Abstract
Little effort has been devoted to characterizing the resting egg banks in tropical lakes. In this study, we evaluated the structure of egg banks across 26 Brazilian lakes located in four geographical regions. We also evaluated cross-taxon concordance in species richness and community similarity between dormant rotifers and dormant cladocerans, and searched for concordant patterns between dormant and active communities. We observed 88 taxa among all the hatchlings that belonged mainly to rotifers and cladocerans. Lakes located in the same geographical region displayed more similar dormant communities. Overall, no concordance was observed between dormant rotifers and dormant cladocerans. Concordance in community similarity was observed between dormant and active organisms but only for rotifers and the entire zooplankton community. Resting egg banks were not associated to a set of environmental variables. Our results demonstrate the occurrence of resting egg banks in several tropical lakes. Due to the weak concordant patterns, rotifers or cladocerans found in egg banks should be used cautiously as a surrogate of the other group in zooplankton surveys. Finally, the lack of strong concordance between the active and dormant stages of cladocerans suggests that some species may not receive appropriate cues to induce diapause.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lake zooplankton is commonly observed in two distinct life stages. Active stages colonize the open water and predominate under favorable conditions for their survival and reproduction. On the other hand, under harsh environmental conditions, a dormant stage is produced by active organisms (Gyllstrom & Hansson, 2004 and references therein; Alekseev et al., 2007). In lakes and other aquatic systems, diapause prevails over other dormancy strategies and results in the production of a resting egg. Diapause is recognized for several rotifers, branchiopods, calanoid copepods, and ostracods (May, 1986; Destasio, 1989; Waterkeyn et al., 2009), while quiescence is observed in bdelloid rotifers and cyclopoid copepods (Ricci, 2001; Frisch, 2002).
Organisms rely on environmental cues to initiate and terminate diapause at the appropriate times. However, not all resting eggs receive the necessary hatching cues in the next growing season and thus accumulate in the sediment (Destasio, 1989). Resting eggs accumulated in the sediment combine to form a persistent resting egg bank where they can remain viable for several years, decades, and even centuries (Hairston et al., 1995; Hairston, 1996). Resting egg banks have myriad ecological and evolutionary consequences for zooplankton, including temporal dispersal, maintenance of genetic diversity, and easier dispersal to and colonization of new habitats (Bilton et al., 2001; Brendonck & De Meester, 2003; Gyllstrom & Hansson, 2004).
The resting egg bank provides an alternative technique to assess local zooplankton diversity (May, 1986). Depending on lake features, sampling the resting egg bank instead of the open water community is easier, cheaper and faster (Duggan et al., 2002; Vandekerkhove et al., 2005b; Garcia-Roger et al., 2008) while still providing feasible data on zooplankton composition. Indeed, some studies have compared the community composition and species richness of rotifers or cladocerans obtained by sampling both the open water and the resting egg bank (Vandekerkhove et al., 2005b; Vandekerkhove et al., 2005c; Garcia-Roger et al., 2008). Overall, the aforementioned studies demonstrated a great overlap in the species list obtained by the two methods, but some species were found exclusively using only one of the two approaches.
Despite inhabiting the same environment, dormant rotifers and dormant cladocerans have never been considered together in the same study when evaluating additional, more recently developed community patterns such as cross-taxon concordance. Cross-taxon concordance refers to the strength and significance of correlation in assemblage-level biodiversity measures between taxonomic groups across a set of sites (Heino, 2010). This concept has been particularly used in conservation approaches aiming to find high biodiversity spots by sampling a few communities (Lopes et al., 2011; Fattorini et al., 2012; Tisseuil et al., 2013). In resting egg banks, cross-taxon concordance would imply that species richness and community composition are similar in a set of sites for both dormant rotifers and dormant cladocerans. If complete surveys of dormant rotifers and cladocerans can be obtained using resting egg banks, then finding cross-taxon concordance patterns in species richness and community similarity could make one group a surrogate for another (Heino, 2010).
In addition, because most zooplankton might occur in the open water and in the sediment (resting eggs) compartments, concordance between active and dormant stages might exist, indicating that species richness and community composition are similar in a set of sites for both dormant and active organisms. This type of concordance could be expected because active and dormant communities have reciprocal influences on each other (Hairston et al., 2000; Gyllstrom & Hansson, 2004). Finding similar community patterns between dormant and active zooplankton would also allow for a more appropriate use of the resting egg bank as a surrogate for the identification of highly diverse zooplankton communities.
Brazil has different types of aquatic systems, ranging from coastal wetlands to river floodplains, periodically flooded forests and savannas (Junk et al., 2014). In this study, we evaluated the dormant and the open water zooplankton communities of 26 Brazilian lakes located in four geographical regions, encompassing coastal lakes, floodplain lakes, and upland lakes. We determined the abundance (number of hatchlings), composition and species richness of resting egg banks, and the composition and species richness of active communities. More importantly, because diapause is commonly adopted by many zooplankters, we expected that the species richness and community similarity of dormant rotifers and dormant cladocerans would be concordant. In addition, due to the previously established similarities between resting egg banks and open water communities (Vandekerkhove et al., 2005c; Garcia-Roger et al., 2008), we predicted that the species richness and community similarity between dormant and active organisms would also be concordant. Finally, we assessed the role of some environmental variables on the composition of resting egg banks. Using this approach, we may better evaluate the role of resting egg banks in assessing zooplankton diversity.
