Abstract
Running waters are among the most threatened ecosystems globally, having altered hydrological regimes, homogenized habitat, and impaired water quality. These multiple stressors impact aquatic biodiversity and ecosystem function across space and time, although a clear mechanistic understanding is still lacking. Here, we examined the trophic response of macroinvertebrates among streams in a Swiss lowland catchment encompassing a gradient of land uses. Clear compositional changes were observed as anthropogenic impacts increased from least-impacted to agricultural and urbanized sites. Taxonomic diversity was lowest at sites with morphological and water quality impairment (agricultural sites), whereas taxonomic identity (susceptible vs. generalist species) mainly changed due to water quality degradation (agricultural and urban sites) based on the SPEAR (pesticides) index. Using stable isotopes (δ13C, δ15N), a simplification in macroinvertebrate trophic structure was evident along the land use gradient. At a site receiving wastewater treatment effluent, stable isotopes also revealed trophic shifts in primary consumers that corresponded to changes in available food resources. Results further showed that some taxa losses, e.g., the mayfly Ecdyonurus, to land- use effects may be due to low trophic plasticity. The combination of analyses, including stable isotopes, provided an improved mechanistic understanding of community and population responses to land-use changes along river networks.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As the importance of stream ecosystem integrity gained awareness (Palmer et al. 1997), stream surveillance was implemented in many countries to mitigate impacts and preserve running waters from further degradation (Friberg et al. 2011), as in line with the EU Water Framework Directive (European Commission 2000). In this regard, aquatic macroinvertebrates have been used as proxies for water quality and ecological integrity for decades, mainly focusing on species presence-absence, abundance and various biotic indices (reviewed by Birk et al. 2012). Macroinvertebrates have many advantages as indicators, as they are diverse, ubiquitous, easily collected, and sensitive to a range of stressors (Bonada et al. 2006). For instance, the trait-based approach using macroinvertebrates was augmented through the development of the Species At Risk (SPEAR) index to identify stream pollution (Liess and von der Ohe 2005). SPEAR incorporates the inherent susceptibility of macroinvertebrate taxa to pesticide toxicity as well as their potential resilience to pesticide peaks (Beketov and Liess 2008; Schletterer et al. 2010). Although various large data sets acquired over a long period of time have shown the impacts of human activities on lotic macroinvertebrates (e.g., Jackson et al. 2015), the underlying mechanisms often stay unknown because the threats to running waters transcend multiple spatio-temporal scales (Malmqvist et al. 2008). Moreover, multiple anthropogenic stressors often auto-correlate as they cumulate along the course of a stream network in conjunction with land-use change (Allan 2004; Statzner and Beche 2010).
Biotic interactions also play a central role in general ecology (Fretwell 1987) and can constitute a major driver of macroinvertebrate community assembly in streams (Poff 1997; Schuwirth et al. 2015). Common biotic interactions among macroinvertebrates include both negative (competition, predation, parasitism) and positive (indirect facilitation) effects (reviewed by Holomuzki et al. 2010). As anthropogenic pressures not only act directly on species but also indirectly on species interactions, shifts in many biotic processes can be induced via land-use change (Folt et al. 1999; Allan 2004). For example, Ledger and Hildrew (2005) found that under human-induced acidic conditions in UK streams, the dominant chironomid and ephemeropteran grazers dramatically decreased and that putative shredders consequently shifted to consuming large amounts of algae, thereby limiting re-colonization of other grazer taxa after habitat restoration via competitive exclusion. However, even these trait-based approaches still lack a fundamental understanding of the mechanisms underlying such biotic responses (e.g., Schuwirth et al. 2015).
The study of trophic interactions using stable isotope analysis has been common in terrestrial ecology for many years (Dawson et al. 2002) and in the last decades has gained momentum in freshwater ecosystems as well (Peterson and Fry 1987; Post 2002; Pastor et al. 2013). Stable isotope results have demonstrated that omnivory is a common trait for many stream macroinvertebrates (Lancaster et al. 2005; Anderson and Cabana 2007), reflecting the ability of invertebrates to adjust diets to available resources (Cabana and Rasmussen 1996; Peipoch et al. 2012). For instance, Leberfinger et al. (2011) found macroinvertebrate shredders opportunistically consumed larger fractions of periphyton at open canopy sites compared to forested sites, whereas Kraus (2010) observed a trophic shift in lentic dragonfly larvae towards aquatic prey after exclusion of terrestrial arthropod subsidies. From a stressor perspective, Layer et al. (2013) found that generalist herbivore-detritivores persisting across a broad pH gradient consumed more periphyton at high pH sites than at low pH sites where algal resources were scarce. The question arises as to whether similar response patterns occur for macroinvertebrates exposed to a gradient of land uses as found in many human-dominated river networks (Jackson et al. 2015).
We used a variety of biotic measures in this study, including stable isotopes to elucidate trophic shifts, to gain a better mechanistic understanding of community and population response patterns to changes in land use and associated stressors in the river network. Macroinvertebrate assemblages were examined among streams in a lowland catchment comprising a gradient of land uses and stressors (i.e., agriculture, urbanization, and a wastewater treatment plant as a point source of pollution). We expected that the information derived from the different measures would differ depending on the response of that variable to a particular stressor as related to land use. We used stable isotope data to provide causal information to better explain the response patterns observed among examined biotic metrics.
Materials and methods
Study sites
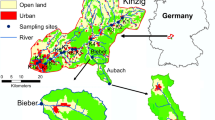
The study was conducted in the Mönchaltorfer Aa catchment situated ca. 20 km southeast of Zürich, Switzerland with an average annual precipitation ca. 1300 mm (20-year mean) (Fig. 1). It covers a drainage area of 46 km2 with a moderate topography (headwater slopes of usually <15 %) and elevations ranging from 445 to 853 m a.s.l. (Robinson et al. 2014). Forests cover about 17 % of the catchment and mostly in headwater areas where most near-natural streams occur. Much of the area is used for agriculture (63 %), whereas urban areas cover around 11 %. Various studies suggest an urbanization threshold effect on streams at ca. 10 % urbanized land cover (see Allan 2004). Lower catchment streams have been mostly channelized for agricultural and flood protection purposes.
