Abstract
In shallow lakes with large littoral zones, epiphytes and submerged macrophytes can make an important contribution to the total annual primary production. We investigated the primary production (PP) of phytoplankton, submerged macrophytes, and their epiphytes, from June to August 2005, in two large shallow lakes. The production of pelagic and littoral phytoplankton and of the dominant submerged macrophytes in the littoral zone (Potamogeton perfoliatus in Lake Peipsi and P. perfoliatus and Myriopyllum spicatum in Lake Võrtsjärv) and of their epiphytes was measured using a modified 14C method. The total PP of the submerged macrophyte area was similar in both lakes: 12.4 g C m−2 day−1 in Peipsi and 12.0 g C m−2 day−1 in Võrtsjärv. In Peipsi, 84.2% of this production was accounted for by macrophytes, while the shares of phytoplankton and epiphytes were low (15.6 and 0.16%, respectively). In Võrtsjärv, macrophytes contributed 58%, phytoplankton 41.9% and epiphytes 0.1% of the PP in the submerged macrophyte area. Epiphyte production in both lakes was very low in comparison with that of phytoplankton and macrophytes: 0.01, 5.04, and 6.97 g C m−2 day−1, respectively, in Võrtsjärv, and 0.02, 1.93, and 10.5 g C m−2 day−1, respectively, in Peipsi. The PP of the littoral area contributed 10% of the total summer PP of Lake Peipsi sensu stricto and 35.5% of the total summer PP of Lake Võrtsjärv.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Littoral zones of aquatic ecosystems are among the most productive communities on earth (Goldsborough et al. 2005). In a shallow lake with a large biomass of submerged macrophytes and epiphytes, the littoral zone may be an important contributor to total lake primary production (PP) and an important regulator of nutrient fluxes (Galanti and Romo 1997). Attached microalgae can make important contributions to the total annual PP, especially in shallow lakes with large littoral zones. In the large, shallow, and alkaline Borax Lake (California), benthic periphyton contributed 69% of the total annual PP (Wetzel 1964). Epiphytic algae have been reported to contribute 6–71% toward the total littoral PP (Müller 2000).
Several papers report the relative contributions of macrophytes, periphyton, and phytoplankton to total lake PP. Sand-Jensen and Borum (1991) determined that phytoplankton, periphyton, macroalgae, and rooted macrophytes contributed about 35–55, 10–15, 25–35 and 15–20%, respectively, toward the total PP in Roskilde Fjord, Denmark. In comparison, models for lacustrine wetlands in the semi-arid Laramie Basin (western United States) estimated that the relative contributions of phytoplankton, epiphytes, epipelon, submerged macrophytes and emergent macrophytes to the total littoral PP were 3–15, 20–32, 1–10, 15–67 and 0–50%, respectively (Hart and Lovvorn 2000). In a large widening of the St. Lawrence River known as Lac St. Pierre, the modeled contributions of phytoplankton, submerged macrophytes, and emergent macrophytes to the total PP were 29–38, 14–19, 25–29 and 23%, respectively (Vis et al. 2007).
Productivity studies in shallow water environments demonstrate that epiphytic algae can provide an abundant, rapidly renewed, and easily assimilated food resource that can be more important than that of macrophytes (Wetzel 2001). Epiphytes represent a food resource complementary to that of phytoplankton for consumers and increase the biological diversity of all trophic levels (Galanti and Romo 1997). The macrophyte–epiphyte complex has been described as a unique ecological unit within shallow aquatic ecosystems, possessing complex inter-relationships not found in open water zones (Goldsborough et al. 2005).
Although epiphyte productivity contributes significantly to the total annual PP in the littoral zones, its relative importance varies seasonally owing to species phenology. In spring, diatoms are commonly the dominant group in epiphyton, but in summer blue–green or green algae may be dominant epiphytes (Cattaneo 1983; Meulemans 1988; Müller 1994). Epiphyte biomass also increases during the growing season (Borum and Wium-Andersen 1980; Devyatkin 1979; Jenkerson and Hickman 1986; Müller 1995).
Epiphyte biomass and growth are strongly influenced by abiotic factors such as nutrient and light availability (Sand-Jensen and Borum 1991). Light availability can control the rate and vertical distribution of PP, while extremely high light intensities can inhibit photosynthesis (Hansson 1992). For periphyton it is important to consider the self-shading effect if their biofilms grow too dense (Boston and Hill 1991). High phytoplankton densities can also severely reduce the availability of light for periphyton and macrophytes (Sand-Jensen and Borum 1991; Hansson 1992).