Methods
Sampled lakes
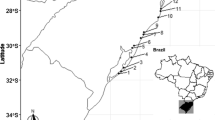
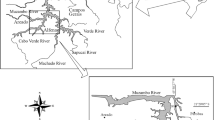
We sampled 26 lakes from four geographical regions in Brazil to evaluate the abundance (number of hatchlings), species richness and composition of resting egg banks. Six upland lakes were sampled in Serra dos Carajás (Amazon region, northern Brazil). Nine floodplain lakes and six floodplain lakes were sampled in the Trombetas River (Amazon River basin, northern Brazil) and at the upper Paraná River (Paraná River basin, southern Brazil), respectively. Finally, five coastal lakes were sampled in southeast Brazil. The prevailing limnological features and zooplankton communities in lakes from the four geographical regions are described elsewhere for the Amazonian upland lakes (Lopes et al., 2011), Trombetas River floodplain lakes (Bozelli, 1992; Thomaz et al., 2007), upper Paraná River floodplain lakes (Simões et al., 2013), and coastal lakes (Santangelo et al., 2007; Caliman et al., 2010).
Resting egg bank sampling and hatching procedure
Sediment sampling was carried out at the 26 lakes in December 2007 or September 2008 (upper Paraná River). We adopted a single-survey (snapshot) approach to test our hypothesis for two main reasons. First, the season when resting eggs are produced most intensively in the tropics remains unknown. In addition, a fraction of the resting eggs produced do not hatch in the next growing season (Destasio, 1990) and thus accumulate in the sediment.
Sediment was sampled in three random stations at each lake with an 8-cm diameter sediment corer. Only the top 3 cm, i.e., the active egg bank, was retained (Caceres & Hairston, 1998). Sediment at the sampling sites was predominantly silt and clay, and the oxygen concentration at the time of sampling was above 3 mg l−1 at all sampled lakes. Sediment samples were stored in individual plastic bags and maintained under room temperature and dark conditions for 2 months before use. Sediment samples were kept wet for two main reasons. First, most of the systems we sampled never dry up completely. This is observed for coastal lakes and floodplain lakes. Additionally, there is evidence that drying up the sediments of permanent lakes reduces the hatching rate of zooplankton (Santangelo et al., 2011a).
For hatching experiments, a 100 g sample of wet sediment from each of the three stations within a lake was mixed to create a single (300 g weight) sediment sample. From this mixed sample, 100 g of wet sediment was used to isolate the resting eggs. Resting eggs were isolated using the sugar flotation method (Onbe, 1978; Vandekerkhove et al., 2004). The sediment/sugar mixture was centrifuged in 50 ml tubes for 3 min at 3000 rpm. After centrifugation, all supernatant was filtered through a 20-μm mesh size net and washed with distilled water to remove the sugar. For each lake, all supernatants where resting eggs accumulate after centrifugation were incubated in small flasks with 250 ml of an artificial media (Tollrian, 1993). Flasks were kept under 24 °C in a 12:12 h light:dark cycle for 20 days.
Hatching was determined every other day. For hatchling quantification, all the water content in a flask was filtered in a 20-µm mesh size net, and the retained material was observed under a stereomicroscope in Petri dishes (Santangelo et al., 2011a, 2014). Active zooplankton organisms were removed, quantified and identified to the lowest taxonomic unit (Koste, 1978; Elmoor-Loureiro, 1997). The remaining material was returned to the original flasks. Calanoid nauplii were considered as different species within each geographical region due to the limited latitudinal distribution of Calanoid in Brazil (Matsumura-Tundisi, 1986).
Open water zooplankton and limnological variables sampling and analyses
The open water zooplankton of 25 lakes was sampled using a 50-µm mesh plankton net in 2007 (or in 2008 for the upper Paraná River lakes) to compare it to the dormant community. Sampling was carried out at one station in two distinct phases: summer and winter for coastal and upland lakes; and low- and high-water phases for floodplain lakes. This approach aimed to encompass the highest variability in zooplankton communities.
Samples were preserved in 4 % formalin solution. In the laboratory, samples were scanned to look for all species in open chambers under a stereomicroscope. Species richness and composition were determined. Data obtained from the two samples of the active community in each lake were merged into a single dataset for each sampled lake. Calanoid copepods were counted as a single species in each lake because we were not able to identify hatched nauplii from the resting egg banks at the species level. Usually, no more than two Calanoid species are found in the samples from the studied lakes. Cyclopoid copepods were not considered, as they are not able to produce resting eggs (Dussart & Defaye, 2001). One lake from the Trombetas River could not be sampled for the active community.