Eight sites were chosen to provide a gradient of anthropogenic stressors on stream morphology and water quality. These included three upper basin sites with low-moderate anthropogenic impact (Li1, Li2, Li3), two sites mainly affected by agriculture (Ag1, Ag2), and three sites affected by agriculture and urbanization (Ur1, Ur2, Ur3) (Fig. 1). The least-impacted (Li) sites showed intact stream morphology with highest width and depth variability. They were situated in forested areas but also drained agricultural lands. At agricultural (Ag) sites, streams were channelized and had uniform flow. Here, stream habitats lacked larger cobbles or water-protruding stones, and the river bed was highly embedded. The urbanized (Ur) sites further downstream had moderately degraded morphology and water quality was affected by agricultural as well as urban runoff. Site Ur2, in particular, was morphologically restored in 2005 but received effluent from a waste-water treatment plant.
Field procedures
A detailed habitat assessment at each site was performed in March 2011 following methods described in Robinson et al. (2014). The area covered by the three land use types (forest, agriculture, urban) were calculated from GIS data (Robinson et al. 2014). Stream depth, substrate size (b-axis), and flow velocity at 0.6 depth were recorded every 0.2 m along 5 transects, each situated 20-m equidistant from another transect at each site (n = 50/variable/site). Quartile percent substrate embeddedness, percent shading by canopy cover, and percent river bed surface covered by macrophytes were estimated visually at each site. Hourly temperatures were recorded using temperature loggers (TidbiT v2, Onset Corp., USA) installed at each site. Water samples were collected on each sample date and analyzed for dissolved organic carbon (DOC), particulate organic carbon (POC), alkalinity, total inorganic carbon (TIC), nitrite (NO2-N), nitrate (NO3-N), dissolved nitrogen (DN), particulate nitrogen (PN), phosphorous (PO4-P), dissolved phosphorous (DP), and particulate phosphorous (PP) following methods detailed in Tockner et al. (1997). Electrical conductivity (at 20 °C) and pH were recorded on each visit at each site using a field portable meter (WTW, Germany).
Macroinvertebrate sampling in April, August, and November 2011 followed the methods of the Swiss Modular Concept for Stream Assessment (Stucki 2010). At each site, a reach of ca. 10× the stream width was used for sampling on each date (n = 24). All meso-habitat types present within the study reach were identified visually and included different substrates and levels of embeddedness, variable flow characteristics, aggregations of woody debris and detritus, and presence of filamentous algae and macrophytes. All meso-habitats were then kick-sampled (250 μ mesh, D-frame net) with larger stones and woody debris sampled separately by hand. Multiple kick-samples were combined for collecting taxa at a site used in isotope analysis (n = 24 as above). All sampled material was placed in a white sorting pan and macroinvertebrates immediately handpicked while alive using soft forceps and placed into flasks filled with stream water. Macroinvertebrates were pre-sorted in the field to keep large predatory taxa from consuming other specimens.
2 h were spent per site for sampling and pre-sorting, after which usually no additional taxa were found. For isotope analysis (see below), the aim of at least 20 random individuals per taxon, encompassing the range of sizes for that taxon on that sample date, was met for common taxa but not for larger, usually rarer taxa. To acquire enough material of small taxa (mostly dipterans and mainly Chironomidae), the remaining sample material from each site was returned to the laboratory for additional sorting using a binocular scope at 10× magnification. Macroinvertebrate abundance for each site was qualitatively recorded in abundance classes: no occurrence, 1–3 individuals, 3–10 individuals, 10–30 individuals, 30–100 individuals, and >100 individuals.
After macroinvertebrate sampling, basic food resources were sampled and returned to the laboratory. Leaf litter, FPOM, macrophytes, and filamentous algae were gathered from at least 5 different areas within each study reach and stored separately in 25 ml centrifuge tubes. For periphyton, five stones were haphazardly collected and stored in plastic bags for processing in the laboratory.
Laboratory procedures
Macroinvertebrates were kept alive at 8 °C overnight to allow gut clearance; sufficient oxygen was ensured by air injection via air pumps. The next day, specimens were sorted using a binocular microscope to the lowest reasonable taxonomic level (Ephemeroptera, Plecoptera, Trichoptera, Crustacea and Coleoptera to genus level, Diptera to family level) using standard keys (Tachet et al. 2010; Waringer and Graf 2010). Whenever possible, 15 live individuals per taxon were collected for isotope analysis. An exception was the Chironomidae, which usually needed ca. 50 individuals to have enough material for isotope analysis. All available individuals were used for rare taxa. Once collected, each taxon sample was washed in ultra-pure water, frozen and then freeze-dried in 2 ml round-bottom Eppendorf (Vaudaux-Eppendorf AG, Switzerland) tubes. Each freeze-dried sample was homogenized using a 5-mm stainless steel bead in a bead mill (Qiagen TissueLyserII, Qiagen, Germany) for 5 min at a frequency of 30 Hz.