The aim of the present study was to estimate the contribution of submerged macrophytes and their epiphytes to the total PP in the littoral zones of two large, shallow lakes in the northern temperate region. The contribution of the littoral PP to total lake PP was also estimated.
Study area
Lake Võrtsjärv (58°16′N 26°02′E) is situated in central Estonia. With a surface area (A o) of 270 km2 and catchment area of 3,374 km2, it is the country’s second largest lake. Lake Võrtsjärv is shallow (maximum depth Z m = 6 m, mean depth \( \overline{Z} \)= 2.8 m), highly eutrophic (Tuvikene et al. 2004; mean chlorophyll a = 24 μg l−1) and polymictic (Nõges et al. 2007). Macrophytes cover 50.7 km2 (18.8% A o): 35.2 km2 submerged, 12.3 km2 emergent, and 3.2 km2 floating (Feldmann and Mäemets 2004). In the 1960s, the dominant submerged macrophyte was Potamogeton perfoliatus; however, Myriopyllum spicatum is currently dominant.
Lake Peipsi (58°40′N 27°26′E) has an A o of 3,555 km2 and is located on the border between Estonia and Russia (Jaani 2001). The lake consists of three basins: (1) the northern basin Peipsi s.s. (A o = 2,611 km2, Z m = 12.9 m, \( \overline{Z} \) = 8.3 m), which is meso-eutrophic (Pihu and Haberman 2001), (2) the strait-like middle basin named Lake Lämmijärv (A o = 236 km2, Z m = 15.3 m, \( \overline{Z} \) = 2.5 m), and (3) the southern basin named Lake Pihkva (A o = 708 km2, Z m = 5.3 m, \( \overline{Z} \) = 3.8 m). Lake Peipsi has extensive areas with a depth <3m, which is potentially suitable for macrophyte growth. However, because of intensive wind-induced erosion, macrophyte bottom cover is only about 1.7% (or 44.4 km2: 4.8 km2 emergents, 38.9 km2 submergents, 0.6 km2 floating) in Peipsi s.s. and up to 8% in Lakes Lämmijärv and Pihkva (Mäemets and Mäemets 2001). The dominant submerged species is P. perfoliatus (Mäemets et al. 2006).
A location map of Lakes Peipsi and Võrtsjärv is provided by Nõges et al. (2007).
Materials and methods
Estimates of PP in Lake Võrtsjärv were undertaken within submerged macrophyte stands along the western shoreline. In Peipsi s.s. (referred to hereafter as Peipsi), experiments were undertaken on the western shoreline near Varnja (population 250). Macrophytes for the experiments were sampled during the summer months (June, July and August) of 2005. For measuring the PP of epiphytes and macrophytes, the dominant submerged macrophyte species were selected from both lakes: P. perfoliatus in Peipsi and M. spicatum and P. perfoliatus in Võrtsjärv. PP was estimated in situ using the 14C assimilation technique first introduced by Steeman-Nielsen (1952), following modifications by Kairesalo (1976) and Cattaneo and Kalff (1980).
Macrophytes were carefully removed from the lake and pieces of leaves from upper, middle, and lower sections were placed separately in 30 ml glass bottles filled with lake water with 2 μCi NaH14CO3 (VKI, Denmark). The average (±1 S.D.) dry weight (DW) of macrophyte material per bottle was 0.13 ± 0.09 g. The bottles were incubated for 4 h within macrophyte stands at depths representing natural conditions: 0.2 m for upper macrophyte sections, 0.5 m for middle sections, and 0.7 m for lower sections. Three light replicates were used for each depth. Nonphotosynthetic carbon fixation was measured in darkened bottles and subtracted from the carbon fixed in the light bottles. After incubation, three sub-samples were taken from each bottle and placed in scintillation counter vials. First, 5 ml of water was taken before shaking off the epiphytes. Production in this sub-sample represented production by phytoplankton. Second, epiphytes were removed from macrophytes by vigorous shaking for 2 min (Kassim and Al-Saadi 1995; Galanti and Romo 1997; Cattaneo et al. 1998) and a second 5 ml water sample was taken. Production in this sub-sample represented the sum of phytoplankton and epiphyte production. Lastly, each macrophyte section was removed from the bottle and placed in a vial containing 5 ml of distilled water; 150 μl of 0.5 N HCl was added to each sub-sample to remove 14Cinorg. All vials were held in the laboratory for 24 h to allow the 14Cinorg fraction to evaporate (Lignell 1992), then 10 ml of OptiPhase HiSafe 3 (Perkin Elmer) scintillation fluid was added to each vial and radioactivity was measured using an LSC RackBeta 1211 (Wallac, Finland).