Additionally to zooplankton sampling, water from each lake was analyzed for total phosphorus, total nitrogen and chlorophyll a content. In the field, depth, Secchi depth, temperature, dissolved oxygen saturation, and conductivity were also measured. Details of the methods employed for the determination of these environmental variables are indicated in Lopes et al. (2014). Additionally, lakes were analyzed for their area, hydrological isolation and permanency. Three lakes from the Trombetas River could not be sampled for the environmental variables.
Statistical analyses
Firstly, the relationship between environmental variables from all lake systems was identified by means of multivariate principal component analysis (PCA). This approach aimed to find groups of lakes with similar environmental features that could help to explain patterns of community concordance. PCA analysis was performed with PC-ORD™ 5.0 software (McCune & Mefford, 1999). All data, except pH, was log10(x + 1) transformed before analysis. Hydrological isolation and permanency were used as dummy variables with two levels (connected vs. isolated; temporary × permanent lake systems).
Hatching results were initially compared for hatchling abundance and species richness. Because PCA analysis grouped most lakes belonging to a given geographical area, we used the origin region of the lakes as the grouping variable and abundance and species richness as the response variables. Abundance was compared with one-way ANOVA in GraphPad Prism 5.0 software using log10-transformed data to meet the assumptions of normal distribution and homogeneity of variances. Species richness was compared with individual-based rarefaction curves using 95 % confidence intervals plotted as error bars to infer significant differences among regions (Gotelli & Colwell, 2001) using the program Estimates 8.0 (Colwell, 2005). Rarefaction curves calculate the expected number of species in a random subsample of individuals and compare it to the number of species observed in the less-sampled areas. Because few hatchlings were observed in the upper Paraná River and coastal lakes, a comparison of species richness was performed only between the Amazonian upland (Carajás) lakes and the Trombetas River lakes.
Community structure hatching from the resting egg banks was compared with multivariate analysis performed with PC-ORD™ 5.0 software. Multi-response permutation procedures (MRPP) was applied to determine differences in community structure among different geographical regions. MRPP analysis provides a descriptor (statistic A) of within-group homogeneity compared to the random expectation (McCune & Mefford, 1999). More specifically, when all items are identical within groups, then A = 1, the highest possible value for A. If heterogeneity within groups equals expectation by chance, then A = 0. On the other hand, if there is less agreement within groups than expected by chance, then A < 0. A corrected α was used, according to Bonferroni’s procedure for multiple comparisons (corrected α = 0.05/6 = 0.008). In addition, non-metric multidimensional scaling (NMDS) based on a Bray–Curtis similarity matrix was used to display community data.
Mainly, we evaluated concordance patterns in species richness and community similarity between (1) dormant rotifers and dormant cladocerans and (2) dormant and active organisms (for rotifers only, for cladocerans only and for the entire community). These analyses were performed for all 26 sampled lakes combined and for each individual geographical dataset. Concordance patterns in species richness were tested with Spearman’s correlation using GraphPad Prism 5.0. Concordance in community similarity between dormant rotifers and dormant cladocerans was tested with Mantel tests using both Bray–Curtis (based on log-transformed abundance data) and Jaccard indexes. Community similarity concordance between dormant and active stages was tested using only the Jaccard index. These are the basic approaches used to test for cross-taxon concordance (Heino, 2010).
Finally, in order to evaluate and compare the associations between environmental variables and geographical distances on the composition of dormant and active zooplankton communities, Mantel tests were performed between the zooplankton (for rotifers only, for cladocerans only and for the entire community) and the environmental data, and between the zooplankton and the geographical position of lakes. Environmental and geographical matrices were constructed using Euclidean distances. Before these calculations, environmental variables were standardized to a mean of zero and unit variance. The significance of the relationship between two similarity matrices or between environmental and geographical matrices were based on a permutation test with 9999 runs using PC-ORD™ 5.0 software. Results were considered significant when P < 0.05.
Results
Environmental variables
The lakes belonging to different geographic regions varied in some environmental variables (Table 1) and the PCA analysis indicated what variables were more important for differentiating and grouping the lakes. The first two components of PCA analysis explained 31.2 and 28.3 % of data variation, respectively. Component 1 was correlated negatively with area, depth and permanence but positively with total nitrogen. Component 2 was correlated negatively with total phosphorus and chlorophyll a content but positively with conductivity (see the Online Resource 1 for the results of PCA analysis). Overall, the PCA analysis grouped the lakes belonging to the same geographical region. Lakes belonging to the Trombetas river were correlated negatively to Component 1, while Carajás lakes were correlated positively to Component 1. On the other hand, coastal lakes were correlated positively to component 2. Paraná lakes did not display strong correlations to any axes.
Abundance, species richness, and composition of the resting egg banks
During the experiment, 1525 individuals from 88 taxa hatched from the resting egg banks. Both the abundance and species richness displayed a high variability in the resting egg bank within and among geographical regions (Fig. 1). The abundance of hatchlings ranged from 1 to 294 (median = 30) in 100 g of wet sediment, and the differences were marginally significant among regions (ANOVA, P = 0.06, df = 3, F = 2.88). The species richness ranged from 1 to 18 (median = 7). The rarefaction curves revealed no differences in the species richness between the Amazonian upland (Carajás) and Trombetas floodplain lakes (Fig. 2).