Leaf litter, FPOM, macrophytes, and filamentous algae were washed with ultra-pure water, frozen and then freeze-dried and homogenized as described above. Periphyton was scrubbed off the stones using a steel brush and rinsing with ultra-pure water. A sub-sample of the slurry was dried at 60 °C for 24 h. Preliminary analysis showed inorganic carbonate content in the periphyton samples and thus they were pre-treated with acid (HCL): 0.3 g of material was placed in a 15-ml centrifuge tube and 2.5-ml 1 M HCL was added three times. After gas emergence stopped, the tube was closed and shaken on an orbital shaker (Heidolph Unimax 1010, Merck, Germany) for 12 h. After 5 min centrifugation at 4500 rpm (Eppendorf Centrifuge 5417C, Vaudaux-Eppendorf AG, Switzerland), another 7.5-ml 1 M HCL was added, and the tube again placed on the orbital shaker for 4 h followed by centrifugation. Each sample was washed two times with ultra-pure water, mixed on a vortexer, followed by centrifugation and removal of supernatant liquid by pipetting. The samples were dried overnight at 60 °C before homogenizing in the bead mill as described above.
For isotope analysis, sufficient material (invertebrates: ca. 0.5 mg, basic food resources ca. 1.5 mg) was weighed into 5 × 9 mm tin capsules (Säntis analytical SA76981103, Teufen, Switzerland). Isotope measurements were performed using an EA-Flash-IRMS following Naeher et al. (2012). Data are expressed in delta notation (δ) as \(\left[ {\left( {{\text{R}}_{\text{sample}} /{\text{R}}_{\text{standard}} } \right) \, - 1} \right] \; \times \; 1000 \permil\), where R is the ratio of heavy to light isotope (i.e., 13C: 12C and 15N: 14N, respectively), Rsample is from the sample, and Rstandard is the international standard value.
For estimating periphyton standing crops, each stone was scrubbed with a metal brush and washed with ultra-pure water. A sub-sample was used for isotope analysis as described above. The remaining solution was diluted with ultra-pure water to a volume of 50 ml. Two aliquots of the resulting slurry were each filtered through glass microfiber filters (Whatman GF/F). One filter was dried at 60 °C, weighed, combusted at 500 °C, and reweighed for determination of periphyton ash-free dry mass (AFDM). The other filter was placed into a glass vial with 8-ml 90 % EtOH and heated at 70 °C for 10 min to extract chlorophyll-a. Chlorophyll-a concentration was then measured with a reverse-phase HPLC and diode array-detector. Individual stones were photographed and the surface area sampled was calculated using the open source software ImageJ 1.4q (W. Rasband, National Institute of Health, Bethesda, Maryland, USA) following Reinking (2007).
Statistical analysis
All statistical analyses were performed using the open-source program R3.0.1 (R Core Team 2012).
Community composition
Macroinvertebrate community composition among sites was examined by detrended correspondence analysis (DCA) using the R-package ‘vegan’ (Oksanen et al. 2013) on log-transformed taxa abundance (using the means) data. The measured chemical and physical habitat properties were fitted to the DCA axes as an ordination biplot using independent Pearson correlations (Legendre and Legendre 2012). A preliminary assessment showed a strong influence of the wastewater treatment plant (WWTP), thus sites were further categorized as ‘least-impacted’ (Li1, Li2, Li3), upstream of the WWTP (Ag1, Ag2, Ur1) and downstream of the WWTP (Ur2, Ur3), and standard deviation ellipses for the groups were drawn to better illustrate differences.
Macroinvertebrate indices of taxa richness and EPT taxa richness were compared between site types using generalized linear mixed models (GLMM, R-package ‘lme4’; Bates et al. 2013) with ‘Month’ as a random factor, assuming poisson distribution and applying a Laplace approximation. Post-hoc tests were performed using the R-package ‘LMERConvenienceFunctions’ (Tremblay et al. 2013). The SPEAR pesticides index (Liess and von der Ohe 2005) was calculated using the online SPEAR calculator (http://www.systemecology.eu/spear/spear-calculator/), resulting in a numerical value estimating pesticide contamination at each site. As SPEAR values followed a normal distribution, the GLMM described above was performed using ‘family = gaussian’ to test for site type differences.
Macroinvertebrate trophic structure
Trophic structure differences among site types was further assessed using the total number of links, link density, and connectance calculated using the R-package ‘food web’ and the outputs analyzed by running the GLMMs described above (family = gaussian). In addition, the number of trophic levels (TL) was calculated following the model of Cabana and Rasmussen (1996) in which: TLconsumer = 1 + (δ15Nconsumer−δ15Nbaseline)/Δ, where δ15Nbaseline is the δ15N average of all primary consumers at a site and ∆ is the enrichment factor per trophic level, i.e., 3–4 ‰ (Peterson and Fry 1987; Post 2002). Isotope ratios (δ13C, δ15N) of basal resources and for the most common taxa (Baetis sp., Chironomidae, Ecdyonurus sp., Gammarus sp., Hydropsyche sp., Rhyacophila sp.) were examined among site and site types using GLMMs as described above.
Average consumption proportions of food sources among sites and site types were investigated with DCA; environmental measures were fit as in the community composition DCA described above. The data sets for primary consumers (herbivores/detrivores) and predators/omnivores were analysed separately and standard deviation ellipses were drawn for sites and site types. Lastly, the food sources of the most common herbivore/detrivore taxa between sites were investigated using GLMMs.
Results
Study site differences
Li1, Li2 and Li3 were surrounded mostly by forest (75–100 % canopy coverage). Li1 had the most natural morphology with high variability in width and depth, although having high concentrations of dissolved phosphorous (Supplement Table 1). Around 30 % of the drainage area of Li1 and Li2 was forested and ca. 45 % was agricultural. Nitrate concentrations at Li2 were high (12.3 ± 5.5 mg N/L). Li3 was a special case, as it partly drained a peat area (grey area in Fig. 1) and it drained more agricultural area than the other least impacted sites (ca. 67 %, mostly pasture). Ag1 and Ag2 were channelized and situated in an agricultural landscape (around 80 % agricultural area). They exhibited higher temperatures, higher nutrient levels and higher amounts of benthic organic matter than the other sites (Supplement Table 1). Ag2 had more shading (50 % cover) than Ag1, which had an open canopy.