Macrophyte sections were then removed from the vials and dried for 24 h at 105°C. Epiphyte and macrophyte production were calculated according to Ærtbjerg-Nielsen and Bresta (1984) and were expressed as mg C assimilated per g macrophyte DW per hour (mg C g−1 h−1). Daily values of PP were calculated using an equation relating daily PP (PPday) to hourly PP at midday (PPhour), obtained for Lake Võrtsjärv by Nõges and Nõges (1998): PPday = PP/[0.230−(8.9 × 10−3 DL)], where DL denotes the number of hours of daylight.
Relative epiphyte biomass was determined as mg chlorophyll a (Chl a) in the epiphyte sample per g macrophyte tissue DW (mg Chl a g−1). For relative biomass determinations, macrophytes were collected from the sites at which the production experiments were undertaken (above). Upper (top 20 cm) and lower (lowest 10 cm) macrophyte sections were sampled for epiphyte biomass. Epiphytes were removed from the macrophyte sections by shaking vigorously for 2 min in 500 ml glass bottles with 100 ml distilled water (Kassim and Al-Saadi 1995; Galanti and Romo 1997; Cattaneo et al. 1998). Ten millilitre of each resulting suspension was then filtered through GF/C filters (1.2 μm). Chl a was extracted from the filters with 96% ethanol (Moss et al. 2003), measured spectrophotometrically and calculated as per Arvola (1981). Macrophyte sections were dried for 24 h at 105°C and weighed. To compare the PP of phytoplankton with the littoral production of epiphytes and macrophytes we used the results of depth-integrated pelagic phytoplankton PP estimates. Concurrent phytoplankton PP measurements were carried out by the 14C assimilation technique (see Arst et al. 2008). Areal epiphyte and macrophyte PP (mg C m−2 day−1) were calculated using available information on macrophyte biomass (g m−2, DW) for both lakes. Data in Mäemets et al. (2006) were used for the littoral biomass of P. perfoliatus on the Estonian side of Lake Peipsi s.s. (37.6 g m−2). Littoral biomasses of P. perfoliatus (8.37 g m−2) and M. spicatum (15.96 g m−2) in Lake Võrtsjärv were from databases compiled from routine monitoring programs (Feldmann, unpubl. data).
To calculate the total PP in the littoral and pelagic zones, we applied the estimated littoral zone areas for both lakes. The littoral, defined here as the area covered with macrophytes, made up 44.39 km2 (1.7% of the total area) in Peipsi s.s. (Mäemets and Mäemets 2001) and 50.7 km2 (18.8% of the total area) in Võrtsjärv (Feldmann and Mäemets 2004). We used the measured areal production of submerged macrophytes and their epiphytes to calculate the PP of the entire littoral zones.
We used ANOVA of Statistica for Windows version 7.0 to assess differences in PP among the lakes, taxa, and macrophyte sections, the General Linear Model application of SAS to test the impact of different factors (macrophyte part and month) on PP.
We used Secchi depths provided by the State Monitoring Program of the Estonian Ministry of Environment. Incident photosynthetically active radiation (PAR) was measured by irradiance quantum sensor Li-Cor 190SA (Li-Cor Biosciences) at the Estonian Institute of Hydrology and Meteorology (EMHI).
Results
Macrophyte and epiphyte production
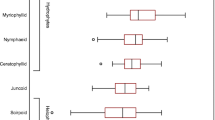
Average P. perfoliatus production in Lake Peipsi was high in June (8.0 mg C g−1 h−1) and August (7.7 mg C g−1 h−1). In July, P. perfoliatus production was lower (4.6 mg C g−1 h−1). In June, production in the middle and lower macrophyte sections was greater (P < 0.05) than in the upper sections (Fig. 1a). Conversely, production was highest in the upper sections in July and August (Fig. 1a). Epiphyte production was highest in August (0.039 mg C g−1 h−1) and lowest in July (0.002 mg C g−1 h−1; Fig. 1d). In June, epiphyte production was high in the lower and middle macrophyte sections, but in July production was greatest in the lower sections and lowest in the middle sections (Fig. 1d). In August, production was highest in the upper sections of P. perfoliatus (Fig. 1d).
