment Quality 38:13–26
Woodward G, Gessner MO, Giller PS, Gulis V, Hladyz S, Lecerf A, Malmqvist B, McKie BG, Tiegs SD, Cariss H, Dobson M, Elosegi A, Ferreira V, Graca MAS, Fleituch T, Lacoursiere JO, Nistorescu M, Pozo J, Risnoveanu G, Schindler M, Vadineanu A, Vought LBM, Chauvet E (2012) Continental-scale effects of nutrient pollution on stream ecosystem functioning. Science 336:1438–1440. doi:10.1126/science.1219534
Xiao Y, Mignolet C, Mari JF, Benoît M (2014) Modeling the spatial distribution of crop sequences at a large regional scale using landcover survey data: a case from France. Comput Electron Agric 102:51–63. doi:10.1016/j.compag.2014.01.010
Zar JH (1996) Biostatistical Analysis. Prentice-Hall, Eryelwood Cliffs, pp 663
Acknowledgments
The authors are grateful to the ‘Agence de l’Eau Rhin-Meuse’ and the ‘Zone Atelier Moselle’ for their financial support to this project. We sincerely thank P. Hartmeyer, A. Iuretig and A. Rivière for their work, fish farmers, the Domaine de Lindre and the National Forests Office that provided access permits for sampling.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Four, B., Arce, E., Danger, M. et al. Catchment land use-dependent effects of barrage fishponds on the functioning of headwater streams. Environ Sci Pollut Res 24, 5452–5468 (2017). https://doi.org/10.1007/s11356-016-8273-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-8273-x