Ur1 and Ur2 were located on a tributary and Ur3 in the main-stream above the town Mönchaltorf (Fig. 1). Ur1 was upstream of a WWTP and was covered by dense riparian vegetation, resulting in 100 % shading. Gravel was scarce, as it was regularly removed further upstream for flood protection reasons. Instream habitat was mainly provided by cobbles and riparian roots. Ur2 is around 150 m downstream of Ur1, but below the WWTP water output. It was morphologically restored in 2005 and showed high variability in width and depth. Habitat was provided by heterogeneous substrate and submerged vascular plants (macrophytes, 50 % coverage). Canopy shading was low (25 %), as the planted riparian shrubs and trees were sparse and not fully grown. High concentrations of dissolved nitrogen (10.1 ± 6.8 mg N/L) and dissolved phosphorous (29.0 ± 6.9 µg P/L) were measured at Ur2. There was a dilution of ca. 1:5 between Ur2 and Ur3, with Ur3 being relatively wide (ca. 5 m) and heterogeneous in substrate and depth. It was flanked by a busy road on one side and agricultural areas on the other.
The DCA revealed that important environmental factors separating the least impacted sites from the other sites were increased shading and DOC concentrations and lower temperatures, depths, macrophyte abundances, benthic organic matter and nitrate concentrations (Fig. 2). Higher chlorophyll-a concentrations and pH were the main environmental factors that separated Ag2 from the other sites. The DCA also showed clear separation of site types as illustrated by the standard deviation ellipses (Fig. 2).
Results from the detrended correspondence analysis (DCA) on macroinvertebrate community composition. The left panel shows the DCA ordination plot with common taxa. The right panel shows sites (green ellipses) and site types (purple ellipses), black arrows are vectors representing significant (r2 = 0.70) environmental factors (abbreviations: BOM benthic organic matter concentration, Macro. macrophyte coverage, Cond. conductivity, Temp. temperature, Chl.a chlorophyll-a concentration, DOC dissolved organic carbon) (color figure online)
Macroinvertebrate communities and trophic structure
Macroinvertebrate community composition was distinct among sites and among site types (Fig. 2). For instance, least-impacted sites had higher DCA1 and lower DCA2 values than agricultural (Ag1, Ag2) and urban sites (intermediate DCA1 and DCA2 scores for Ur1 above the WWTP; lower DCA1 and higher DCA2 scores for Ur2 and Ur3 below the WWTP). Taxa more abundant at least-impacted sites were Nemoura sp. (Plecoptera), Dytiscidae (Coleoptera), Ecdyonurus sp. (Ephemeroptera), and Ephemera sp. (Ephemeroptera). The trichopterans Mystacides sp. and Sericostoma sp. were more abundant at Li1, thus lowering its DCA1 score. Taxa abundant at Ag2 (high DCA1 and DCA2 scores) were Habrophlebia sp. (Ephemeroptera), Limnephilus sp. (Trichoptera), and Asellus sp. (Crustacea). Taxa more abundant in urban sites were Ceratopogonidae (Diptera), Serratella sp. (Ephemeroptera), Piscicolidae (Hirudinea), and Cordulegaster sp. (Odonata) (Fig. 2).
Significantly higher total taxa richness and EPT taxa richness was found at least-impacted sites than at sites above/below the WWTP (Table 1). Furthermore, significantly more sensitive taxa (SPEAR pesticides index) were found at least-impacted sites than at downstream sites above and below the WWTP. Significantly higher total number of links and link density were found at least-impacted sites than at impacted sites, whereas no significant difference in connectance was found between sites (Table 1). The mean number of trophic levels (TLconsumer) were similar among sites, although the range in values was highest at sites downstream of the WWTP (U2, Ur3) and more low values were evident for impacted sites in general (Fig. 3).
The WWTP effluent strongly influenced nitrogen isotope ratios with substantially higher δ15N values at sites downstream of the WWTP (Ur2, Ur3: δ15N = ca. 10) than all sites above the WWTP (δ15N usually <8) (Fig. 4). The least-impacted sites had the lowest δ15N values (usually <6), indicating that agricultural sites also were influenced by inputs from adjacent land use. In general, δ13C values were variable among sites (average range −27.1 to −34.6), although there was a trend of greater δ13C values in the least-impacted sites (mean = −29.9 ± 1.2 (SD) Li versus −32.1 ± 1.9 (SD) for Ag/Ur sites) that likely reflected the differences in adjacent terrestrial vegetation (i.e., higher forest cover).
All common taxa, Baetis sp., Chironomidae, Ecdyonurus sp., Gammarus sp., Hydropsyche sp. and Rhyacophila sp. were enriched in 15N at sites downstream of the WWTP (Fig. 5). These taxa also showed intermediate δ15N values for more agriculturally influenced sites, and these taxa at site Ur1 (above WWTP) surprisingly showed values similar to least-impacted sits Li1 and Li2. The δ15N values for periphyton also were substantially higher in urban sites (and Ag2) than least-impacted sites (Li1, Li2, Li3) and Ag1. Other basal resources (FPOM, leaf material, filamentous algae) had similar δ15N values among sites (Fig. 5). Lastly, the three most common herbivore/detrivore taxa, Baetis sp., Chironomidae and Ecdyonurus sp., showed a clear preference for periphyton (Fig. 6). Baetis sp. and Chironomidae switched to other food sources at Ur1 and Ur2 (leaf litter at Ur1 and filamentous algae at Ur2), whereas Ecdyonurus sp. did not occur at these two sites.
Discussion
The community assembly and trophic structure macroinvertebrates significantly changed at eight sites examined along a land-use gradient of increasing anthropogenic impact within a lowland river catchment. Results showed that a higher resolution analysis using stable isotopes increased the mechanistic understanding of response patterns by stream macroinvertebrates to changes in land use.