Primary production (PP) of different parts (upper, middle, lower) of P. perfoliatus and M. spicatum (a, b, c) and epiphytes on these macrophyte parts (d, e, f) in Lakes Peipsi and Võrtsjärv in 2005. PP was measured from 11.00 to 15.00 h on June 14 in Võrtsjärv; the second measurement series (*) was performed in the afternoon (from 16.00 to 20.00 h). Standard error bars of parallel measurements are denoted
In Lake Võrtsjärv, P. perfoliatus production was highest in July (9.6 mg C g−1 h−1), with production in June and August being slightly lower (9.4 and 7.75 mg C g−1 h−1, respectively) (Fig. 1b). Epiphyte production on P. perfoliatus remained relatively constant throughout the sampling period, remaining within the range of 0.005–0.01 mg C g−1 h−1. In June, P. perfoliatus production was highest in the middle sections, whereas production was highest in the upper sections in July and August (Fig. 1b). Epiphyte production on P. perfoliatus was highest in the middle sections in June and July, but highest in the lower sections in August (Fig. 1e). The lowest epiphyte production occurred in the lower part of the macrophyte in June and July and in the upper part of the macrophyte in August. Production of M. spicatum (Fig. 1c) was highest in June (11.4 mg C g−1 h−1) and much lower in July (3.4 mg C g−1 h−1) and August (3.06 mg C g−1 h−1). Epiphyte production was also highest in June (0.014 mg C g−1 h−1), but only slightly lower in July and August [0.012 and 0.01 mg C g−1 h−1, respectively (Fig. 1f)]. The production of M. spicatum, like that of P. perfoliatus, was highest in the middle part of the macrophyte in June and in the upper part of the macrophyte in July and August (Fig. 1c). In June, epiphyte production on these macrophytes was high in the middle and lower parts of the macrophyte, in July in the middle and upper parts, while in August the PP was almost the same in all parts of the macrophyte (Fig. 1f).
During our measurements incident PAR was 1,600 μmol s−1 m−2 in June and July, and 1,200 μmol s−1 m−2 in August (Fig. 2), Secchi depth in Võrtsjärv (0.5–1 m) was considerably lower than in Peipsi (1.2–2.6 m).
Average daily production of P. perfoliatus in June–August, 2005 was 305 mg C g−1 day−1 in Võrtsjärv and 278 mg C g−1 day−1 in Peipsi. The average daily epiphyte production on P. perfoliatus was 0.303 mg C g−1 day−1 in Võrtsjärv and 0.53 mg C g−1 day−1 in Peipsi. The average daily production of M. spicatum and its epiphytes in Võrtsjärv were 253 and 0.70 mg C g−1 day−1, respectively. Our results of epiphyte production relative to macrophyte biomass are consistent with values reported by other investigators, but our macrophyte production values per unit biomass exceed the literature values quite substantially (Table 1).
The factors that significantly affected the productivity of macrophyes and epiphytes in Lake Peipsi were sampling month and macrophyte section (upper, middle, lower). For epiphyte production the effect of the month was highly significant (P < 0.0001) and the effect of the macrophyte part was moderately significant (P = 0.04). For macrophyte production both month (P = 0.02) and macrophyte part (P = 0.02) were equally significant. The combined effect of month and macrophyte part was highly significant for both epiphyte (P = 0.001) and macrophyte (P < 0.0001) production. In Võrtsjärv, the significant factors for the production of P. perfoliatus were the macrophyte part (P = 0.02) and the combined effect of macrophyte part with month (P = 0.004). For epiphyte production on P. perfoliatus none of these effects proved significant (P = 0.45). For production of M. spicatum and its epiphytes the only statistically significant factor was the month (P < 0.01).
Epiphyte biomass
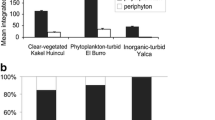
In Lake Peipsi, mean epiphyte biomass was highest in June (56 μg Chl a g−1) and somewhat lower in July and August (36 and 34 μg Chl a g−1). In Lake Võrtsjärv, mean epiphyte biomass on M. spicatum was highest in June (44 μg Chl a g−1), quite similar in August (37 μg Chl a g−1), and lowest in July (13 μg Chl a g−1). Epiphyte biomass on P. perfoliatus did not change much during the study period; in June it was 32 μg Chl a g−1, in July 31 μg Chl a g−1, and in August 34 μg Chl a g−1 (Fig. 3a).