The hatchlings were identified as rotifers, cladocerans, calanoid copepods, ostracods, and anostracans (see the Online Resource 2 for a list of the observed dormant and active species). Cyclopoid copepods were not observed in the sediment of any lake. Rotifers were the most diverse group (62 species), followed by cladocerans (23 species). Rotifers and cladocerans also displayed a greater relative contribution to the species richness and abundance in the four geographical regions, except in Carajás where copepods were more abundant than cladocerans (Fig. 3). The most abundant and species-rich rotifer families were Brachionidae, Lecanidae, and Trichocercidae. For cladocerans, Chydoridae, and Sididae were the most common families. Calanoid nauplii and ostracods were restricted to Amazonian upland and coastal lakes. Anostracans were observed exclusively in Amazonian upland lakes (Fig. 3). Rotifers and cladocerans were observed in all geographical regions, but eventually rotifers or cladocerans were absent from individual lakes of a given geographical region.
The community structure (based on hatchlings) in the resting egg banks differed significantly between the four regions analyzed (MRPP analysis, A value varied from 0.17 to 0.28, P < 0.008 for all combinations). This difference in species composition was also revealed by the NMDS ordination (Fig. 4).
Concordance patterns between resting egg banks of rotifers and cladocerans
When all the lakes were considered together, species richness in the resting egg bank was concordant between rotifers and cladocerans (r Spearman = 0.41, P = 0.03, n = 26) (Table 2), i.e., the resting egg banks with more rotifer species also exhibited more cladoceran species. However, distance matrices of dormant rotifers and dormant cladocerans were only marginally correlated to each other irrespective of the index used and displayed low correlation coefficients (r Mantel for Bray–Curtis index = 0.17, P = 0.06; r Mantel for Jaccard index = 0.18, P = 0.06) (Table 2).
When the geographical datasets were individually analyzed, species richness in the resting egg banks and distance matrices of dormant rotifers and dormant cladocerans were not concordant in any region (Table 2).
Concordance patterns between dormant and active communities
When all the lakes were considered together, the species richness of dormant and active communities were not concordant between rotifers (r Spearman = 0.17, P = 0.40), cladocerans (r Spearman = 0.23, P = 0.25) or all taxonomic groups together (r Spearman = 0.35, P = 0.08) (Table 3). The correlations of active and dormant zooplankton matrices of similarity (based on Jaccard index) showed significant positive correlations for rotifers (r Mantel = 0.44, P < 0.001) and for all taxonomic groups together (r Mantel = 0.53, P < 0.0001) (Table 4), i.e., more similar lakes regarding active communities were also more similar regarding dormant communities. A non-significant correlation was found for cladocerans (r Mantel = 0.17, P = 0.15).
When geographical datasets were individually analyzed, the upper Paraná River lakes displayed a negative correlation between the dormant and active species richness of rotifers (−0.90, P < 0.05) (Table 3). The distance matrices of dormant and active stages were only marginally correlated to each other in the Amazonian upland lakes (Carajás), between rotifers (r Mantel for Jaccard index = 0.60, P = 0.07) and between cladocerans (r Mantel for Jaccard index = 0.70, P = 0.06) (Table 4).
Relationship between zooplankton and environmental and geographical matrices
When all the lakes were considered together, the distance matrices of dormant communities and geographical position were correlated for rotifers (r Mantel = 0.29, P < 0.001), cladocerans (r Mantel = 0.18, P < 0.015) and all taxonomic groups together (r Mantel = 0.45, P < 0.001). However, dormant communities were not correlated to the environmental matrix. When geographical datasets were individually analyzed, no significant correlations were observed either to the geographic or to the environmental matrices (P > 0.05 for all cases) (see the Online Resource 3 for a detail of the correlation coefficients).
Regarding the open water zooplankton communities, when all the lakes were considered together, the distance matrices of communities were correlated to both the environmental and geographic distance matrices. The correlation of zooplankton and geographic distance matrices was significant for rotifers (r Mantel = 0.45, P < 0.001), cladocerans (r Mantel = 0.22, P = 0.002), and all taxonomic groups together (r Mantel = 0.43, P < 0.001). The correlation of zooplankton and environmental matrices was also significant for rotifers (r Mantel = 0.33, P < 0.001), cladocerans (r Mantel = 0.31, P = 0.028), and all taxonomic groups together (r Mantel = 0.34, P = 0.004). When geographical datasets were individually analyzed, only coastal lakes displayed significant correlations, between zooplankton and geographic distance matrices for rotifers (r Mantel = 0.28, P = 0.04) and all taxonomic groups together (r Mantel = 0.56, P < 0.001) (see the Online Resource 3 for a detail of the correlation coefficients).