Macroinvertebrate communities
Community composition changed along the gradient of increasing anthropogenic impact as found worldwide in many studies (e.g., Folt et al. 1999; Murphy and Davy-Bowker 2005; Manfrin et al. 2013). Shifts in community composition have been attributed to general land-use change (forested, agricultural, rural) (Lenat and Crawford 1994; Wang et al. 1997) and associated morphological alterations and fine sediment inputs (Richards et al. 1993; Waters 1995; Matthaei et al. 2006) as well as pesticide (Friberg et al. 2003) and nutrient pollution (Vander Laan et al. 2013). Potential confounding factors are usually inferred in these studies, further emphasizing that co-variation between anthropogenic and natural gradients is a major challenge in understanding the mechanisms underlying response patterns (Allan 2004). Due to natural gradients (stream order, substratum, altitude, etc.) and the dendritic structure of river networks (Altermatt et al. 2013), cumulative environmental factors affecting biotic assemblages are expected in human-dominated catchments (Allan 2004). At regional and local scales, habitat properties (sensu habitat filters) can be additional drivers of macroinvertebrate distribution and abundances (Poff 1997).
The least-impacted sites had more pesticide sensitive taxa (higher SPEAR pesticides index scores) than sites influenced by agriculture and urbanization. For instance, highly sensitive taxa like the stoneflies Nemoura sp. and Leuctra sp. were found only at least-impacted sites, whereas less sensitive, more generalist mayfly taxa like Baetis sp. and Serratella sp. were common below the WWTP. The SPEAR pesticides index is based on life history traits of macroinvertebrates and determines susceptibility to pesticides in terms of sensitivity to toxicants per se (including presence of aquatic stages during periods of maximum pesticide application) as well as the resilience to pesticide pollution (generation time and migration ability) (Liess and von der Ohe 2005). This index is independent of most longitudinally related factors in river networks (Beketov and Liess 2008; Schletterer et al. 2010), thereby highlighting the local effects of water quality degradation. Further, macroinvertebrate diversity indices (total and EPT taxa richness) also decreased between the least-impacted sites and sites above the wastewater treatment plant, but sites below the WWTP were comparable to least-impacted sites. Sites below the WWTP were mainly affected by poor water quality but only moderately by poor morphology. These results suggest that assemblages in agricultural/urban influenced sites may be constrained by a press disturbance of pesticide pollution as a component of land use in the catchment even though local sites may have relatively good morphology (sensu Lake 2000).
Macroinvertebrate trophic structure
The total number of links and link density were higher at least-impacted than impacted sites (above or below the WWTP), suggesting simplification in trophic structure with increasing degradation. Further, the mean number of trophic levels remained similar among sites, although the range in TLconsumer values was highest in urban sites. These results indicate a compositional shift in assemblage structure that compensates for the simplification in trophic structure with increasing degradation. Omnivory is common in lotic systems and this coarse assessment of trophic structure suggests a possible loss in dietary-specialist taxa with increasing degradation. For example, Borrvall et al. (2000) found a stabilizing effect of omnivory on trophic dynamics, and Dunne et al. (2002) showed robustness (measured as secondary extinctions due to species removal) increased with connectance. Connectance in this study was similar between site types, further supporting the importance of omnivory in sustaining trophic dynamics in streams. Closs et al. (1999) argue that the traditional theory of a negative correlation between trophic complexity and stability must be reassessed, incorporating the stabilizing effect of generalist predators and omnivory, which is concordant with functional redundancy theory (Woodward 2009).
We found δ15N values to increase along the stress gradient in land use, with a particularly major increase at sites affected by wastewater treatment effluent. Stable isotopes (i.e., δ15N) have been used to indicate wastewater effects on macroinvertebrates (Cole et al. 2004; Morrissey et al. 2013; Pastor et al. 2014); and Saito et al. (2008) found a correlation of 15N enrichment and toxic PAH concentrations. Similarly, Harrington et al. (1998) and Bergfur et al. (2009) also showed increased δ15N values in response to a nutrient enrichment gradient from agricultural runoff (also see meta-analysis results of Peipoch et al. 2012). Denitrification of inorganic fertilizer can cause increases in δ15N values (Diebel and Vander Zanden 2009). Results of these studies, including ours, support the finding that high δ15N values are good indicators of anthropogenic stress in stream networks. However, it also should be noted that anthropogenic stressors can have opposite effects on nitrogen isotope ratios, e.g., when industrial effluents are depleted of 15N (Ulseth and Hershfy 2005).
Carbon isotope ratios (δ13C) showed higher values at least-impacted than impacted sites. Finlay (2001) found a longitudinal increase in δ13C along a stream network due to increased limitation of algal carbon uptake. In contrast, Turner and Edwards (2012) found non-linear or even reversed longitudinal δ13C patterns in their study system. It is likely that the patterns in the present study were caused by the type of organic matter consumed. Although inputs of allochthonous resources, which are depleted in heavy carbon isotopes (Benner et al. 1987), was high at least-impacted sites situated in mostly forested areas, periphyton was still a proportionately higher food source for macroinvertebrates.