Epiphyte biomass (Be) on M. spicatum and P. perfoliatus in Lakes Peipsi s.s. and Võrtsjärv (a), and primary production (PP) of phytoplankton, macrophytes, and epiphytes in M. spicatum stands in Võrtsjärv (b), and in P. perfoliatus stands in Peipsi (c) and Võrtsjärv (d) in June–August 2005. Standard error bars of parallel measurements are denoted
Statistical analyses showed that the lakes did not differ significantly in the production of P. perfoliatus (P = 0.28) and that the difference between P. perfoliatus and M. spicatum in the same lake was not significant (P = 0.70). Despite the higher (P = 0.01) biomass of epiphytes on P. perfoliatus in Lake Peipsi (42.0 μg Chl a g−1) than in Lake Võrtsjärv (32.3 μg Chl a g−1), epiphyte production did not differ significantly between lakes (P = 0.09). Epiphyte biomass did not differ statistically on the different macrophyte species in Võrtsjärv (P = 0.4), but its production was significantly higher (P = 0.006) on M. spicatum (0.0138 mg C g−1 h−1) than on P. perfoliatus (0.00845 mg C g−1 h−1).
Share of different producers in the total PP of the lakes studied
From June to August in both lakes and both macrophyte stands, epiphyte production was very low in comparison with phytoplankton and macrophyte production (Fig. 3b, c, d); daily averages were, respectively, 0.01, 5.04, and 6.97 g C m−2 day−1 in Võrtsjärv and 0.02, 1.93, and 10.5 g C m−2 day−1 in Peipsi. Average daily total PP of submerged macrophyte area was similar (P = 0.67 for the difference) in the two lakes: 12.4 g C m−2 day−1 in Lake Peipsi and 12.0 g C m−2 day−1 in Lake Võrtsjärv. In Peipsi, 84.2% of this production was accounted for by macrophytes, while the shares of phytoplankton and epiphytes were low (15.6 and 0.16%, respectively). In Võrtsjärv, macrophytes contributed 58%, phytoplankton 41.9%, and epiphytes 0.1% to littoral production. The PP of the littoral area contributed 10% to the total summer PP of Peipsi s.s. and 35.5% to the total summer PP of Võrtsjärv (Table 2).
Discussion
Our results showed that macrophyte and epiphyte production was variable throughout the growing season in both lakes, for the same macrophyte species (P. perfoliatus) in different lakes, and for different macrophyte species (M. spicatum and P. perfoliatus) in the same lake (Fig. 1). In large lakes, strong wave action may adversely affect epiphyte establishment and growth (Devyatkin 1979; Strand and Weisner 1996). However, according to our data, the biomass of epiphytes was greater in the larger Peipsi than in Võrtsjärv. In the shallower Lake Võrtsjärv, stands of submerged macrophytes occupy much larger areas and are more exposed to wind action and mechanical disturbance by waves than in the deeper Lake Peipsi, where submerged macrophytes can develop only in sheltered areas close to the shoreline.
Owing to differences in leaf architecture, M. spicatum offers a larger leaf area suitable for epiphyte attachment than P. perfoliatus. Differences in epiphyte production between macrophyte species may occur because of these different macrophyte structures (Romo and Galanti 1998). Our analysis in Võrtsjärv showed that the average epiphyte production was significantly higher on M. spicatum than on P. perfoliatus, although the epiphyte biomass did not differ statistically between the macrophyte species.
Different macrophyte species may exhibit seasonally variable growth patterns (Wetzel 2001). In our study, M. spicatum production was highest in June and decreased toward August, while the production of P. perfoliatus was relatively constant during the study period. The distribution of production between the different macrophyte parts was quite similar in both macrophyte species. The differences in production among macrophyte parts could be explained by differences in the light conditions to which those parts were exposed. Generally, greater light availability should increase photosynthesis for the upper macrophyte part (Wetzel 2001), while light that is too intense may also inhibit photosynthesis (Rae et al. 2001). In our study, the light intensities in June and July were quite similar, while the water was more transparent in June (Fig. 2). Therefore, the upper macrophyte parts were exposed to more intense light in June and production was likely to be photoinhibited. In August, the light intensity was lower, Secchi depth was quite small and, consequently, photoinhibition did not occur.