Discussion
Structure of the resting egg bank
Our results demonstrated that the resting egg banks of zooplankton are widespread in variable tropical aquatic systems, but their abundance and composition is highly uneven among and within a geographical region. Such variability might reflect different production rates, long-term viability and hatching patterns of the resting eggs that are related to either regional (e.g., hydrology, climate, and degree of isolation) or local factors (e.g., abiotic factors and biotic interactions). Indeed, the production of resting eggs is highly dependent on the fluctuations of environmental factors (Alekseev et al., 2007), in addition to the ability of active organisms to detect and properly react to environmental cues (Caceres, 1998; Caceres & Tessier, 2004). Moreover, hatching cues vary between and within species and populations, which might also contribute to distinct hatchling communities (Vandekerkhove et al., 2005a).
The absence of strong differences in the abundance of hatchlings among different regions might reflect the high variability observed among lakes within the same region, in addition to the possible aforementioned different cues indicating the production and termination of diapause. However, despite the high variability among lakes, some general patterns emerged. Overall, rotifers were the most abundant and species-rich group in the resting egg bank, a pattern that corroborates earlier studies from other freshwater systems (Maia-Barbosa et al., 2003; Ning & Nielsen, 2011; Santangelo et al., 2011b). The prevalence of rotifers among hatchlings might reflect their numerical dominance in the open water communities of the studied lakes (e.g., Bozelli, 1994; Santangelo et al., 2007) and high species richness (Segers, 2008). In addition, perhaps owing to their smaller body size, rotifers have higher rates of production of resting eggs (Gyllstrom & Hansson, 2004). Indeed, despite the possible research and publication bias, it has been demonstrated that both biotic and abiotic cues may induce diapause in rotifers. On the other hand, cladocerans and copepods produce resting eggs at a lower rate and may require more specific cues to initiate diapause (Gyllstrom & Hansson, 2004 and references therein).
The low number of hatchlings in the Paraná and coastal lakes impaired the appropriate comparison of species richness among all geographical regions, but the species richness of Amazonian upland lakes and Trombetas floodplain lakes did not differ. This similarity in species richness in the resting egg banks could be a consequence of the widespread diapause strategy evolved in most freshwater invertebrates, such as zooplankton (Caceres, 1997). In addition, lakes within a geographical region were more similar than lakes belonging to different regions. The short distance between isolated lakes within a geographical region and the hydrological connectivity among floodplain lakes might explain this pattern. Dispersal is facilitated under small distances (Havel & Shurin, 2004), and resting eggs are recognized as an important mechanism of dispersal for zooplankton (Bilton et al., 2001). Moreover, species turnover rates are usually shorter among closer sites (Soininen et al., 2007), implying that more distant lakes (i.e., those belonging to different geographical regions) are potentially less similar when compared to nearby lakes. Finally, lakes in the same geographical region are likely subjected to the action of similar local and regional factors, implying that more similar communities might develop.
The hatching cues most likely differed among the lakes and the populations analyzed in this study, and our hatching conditions may not have encompassed all the cues necessary to stimulate hatching. Indeed, different combinations of hatching cues might produce distinct hatchling communities (Vandekerkhove et al., 2005a; Garcia-Roger et al., 2008). However, the hatching procedure used here was successfully applied in a tropical coastal lake (Santangelo et al., 2011a, 2014). Thus, we believe our hatching experiment produced reliable results.
Concordance patterns between the resting egg banks of rotifers and cladocerans
Several reasons are highlighted to explain the concordance among different taxonomic groups of aquatic organisms. These reasons include similar responses to the same environmental gradient(s); responses to different but correlated environmental gradients; and the biotic interactions between them (Heino, 2010). When considering active freshwater zooplankton (rotifers, cladocerans, and copepods), a similar response to limnological features has been noted to explain the concordance among the aforementioned taxonomic groups in Brazilian reservoirs (Bini et al., 2008). Strong biotic interactions are expected between phyto- and zoo-plankton or between fish and zooplankton (Padial et al., 2012), but they are not expected among organisms within the zooplankton community.
For resting egg banks, we found concordant patterns in species richness and community similarity between the rotifers and cladocerans across all the sampled lakes. These concordant patterns imply that both taxonomic groups received the necessary cues for the induction of diapause, but different species and taxonomic groups (rotifers and cladocerans) might differ in the required cues to initiate diapause (Gyllstrom & Hansson, 2004). However, due to the low correlation coefficients (≤0.41) and the lack of correlation at individual geographical regions, rotifers, or cladocerans found in the resting egg banks should be used cautiously as a surrogate of the other group in zooplankton assays. Further studies are necessary to assess the generality of this finding.
Concordance patterns between dormant and active communities
Because resting egg banks usually mirror the active community of a given lake relatively well (Duggan et al., 2002; Vandekerkhove et al., 2005c), we expected to find high concordance patterns in the community similarity of active and dormant communities. This expectation was corroborated only when rotifers and all taxonomic groups were analyzed across all lakes. Two main reasons might be responsible for explaining the concordance between dormant and active stages. First, the composition of the resting egg bank largely depends on the composition of the active community (May, 1986; Vandekerkhove et al., 2005c). In addition, extinctions at the water column might be quickly compensated by the emergence of resting eggs, which in turn would increase the similarity between dormant and active communities.