Shifts in carbon isotopes infer changes in resources available to consumers and potentially give insight to the loss of taxa with changes in land use. For example, the most common primary consumer taxa, Baetis sp., Chironomidae, and Ecdyonurus sp., used periphyton as a main food source at all sites except Ur1 and Ur2. Ur1 was the site above the WWTP with a full canopy that limited periphyton development. Here, diet-generalists Baetis sp. and Chironomidae switched to leaf litter as a primary resource. In contrast, Ur2 is just below the WWTP with an open canopy and high levels of filamentous algae. Here, Baetis sp. and Chironomidae switched to filamentous algae as the primary resource. Lastly, at both sites where these shifts were evident, the dietary specialist Ecdyonurus sp. was absent. Changes in habitat properties (Ur1) or water quality (Ur2) as an explanation for the lack of Ecdyonurus sp. are unlikely as Ecdyonurus sp. was found further upstream (authors, personal observation). The isotope data suggest that Ecdyonurus sp. has low trophic plasticity and may be physiologically limited by resource availability (i.e., diet specialist), whereas Baetis sp. and Chironomidae can shift their diet in response to resources availability (i.e., diet generalists) (see Wögerbauer and Kelly-Quinn 2013), thus providing a mechanistic explanation for the loss of Ecdyonurus sp. at these sites. A similar mechanism may explain the loss of other taxa along a land-use gradient in human-dominated catchments. For instance, changes in nutritional composition or abundances of basal food sources can induce shifts in primary consumers or even their exclusion (Hall 2004). Indeed, Scherber et al. (2010) found that bottom-up effects exerted stronger impacts on lower than higher trophic levels.
General conclusions and implications
Running waters are highly significant, not only for global biodiversity (Dudgeon et al. 2006) and terrestrial ecosystems transcending stream boundaries (Richardson et al. 2010), but also for human economy and society (Dudgeon et al. 2006). Assessment of stream integrity has been an important part of lotic ecosystem research for decades. Different methodologies have been implemented and applied, mostly concentrating on water quality, river morphology, catchment land use, and flora and fauna (e.g., macroinvertebrates as indicators), and combinations of the above (Birk et al. 2012). Using macroinvertebrate presence, abundance, evenness and other indices, and also their interactions, in an ecological network approach is a more recent development (Woodward 2009). The present study also used stable isotopes to improve the mechanistic understanding of response patterns by macroinvertebrates along a land-use gradient.
Agricultural land use strongly affected all macroinvertebrate properties due to degradation in water quality and habitat morphology. Sites impacted by urban settlements (especially below the WWTP) had low water quality but moderately impacted morphology. As a result, macroinvertebrate diversity indices failed to detect anthropogenic stressors at these sites, whereas the SPEAR pesticides index indicated poor water quality. Increasing the resolution to taxa identity (community composition) confirmed this finding. Incorporating trophic interactions further revealed more complex assemblages at least-impacted sites. Stable isotope analysis additionally revealed the possibility of anthropogenic stressors indirectly affecting macroinvertebrate assemblages by excluding taxa lacking dietary plasticity. The different approaches led to a more complete understanding of macroinvertebrate assemblage shifts along an anthropogenic stress gradient. The current study demonstrates how stable isotope analysis can advance our mechanistic understanding of factors driving changes in community assembly along anthropogenic stress gradients.
References
Allan JD (2004) Landscapes and riverscapes: the influence of land use on stream ecosystems. Annu Rev Ecol Evol Syst 35:257–284
Altermatt F, Seymour M, Martinez N (2013) River network properties shape alpha-diversity and community similarity patterns of aquatic insect communities acros major drainage basins. J Biogeogr 40:2249–2260
Anderson C, Cabana G (2007) Estimating the trophic position of aquatic consumers in river food webs using stable nitrogen isotopes. J N Am Bentholo Soc 26:273–285
Bates D, Maechler M, Bolker B (2013) Lme4: linear mixed-effects models using S4 classes. R package version 0.999999-2. http://CRAN.R-project.org/package=lme4
Beketov MA, Liess M (2008) An indicator for effects of organic toxicants on lotic invertebrate communities: independence of confounding environmental factors over an extensive river continuum. Environ Pollut 156:980–987
Benner R, Fogel ML, Sprague EK, Hodson RE (1987) Depletion of C-13 lignin and its implications for stable carbon isotope studies. Nature 329:708–710
Bergfur J, Johnson RK, Sandin L, Goedkoop W (2009) Effects of nutrient enrichment on C and N stable isotope ratios of invertebrates, fish and their food resources in boreal streams. Hydrobiologia 628:67–79
Birk S, Bonne W, Borja A, Brucet S, Courrat A, Poikane S, Solimini A, van de Bund WV, Zampoukas N, Hering D (2012) Three hundred ways to assess Europe’s surface waters: an almost complete overview of biological methods to implement the water framework directive. Ecol Ind 18:31–41
Bonada N, Prat N, Resh VH, Statzner B (2006) Developments in aquatic insect biomonitoring: a comparative analysis of recent approaches. Annu Rev Entomol 51:495–523
Borrvall C, Ebenman B, Jonsson T (2000) Biodiversity lessens the risk of cascading extinction in model food webs. Ecol Lett 3:131–136