In spite of the generally positive correlation between macrophyte and epiphyte production in our study, the seasonal production pattern of epiphytes differed from that of the macrophytes. Besides the influence of light and nutrient availability, fish and invertebrates can graze epiphyton, reducing its biomass (Cattaneo 1983). At the same time this grazing may increase the specific production of epiphytes by diminishing self-shading and competition for nutrients (Cattaneo and Kalff 1980; Hatcher 1983; Hay 1991). Changes in epiphyte production can also be induced by changes in the epiphyte algal community (Cattaneo and Kalff 1979).
Our comparison of the PP of different producers (epiphytes, macrophytes and phytoplankton) showed that macrophytes are important primary producers in the littoral zone in both studied lakes, but epiphytes had a very low share of production, only 0.1–0.2%. Although the calculated daily summer PP of the submerged macrophyte area was similar (about 12 g C m−2) in both lakes, different producers had different shares in this PP. In highly eutrophic Võrtsjärv the share of macrophytes (58%) was lower and the share of phytoplankton (41.9%) was higher than in meso-eutrophic Peipsi s.s. where macrophytes and phytoplankton, respectively, contributed 84.2 and 15.6% of the daily summer PP in the submerged macrophyte area. In more eutrophic lakes, high phytoplankton biomass may shade macrophytes and epiphytes (Romo et al. 2007), causing a reduction in their share in the total PP. However, the share of littoral PP in the total PP of the lake was 3.6 times greater in Võrtsjärv than in Peipsi. In the larger and deeper Peipsi the littoral area is smaller than in Võrtsjärv (Table 2), and on a relative scale the difference between the two lakes is more than tenfold: the littoral zone occupies about 1.7% of the total area of Peipsi s.s. and 19% in Võrtsjärv. Therefore, the share of littoral PP in the total PP of the lake was also much greater in Võrtsjärv.
Our results on macrophyte production exceed the literature values quite substantially (Table 1). Moreover, the share of epiphytic algal PP in the total littoral primary production estimated in our study (0.1–0.2%) is substantially lower than the values reported in the literature (5.5–71% as reviewed by Müller 2000). In Lake Lawrence (Michigan), epiphytic algae were responsible for 31.3% of the total littoral production and for 21.4% of the total annual production of the whole lake (Allen 1971). However, the A o of Lake Lawrence is only 0.05 km2, and most of the other studies reviewed have also been conducted in small lakes. Lake Memphremagog (Québec) is a large but very long and narrow lake, and McPherson Bay, where the study of Cattaneo and Kalff (1980) was conducted, has a rather small area. Lakes Peipsi and Võrtsjärv are large lakes with quite simple shorelines, and our measurements were made in the littoral adjoined to the large open water area. We assume that in such large lakes as Peipsi and Võrtsjärv, the macrophyte stands are much more actively disturbed by wave action, which interferes with the colonization of macrophytes by epiphytic algae and at the same time supplies nutrients to macrophytes. Therefore, the productivity of macrophytes in such systems is much higher and the contribution of epiphytes to the total primary productivity is much less important than in small lakes.
As the main aim of our study was to estimate the contribution of submerged macrophytes and their epiphytes to the total PP of large and shallow lakes, our most important result was that for the first time the total primary productivity and the share of different producers (epiphytes, macrophytes, and phytoplankton) was estimated in large shallow eutrophic temperate lakes. These results would give a basis for the further intra- and supra-regional comparisons and will also serve as the basis of the calculation of the carbon budget of these large lakes. In our further studies, we plan to use more sophisticated equipment for the measurements of seasonal and vertical distribution of light in the macrophyte beds and to discuss more thoroughly the causes of the seasonal and vertical variations of the productivity.