Exceptions to this pattern, such as that observed for cladocerans, could be found in lakes where dispersal is bringing new species (in a dormant stage) but the dormant disperser remains dormant. Two additional plausible explanations for the lack of concordance among cladocerans are the lower rates of production of resting eggs when compared to rotifers (Gyllstrom & Hansson, 2004) or even the absence of cues inducing diapause.
Among individual geographic datasets, only Carajás upland lakes (marginally) displayed concordance, both for rotifers and cladocerans. Among the regions analyzed in this study, Carajás is the only region whose lakes are prone to desiccation (Lopes et al., 2011). The predictable behavior of desiccation in lakes makes dormancy more common among zooplankton (Walsh, 2013) and could explain the strongest concordance observed at the Carajás region. Among the geographical regions analyzed, the resting egg bank is possibly more important for the recovery of active communities in those upland lakes.
Relationship between zooplankton and environmental and geographical matrices
Clear associations were observed between zooplankton and the environmental variables, but only for the active stages. This finding indicates that different factors explain the composition of dormant and active communities. The main reasons explaining this pattern are the high variability in the structure of resting egg banks observed in this study and possibly the way resting egg banks are formed, by the combination of processes that add to or remove eggs from the sediment over time (Destasio, 1989). Resting eggs may be continuously added to the sediment, as different species react in distinct ways to changes in water quality, implying that no singular set of limnological conditions might predict the composition of resting egg banks. Significant associations between active zooplankton and environmental variables are commonly observed (e.g. Bini et al., 2008). Active zooplankton, owing to the short life cycle, may respond quickly to changes in water quality.
Finally, when all lakes were evaluated together, both dormant and active zooplankters were significantly associated to the geographical matrices. This finding suggests that closer communities are more similar to each other, probably because dormant and active communities influence each other (May, 1986; Vandekerkhove et al., 2005c), and closer communities usually share similar species.
Conclusions
In conclusion, our data demonstrate a high variability in the abundance, species richness and composition of resting egg banks in tropical lakes, both within and among geographical regions. The structure of resting egg banks was not associated to the environmental variables. Overall, the species richness and community similarity were not concordant between dormant rotifers and dormant cladocerans, suggesting that one taxonomic group may not be used as a surrogate of the other. In addition, even when active and dormant communities were compared, the species richness and community similarity were not necessarily concordant for all the individual or combined taxonomic groups. Stronger concordance among active and dormant organisms might be expected in lakes that clearly select for diapause, such as ephemeral lakes (Walsh, 2013).
References
Alekseev, V., O. Ravera & B. T. DeStasio, 2007. Introduction to diapause. In Alekseev, V., B. T. DeStasio & J. J. Gilbert (eds), Diapause in Aquatic Invertebrates. Springer, Netherlands, Dordrecht: 3–10.
Bilton, D. T., J. R. Freeland & B. Okamura, 2001. Dispersal in freshwater invertebrates. Annual Review of Ecology and Systematics 32: 159–181.
Bini, L. M., L. C. F. da Silva, L. F. M. Velho, C. C. Bonecker & F. A. LansacToha, 2008. Zooplankton assemblage concordance patterns in Brazilian reservoirs. Hydrobiologia 598: 247–255.
Bozelli, R. L., 1992. Composition of the zooplankton community of Batata and Mussura lakes and of the Trombetas River, State of Para, Brazil. Amazoniana 12: 239–261.
Bozelli, R. L., 1994. Zooplankton community density in relation to water-level fluctuations and inorganic turbidity in an Amazonian lake, Lago-Batata, State of Para, Brazil. Amazoniana 13: 17–32.
Brendonck, L. & L. De Meester, 2003. Egg banks in freshwater zooplankton: evolutionary and ecological archives in the sediment. Hydrobiologia 491: 65–84.
Caceres, C. E., 1997. Dormancy in invertebrates. Invertebrate Biology 116: 371–383.
Caceres, C. E., 1998. Interspecific variation in the abundance, production, and emergence of Daphnia diapausing eggs. Ecology 79: 1699–1710.
Caceres, C. E. & N. G. Hairston, 1998. Benthic-pelagic coupling in planktonic crustaceans: the role of the benthos. Archiv fur Hydrobiologie 52: 163–174.
Caceres, C. E. & A. J. Tessier, 2004. To sink or swim: Variable diapause strategies among Daphnia species. Limnology and Oceanography 49: 1333–1340.
Caliman, A., L. S. Carneiro, J. M. Santangelo, R. D. Guariento, A. P. F. Pires, A. L. Suhett, L. B. Quesado, V. Scofield, E. S. Fonte, P. M. Lopes, L. F. Sanches, F. D. Azevedo, C. C. Marinho, R. L. Bozelli, F. A. Esteves & V. F. Farjalla, 2010. Temporal coherence among tropical coastal lagoons: a search for patterns and mechanisms. Brazilian Journal of Biology 70: 803–814.
Colwell, R. K., 2005. Estimates: Statistical Estimation of Species Richness and Shared Species from Samples. Version 8.0. User’s Guide and application published at http://viceroy.eeb.uconn.edu/EstimateS.