Cabana G, Rasmussen JB (1996) Comparsion of aquatic food chains using nitrogen isotopes. Proc Natl Acad Sci 93:10844–10847
Closs GP, Balcombe SR, Shirley MJ (1999) Generalist predators, interaction strength and food-web stability. Adv Ecol Res 28:93–126
Cole ML, Valiela I, Kroeger KD, Tomasky GL, Cebrian J, Wigand C, McKinney RA, Grady SP, da Silva MHC (2004) Assessment of a delta N-15 isotopic method to indicate anthropogenic eutrophication in aquatic ecosystems. J Environ Qual 33:124–132
Dawson TE, Mambelli S, Plamboeck AH, Templer PH, Tu KP (2002) Stable isotopes in plant ecology. Annu Rev Ecol Syst 33:507–559
Diebel MW, Vander Zanden MJ (2009) Nitrogen stable isotopes in streams: effects of agricultural sources and transformations. Ecol Appl 19:1127–1134
Dudgeon D, Arthington AH, Gessner MO, Kawabata ZI, Knowler DJ, Leveque C, Naiman RJ, Prieur-Richard AH, Soto D, Stiassny MLJ, Sullivan CA (2006) Freshwater biodiversity: importance, threats, status and conservation challenges. Biol Rev 81:163–182
Dunne JA, Williams RJ, Martinez ND (2002) Network structure and biodiversity loss in food webs: robustness increases with connectance. Ecol Lett 5:558–567
European Commission (2000) Directive 2000/60/EEC, establising a framework for community acton in the field of water policy. Official Journal of the European Communities L327. Brussels, p 1–71
Finlay JC (2001) Stable-carbon-isotope ratios of river biota: implications for energy flow in lotic food webs. Ecology 82:1052–1064
Folt CL, Chen CY, Moore MV, Burnaford J (1999) Synergism and antagonism among multiple stressors. Limnol Oceanogr 44:864–877
Fretwell SD (1987) Food-chain dynamics—the central theory of ecology. Oikos 50:291–301
Friberg N, Lindstrom M, Kronvang B, Larsen SE (2003) Macroinvertebrate/sediment relationships along a pesticide gradient in Danish streams. Hydrobiologia 494:103–110
Friberg N, Bonada N, Bradley DC, Dunbar MJ, Edwards FK, Grey J, Hayes RB, Hildrew AG, Lamouroux N, Trimmer M, Woodward G (2011) Biomonitoring of human impacts in freshwater ecosystems: the good, the bad and the ugly. Adv Ecol Res 44:1–68
Hall SR (2004) Stoichiometrically explicit competition between grazers: species replacement, coexistence, and priority effects along resource supply gradients. Am Nat 164:157–172
Harrington RR, Kennedy BP, Chamberlain CP, Blum JD, Folt CL (1998) N-15 enrichment in agricultural catchments: field patterns and applications to tracking Atlantic salmon (Salmo salar). Chem Geol 147:281–294
Holomuzki JR, Feminella JW, Power ME (2010) Biotic interactions in freshwater benthic habitats. J N Am Benthol Soc 29:220–244
Jackson MC, Loewen CJG, Vienbrook RD, Chimimba CT (2015) Net effects of multiple stressors in freshwater ecosytems. A meta-analysis. Glob Chang Biol. doi:10.1111/gcb.13028
Kraus JM (2010) Diet shift of lentic dragonfly larvae in response to reduced terrestrial prey subsidies. J N Am Benthol Soc 29:602–613
Lake PS (2000) Disturbance, patchiness, and diversity in streams. J N Am Benthol Soc 19:573–592
Lancaster J, Bradley DC, Hogan A, Waldron S (2005) Intraguild omnivory in predatory stream insects. J Anim Ecol 74:619–629
Layer K, Hildrew AG, Woodward G (2013) Grazing and detritivory in 20 stream food webs across a broad pH gradient. Oecologia 171:459–471
Leberfinger K, Bohman I, Herrmann J (2011) The importance of terrestrial resource subsidies for shredders in open-canopy streams revealed by stable isotope analysis. Freshw Biol 56:470–480
Ledger ME, Hildrew AG (2005) The ecology of acidification and recovery: changes in herbivore-algal food web linkages across a stream pH gradient. Environ Pollut 137:103–118
Legendre P, Legendre L (2012) Numerical ecology. Elsevier, The Netherlands
Lenat DR, Crawford JK (1994) Effects of land-use on water quality and aquatic biota of three North Carolina Piedmont streams. Hydrobiologia 294:185–199
Liess M, von der Ohe PC (2005) Analyzing effects of pesticides on invertebrate communities in streams. Environ Toxicol Chem 24:954–965
Malmqvist B, Rundle S, Covich AP, Hildrew AG, Robinson CT, Townsend CR (2008) Prospects for streams and rivers: an ecological perspective. In: Polunin NP (ed) Aquatic ecosytems: trends and globel perspectives. Cambridge University Press, Cambridge, pp 19–29
Manfrin A, Larsen S, Traversetti L, Pace G, Scalici M (2013) Longitudinal variation of macroinvertebrate communities in a Mediterranean river subjected to multiple anthropogenic stressors. Int Rev Hydrobiol 98:155–164
Matthaei CD, Weller F, Kelly DW, Townsend CR (2006) Impacts of fine sediment addition to tussock, pasture, dairy and deer farming streams in New Zealand. Freshw Biol 51:2154–2172
Morrissey CA, Boldt A, Mapstone A, Newton J, Ormerod SJ (2013) Stable isotopes as indicators of wastewater effects on the macroinvertebrates of urban rivers. Hydrobiologia 700:231–244
Murphy JF, Davy-Bowker J (2005) Spatial structure in lotic macroinvertebrate communities in England and Wales: relationship with physical, chemical and anthropogenic stress variables. Hydrobiologia 534:151–164
Naeher S, Smittenberg RH, Gilli A, Kirilova EP, Lotter AF, Schubert CJ (2012) Impact of recent lake eutrophication on microbial community changes as revealed by high resolution lipid biomarkers in Rotsee (Switzerland). Org Geochem 49:86–95
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Henry M, Stevens H, Wagner H (2013) Vegan: Community Ecology Package. R package version 2.0–7. http://CRAN.R-project.org/package=vegan
Palmer MA, Ambrose RF, Poff NL (1997) Ecological theory and community restoration ecology. Restor Ecol 5:291–300
Pastor A, Peipoch M, Canas L, Chappius E, Ribot M, Gacia E, Riera JL, Marti E, Sabater F (2013) Nitrogen stable isotopes in primary uptake compartments across streams differing in nutrient availability. Environ Sci Technol 47:10155–10162