References
Ærtbjerg-Nielsen G, Bresta AM (eds) (1984) Guidelines for the measurement of phytoplankton primary production, 2nd edn. The Baltic Marine Biologists Publ 1, Charlottenlund
Allen HL (1971) Primary productivity, chemo-organotrophy, and nutritional interactions of epiphytic algae and bacteria on macrophytes in the littoral of a lake. Ecol Monogr 41:98–127. doi:10.2307/1942387
Arst H, Nõges T, Nõges P, Paavel B (2008) Relationships between primary phytoplankton production in situ, chlorophyll concentration and underwater irradiance in turbid lakes. Hydrobiologia 599:169–176. doi:10.1007/s10750-007-9213-z
Arvola L (1981) Spectrophotometric determination of chlorophyll a and phaeopigments in ethanol extraction. Ann Bot Fenn 18:221–227
Borum J, Wium-Andersen S (1980) Biomass variation and autotrophic production of an epiphyte-macrophyte community in a costal Danish area: II. Epiphyte series composition, biomass and production. Ophelia 23:165–179
Boston HL, Hill WR (1991) Photosynthesis-light relations of stream periphyton communities. Limnol Oceanogr 36:644–656
Cattaneo A (1983) Grazing on epiphytes. Limnol Oceanogr 28:124–132
Cattaneo A, Kalff J (1979) Primary production of algae growing on natural and artificial plants: a study of interactions between epiphytes and their substrate. Limnol Oceanogr 24:1031–1037
Cattaneo A, Kalff J (1980) The relative contribution of aquatic macrophytes and their epiphytes to the production of macrophyte beds. Limnol Oceanogr 25:280–289
Cattaneo A, Galanti G, Gentinetta S, Romo S (1998) Epiphytic algae and macroinvertebrates on submerged and floating-leaved macrophytes in an Italian lake. Freshw Biol 39:725–740. doi:10.1046/j.1365-2427.1998.00325.x
Devyatkin VG (1979) Dinamika razvitiya al’goflory obrastanij v Rybinskom vodokhranilishche. In: Flora I rastitel’nost vodoemov bassejna Verkhnej Volgi. Trudy IBVV RAN 42: 78–108 (in Russian)
Feldmann T, Mäemets H (2004) Macrophytes. In: Haberman J, Pihu E, Raukas A (eds) Lake Võrtsjärv. Estonian Encyclopaedia Publishers, Tallinn, pp 185–205
Galanti G, Romo S (1997) Epiphyton biomass on the floating leaved water chestnut (Trapa natans) and its importance for the carbon balance in the eutrophic Lake Candia (N. Italy). Mem Ist Ital Idrobiol 56:95–111
Goldsborough LG, McDougal RL, North AK (2005) Periphyton in freshwater lakes and wetlands. In: Azim ME, Verdegem MCJ, van Dam AA, Beveridge MCM (eds) Periphyton: ecology, exploitation and management. CABI Publishing, London, pp 71–89
Hansson L-A (1992) Factors regulating periphytic algal biomass. Limnol Oceanogr 37:322–328
Hart EA, Lovvorn JR (2000) Vegetation dynamics and primary production in saline, lacustrine wetlands of a Rocky Mountain basin. Aquat Bot 66:21–39. doi:10.1016/S0304-3770(99)00021-2
Hatcher BG (1983) Grazing in coral substrate ecosystems. In: Barnes DJ (ed) Perspectives on coral substrates. Brian Clouston Publishers, Manuka, pp 164–179
Hay ME (1991) Fish-seaweed interactions on coral substrates: effects of herbivorous fishes and adaptations of their prey. In: Sale PF (ed) The ecology of fishes on coral substrates. Academic Press, London, pp 96–119
Jaani A (2001) The location, size and general characterization of Lake Peipsi. In: Nõges T (ed) Lake Peipsi: meteorology, hydrology, hydrochemistry. Sulemees Publishers, Tartu, pp 10–17
Jenkerson CG, Hickman M (1986) Interrelationships among the epipelon, epiphyton and phytoplankton in a eutrophic lake. Int Rev Gesamten Hydrobiol 71:557–579. doi:10.1002/iroh.19860710409
Kairesalo T (1976) Measurements of production of epilithiphyton and littoral plankton in Lake Pääjärvi, southern Finland. Ann Bot Fenn 13:114–118
Kassim TI, Al-Saadi HA (1995) Seasonal variation of epiphytic algae in a marsh area (southern Iraq). Acta Hydrobiol 37:153–161
Lignell R (1992) Problems in filtration fractionation of 14C primary productivity samples. Limnol Oceanogr 37:172–178
Luup H (2003) Spatial and temporal development of epiphytic community in some Estonian lakes. Master thesis, Estonian Agricultural University, Tartu