Destasio, B. T., 1989. The seed bank of a fresh-water crustacean – copepodology for the plant ecologist. Ecology 70: 1377–1389.
Destasio, B. T., 1990. The role of dormancy and emergence patterns in the dynamics of a fresh-water zooplankton community. Limnology and Oceanography 35: 1079–1090.
Duggan, I. C., J. D. Green & R. J. Shiel, 2002. Rotifer resting egg densities in lakes of different trophic state, and their assessment using emergence and egg counts. Archiv fur Hydrobiologie 153: 409–420.
Dussart, B. H. & D. Defaye, 2001. Introduction to the Copepoda. Backhuys Publishers, Leiden.
Elmoor-Loureiro, L. M. A., 1997. Manual de Identificação de Cladóceros Límnicos do Brasil. Universa, Taguatinga.
Fattorini, S., R. L. H. Dennis & L. M. Cook, 2012. Use of cross-taxon congruence for hotspot identification at a regional scale. PLoS One 7: e40018.
Frisch, D., 2002. Dormancy, dispersal and the survival of cyclopoid copepods (Cyclopoida, Copepoda) in a lowland floodplain. Freshwater Biology 47: 1269–1281.
Garcia-Roger, E. M., X. Armengol, M. J. Carmona & M. Serra, 2008. Assessing rotifer diapausing egg bank diversity and abundance in brackish temporary environments: an ex situ sediment incubation approach. Fundamental and Applied Limnology 173: 79–88.
Gotelli, N. J. & R. K. Colwell, 2001. Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecology Letters 4: 379–391.
Gyllstrom, M. & L. A. Hansson, 2004. Dormancy in freshwater zooplankton: induction, termination and the importance of benthic-pelagic coupling. Aquatic Sciences 66: 274–295.
Hairston, N. G., 1996. Zooplankton egg banks as biotic reservoirs in changing environments. Limnology and Oceanography 41: 1087–1092.
Hairston, N. G., A. V. B. Robert, C. M. Kearns & D. R. Engstrom, 1995. Age and survivorship of diapausing eggs in a sediment egg bank. Ecology 76: 1706–1711.
Hairston, N. G., A. M. Hansen & W. R. Schaffner, 2000. The effect of diapause emergence on the seasonal dynamics of a zooplankton assemblage. Freshwater Biology 45: 133–145.
Havel, J. E. & J. B. Shurin, 2004. Mechanisms, effects, and scales of dispersal in freshwater zooplankton. Limnology and Oceanography 49: 1229–1238.
Heino, J., 2010. Are indicator groups and cross-taxon congruence useful for predicting biodiversity in aquatic ecosystems? Ecological Indicators 10: 112–117.
Junk, W. J., M. T. F. Piedade, R. Lourival, F. Wittmann, P. Kandus, L. D. Lacerda, R. L. Bozelli, F. A. Esteves, C. Nunes da Cunha, L. Maltchik, J. Schongart, Y. Schaeffer-Novelli & A. A. Agostinho, 2014. Brazilian wetlands: their definition, delineation, and classification for research, sustainable management, and protection. Aquatic Conservation: Marine and Freshwater Ecosystems 24: 5–22.
Koste, W., 1978. Die Radertiere mitteleuropas begrundet von Max Voigt. Gebruder Borntraeger, Stuttgart.
Lopes, P. M., A. Caliman, L. S. Carneiro, L. M. Bini, F. A. Esteves, V. F. Farjalla & R. L. Bozelli, 2011. Concordance among assemblages of upland Amazonian lakes and the structuring role of spatial and environmental factors. Ecological Indicators 11: 1171–1176.
Lopes, P. M., L. M. Bini, S. A. J. Declerk, V. F. Farjalla, L. C. G. Vieira, C. C. Bonecker, F. A. Lansac-Toha, F. A. Esteves & R. L. Bozelli, 2014. Correlates of zooplankton beta diversity in tropical lake systems. PLoS One 9: e109581.
Maia-Barbosa, P. M., E. M. Eskinazi-Sant’Anna, C. F. Valadares & G. C. D. Pessoa, 2003. The resting eggs of zooplankton from a tropical, eutrophic reservoir (Pampulha Reservoir, south-east Brazil). Lakes & Reservoirs: Research and Management 8: 269–275.
Matsumura-Tundisi, T., 1986. Latitudinal distribution of Calanoida copepods in freshwater aquatic systems of Brazil. Revista Brasileira de Biologia 46: 527–553.
May, L., 1986. Rotifer sampling – a complete species list from one sampling. Hydrobiologia 134: 117–120.
McCune, B. & M. J. Mefford, 1999. PC-ORD: Multivariate Analysis of Ecological Data, version 4. MjM Software Design Gleneden Beach, Oregon, USA.
Ning, N. S. P. & D. L. Nielsen, 2011. Community structure and composition of microfaunal egg bank assemblages in riverine and floodplain sediments. Hydrobiologia 661: 211–221.