Pastor A, Riera JL, Peipoch M, Canas L, Ribot M, Gacia E, Marti E, Sabater F (2014) Temporal variability in nitrogen stable isotopes in primary uptake compartments in four streams differing in human impacts. Environ Sci Technol 48:6612–6619
Peipoch M, Marti E, Garcia E (2012) Variability in δ15 N natural abundance of basal resources in fluvial ecosystems: a meta-analysis. Freshw Sci 31:1003–1015
Peterson BJ, Fry B (1987) Stable isotopes in ecosystem studies. Annu Rev Ecol Syst 18:293–320
Poff NL (1997) Landscape filters and species traits: towards mechanistic understanding and prediction in stream ecology. J N Am Benthol Soc 16:391–409
Post DM (2002) Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83:703–718
Reinking L (2007) Examples of image analysis using ImageJ. http://rsbweb.nih.gov/ij/docs/pdfs/examples.pdf. Department of Biology, Millersville University
Richards C, Host GE, Arthur JW (1993) Identification of predominant environmental factors structuring stream macroinvertebrate communities within a large agricultural catchment. Freshw Biol 29:285–294
Richardson JS, Zhang YX, Marczak LB (2010) Resource subsidies across the land-freshwater interface and responses in recipient communities. River Res Appl 26:55–66
Robinson CT, Schuwirth N, Baumgartner S, Stamm C (2014) Spatial relationships between land-use, habitat, water quality and lotic macroinvertebrates in two Swiss catchments. Aquat Sci 76:375–392
Saito L, Rosen MR, Chandra S, Fritsen CH, Arufe JA, Redd C (2008) Using semi-permeable membrane devices and stable nitrogen isotopes to detect anthropogenic influences on the Truckee River, USA. Environ Eng Sci 25:585–600
Scherber C, Eisenhauer N, Weisser WW, Schmid B, Voigt W, Fischer M, Schulze ED, Roscher C, Weigelt A, Allan E, Bessler H, Bonkowski M, Buchmann N, Buscot F, Clement LW, Ebeling A, Engels C, Halle S, Kertscher I, Klein AM, Koller R, Konig S, Kowalski E, Kummer V, Kuu A, Lange M, Lauterbach D, Middelhoff C, Migunova VD, Milcu A, Muller R, Partsch S, Petermann JS, Renker C, Rottstock T, Sabais A, Scheu S, Schumacher J, Temperton VM, Tscharntke T (2010) Bottom-up effects of plant diversity on multitrophic interactions in a biodiversity experiment. Nature 468:553–556
Schletterer M, Fureder L, Kuzovlev VV, Beketov MA (2010) Testing the coherence of several macroinvertebrate indices and environmental factors in a large lowland river system (Volga River, Russia). Ecol Ind 10:1083–1092
Schuwirth N, Dietzel A, Reichert P (2015) The importance of biotic interactions for the prediction of macroinvertebrate communities under multiple stressors. Funct Ecol. doi:10.1111/1365-2435.12605
Statzner B, Beche LA (2010) Can biological invertebrate traits resolve effects of multiple stressors on running water ecosystems? Freshw Biol 55:80–119
Stucki P (2010) Methoden zur Untersuchung und Buerteilung der Fliessgewässer. Makrozoobenthos Stufe F. Bundesamt für Umwelt, Bern. Umwelt-Vollzug Nr. 1026: 61 S
Tachet H, Richoux P, Bournaud M, Usseglio-Polatera P (2010) Invertébrés d’eau douces. CNRS, Paris
R Core Team (2012) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL: http://www.R-project.org/
Tockner K, Malard F, Burgherr P, Robinson CT, Uehlinger U, Zahand R, Ward JV (1997) Characteristics of channel types in a glacial floodplain ecosystem (Val Roseg, Switzerland). Archiv für Hydrobiologie 140:433–463
Tremblay A, Ransijn J, University of Copenhagen (2013) LMERConvenienceFunctions: A suite of functions to back-fit fixed effects and forward-fit random effects, as well as other miscellaneous functions. R package version 2.0. http://CRAN.R-project.org/package=LMERConvenienceFunctions
Turner TF, Edwards MS (2012) Aquatic foodweb structure of the Rio Grande assessed with stable isotopes. Freshw Sci 31:825–834
Ulseth AJ, Hershfy AE (2005) Natural abundances of stable isotopes trace anthropogenic N and C in an urban stream. J N Am Benthol Soc 24:270–289
Vander Laan JJ, Hawkins CP, Olson JR, Hill RA (2013) Linking land use, in-stream stressors, and biological condition to infer causes of regional ecological impairment in streams. Freshw Sci 32:801–820
Wang LZ, Lyons J, Kanehl P, Gatti R (1997) Influences of watershed land use on habitat quality and biotic integrity in Wisconsin streams. Fisheries 22:6–12
Waringer J, Graf W (2010) Atlas of Central European Trichoptera Larvae. Erik Mauch, Dinkelscherben
Waters TF (1995) Sediment in streams: sources, biological effects and control. American fisheries society monograph 7. American Fisheries Society, Maryland
Wögerbauer CM, Kelly-Quinn M (2013) Seasonal variation in diet and feeding strategy of three mayfly species. Biol Environ Proc Royal Irish Acad 113B:1–16
Woodward G (2009) Biodiversity, ecosystem functioning and food webs in fresh waters: assembling the jigsaw puzzle. Freshw Biol 54:2171–2187
Acknowledgments
Funding was provided by the Swiss Federal Institute of Environment (FEON). We thank C. Schubert for providing the laboratory equipment for stable isotope analysis and G. Nobbe for his extensive help and shared insights on stable isotope sample preparation and analysis. We thank S. Blaser, C. Jolidon, S. Kaeser, K. Krähenbühl, and C. Baumgartner for assistance in the field, and the AUA chemical laboratory at Eawag for water chemistry analysis. We thank two highly constructive reviewers for comments that improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Baumgartner, S.D., Robinson, C.T. Changes in macroinvertebrate trophic structure along a land-use gradient within a lowland stream network. Aquat Sci 79, 407–418 (2017). https://doi.org/10.1007/s00027-016-0506-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00027-016-0506-z