Mäemets A, Mäemets H (2001) Macrophytes. In: Pihu E, Haberman J (eds) Lake Peipsi. Flora and Fauna. Sulemees Publishers, Tartu, pp 9–22
Mäemets H, Freiberg L, Haldna M, Möls T (2006) Inter-annual variability of Potamogeton perfoliatus stands. Aquat Bot 85:177–183. doi:10.1016/j.aquabot.2006.03.008
Meulemans JT (1988) Seasonal changes in biomass and production of periphyton growing upon reed in Lake Maarsseveen I. Arch Hydrobiol 112:21–42
Moss B, Stephen D, Alvarez C et al (2003) The determination of ecological quality in shallow lakes - a tested system (ECOFRAME) for implementation of the European Water Framework Directive. Aquat Conserv: Mar Freshwat Ecosyst 13:507–549. doi:10.1002/aqc.592
Müller U (1994) Seasonal development of epiphytic algae on Phragmites australis (Cav.) Trin. ex Sten. in a eutrophic lake. Arch Hydrobiol 129:273–292
Müller U (1995) Vertical zonation and production rates of epiphytic algae on Phragmites australis. Freshw Biol 34:69–80. doi:10.1111/j.1365-2427.1995.tb00424.x
Müller U (2000) Periphytic primary production during spring. A sink or source of oxygen in the littoral zone? Limnologica 30:169–174. doi:10.1016/S0075-9511(00)80012-2
Nõges P, Nõges T (1998) Stratification of Estonian lakes studied during hydrooptical expeditions in 1995–97. Proc Estonian Acad Sci Biol Ecol 47:268–281
Nõges T, Järvet A, Kisand A, Laugaste R, Loigu E, Skakalski B, Nõges P (2007) Reaction of large and shallow lakes Peipsi and Võrtsjärv to the changes of nutrient loading. Hydrobiologia 584:253–264. doi:10.1007/s10750-007-0603-z
Pihu E, Haberman J (eds) (2001) Lake Peipsi. Flora and Fauna. Sulemees Publishers, Tartu
Rae R, Hanelt D, Hawes I (2001) Sensitivity of freshwater macrophytes to UV radiation: relationship to depth zonation in an oligotrophic New Zealand lake. Mar Freshw Res 52:1023–1032. doi:10.1071/MF01016
Romo S, Galanti G (1998) Vertical and seasonal distribution of epiphytic algae on water chestnut (Trapa natans). Arch Hydrobiol 141:483–504
Romo S, Villena M-J, Garcia-Murica A (2007) Epiphyton, phytoplankton and macrophyte ecology in a shallow lake under in situ experimental conditions. Arch Hydrobiol 170(3):197–209
Sand-Jensen K, Borum J (1991) Interactions among phytoplankton, epiphyton and macrophytes in temperate freshwaters and estuaries. Aquat Bot 41:137–175. doi:10.1016/0304-3770(91)90042-4
Steeman-Nielsen E (1952) The use of radioactive carbon (C14) for measuring organic production in the sea. J Cons Perm Int Explor Mer 18:117–140
Strand JA, Weisner SEB (1996) Wave exposure related growth of epiphyton: implications for the distribution of submerged macrophytes in eutrophic lakes. Hydrobiologia 325:113–119. doi:10.1007/BF00028271
Tuvikene L, Kisand A, Tõnno I, Nõges P (2004) Chemistry of lake water and bottom sediments. In: Haberman J, Pihu E, Raukas A (eds) Lake Võrtsjärv. Estonian Encyclopaedia Publishers, Tallinn, pp 89–102
Vis C, Hudon C, Carignan R, Gagnon P (2007) Spatial analysis of production by macrophytes, phytoplankton and epiphyton in a large river system under different water-level conditions. Ecosystems (N Y, Print) 10:293–310. doi:10.1007/s10021-007-9021-3
Wetzel RG (1964) A comparative study of the primary productivity of higher plants, periphyton and phytoplankton in large shallow lake. Int Rev Gesamten Hydrobiol 49:1–61. doi:10.1002/iroh.19640490102
Wetzel RG (2001) Limnology, third edition. Academic Press Elsevier, San Diego
Acknowledgments
Funding was provided by the Estonian Ministry of Education (SF 0170011508) and Estonian Science Foundation grants 7156 and 7600. We thank Dr. Margus Pihlak for statistical advice, and we are grateful to four anonymous reviewers for their patience and valuable comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nõges, T., Luup, H. & Feldmann, T. Primary production of aquatic macrophytes and their epiphytes in two shallow lakes (Peipsi and Võrtsjärv) in Estonia. Aquat Ecol 44, 83–92 (2010). https://doi.org/10.1007/s10452-009-9249-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-009-9249-4