Onbe, T., 1978. Sugar flotation method for sorting the resting eggs of marine cladocerans and copepods from sea-bottom sediment. Bulletin of the Japanese Society of Scientific Fisheries 44: 1411.
Padial, A. A., S. Declerck, L. de Meester, C. C. Bonecker, F. A. LansacToha, L. C. Rodrigues, A. Takeda, S. Train, L. F. M. Velho & L. M. Bini, 2012. Evidence against the use of surrogates for biomonitoring of Neotropical floodplains. Freshwater Biology 57: 2411–2423.
Ricci, C., 2001. Dormancy patterns in rotifers. Hydrobiologia 446: 1–11.
Santangelo, J. M., A. D. M. Rocha, R. L. Bozelli, L. S. Carneiro & F. D. A. Esteves, 2007. Zooplankton responses to sandbar opening in a tropical eutrophic coastal lagoon. Estuarine Coastal and Shelf Science 71: 657–668.
Santangelo, J. M., L. R. Araújo, F. A. Esteves, M. Manca & R. L. Bozelli, 2011a. Method for hatching resting eggs from tropical zooplankton: effects of drying or exposing to low temperatures before incubation. Acta Limnologica Brasiliensia 23: 42–47.
Santangelo, J. M., F. A. Esteves, M. Manca & R. L. Bozelli, 2011b. Abundance, composition and spatial variation in the egg bank of a tropical zooplankton community. Studies on Neotropical Fauna and Environment 46: 225–232.
Santangelo, J. M., F. A. Esteves, M. Manca & R. L. Bozelli, 2014. Disturbances due to increased salinity and the resilience of zooplankton communities: the potential role of the resting egg bank. Hydrobiologia 722: 103–113.
Segers, H., 2008. Global diversity of rotifers (Rotifera) in freshwater. Hydrobiologia 595: 49–59.
Simões, N. R., J. D. Dias, C. M. Leal, L. S. M. Braghin, F. A. Lansac-Tôha & C. C. Bonecker, 2013. Floods control the influence of environmental gradients on the diversity of zooplankton communities in a neotropical floodplain. Aquatic Sciences 75: 607–617.
Soininen, J., R. McDonald & H. Hillebrand, 2007. The distance decay of similarity in ecological communities. Ecography 30: 3–12.
Thomaz, S. M., L. M. Bini & R. L. Bozelli, 2007. Floods increase similarity among aquatic habitats in river-floodplain systems. Hydrobiologia 579: 1–13.
Tisseuil, C., J. Cornu, O. Beauchard, S. Brosse, W. Darwall, R. Holland, B. Hugueny, P. A. Tedesco & T. Oberdorff, 2013. Global diversity patterns and cross-taxa convergence in freshwater systems. Journal of Animal Ecology 82: 365–376.
Tollrian, R., 1993. Neckteeth formation in Daphnia pulex as an example of continuous phenotypic plasticity – morphological effects of Chaoborus kairomone concentration and their quantification. Journal of Plankton Research 15: 1309–1318.
Vandekerkhove, J., B. Niessen, S. Declerck, E. Jeppesen, J. M. C. Porcuna, L. Brendonck & L. De Meester, 2004. Hatching rate and hatching success with and without isolation of zooplankton resting stages. Hydrobiologia 526: 235–241.
Vandekerkhove, J., S. Declerck, L. Brendonck, J. M. Conde-Porcuna, E. Jeppesen & L. De Meester, 2005a. Hatching of cladoceran resting eggs: temperature and photoperiod. Freshwater Biology 50: 96–104.
Vandekerkhove, J., S. Declerck, L. Brendonck, J. M. Conde-Porcuna, E. Jeppesen, L. S. Johansson & L. De Meester, 2005b. Uncovering hidden species: hatching diapausing eggs for the analysis of cladoceran species richness. Limnology and Oceanography-Methods 3: 399–407.
Vandekerkhove, J., S. Declerck, E. Jeppesen, J. M. Conde-Porcuna, L. Brendonck & L. De Meester, 2005c. Dormant propagule banks integrate spatio-temporal heterogeneity in cladoceran communities. Oecologia 142: 109–116.
Walsh, M. R., 2013. The link between environmental variation and evolutionary shifts in dormancy in zooplankton. Integrative and Comparative Biology 53(4): 713–732.
Waterkeyn, A., P. Grillas, E. R. M. De Roeck, L. Boven & L. Brendonck, 2009. Assemblage structure and dynamics of large branchiopods in Mediterranean temporary wetlands: patterns and processes. Freshwater Biology 54: 1256–1270.
Acknowledgments
We thank the staff of UFRJ and UEM for assistance during this study. Financial support was provided by CNPq, Petrobras, Vale and MRN. A fellowship for J. M. Santangelo was provided by CAPES.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Karl E. Havens
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Santangelo, J.M., Lopes, P.M., Nascimento, M.O. et al. Community structure of resting egg banks and concordance patterns between dormant and active zooplankters in tropical lakes. Hydrobiologia 758, 183–195 (2015). https://doi.org/10.1007/s10750-015-2289-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-015-2289-y