Abstract
Dormancy may be an important aspect influencing the ecology of riverine microfauna, yet fundamental knowledge concerning riverine egg bank communities is still scant compared with that for communities in floodplain habitats. We investigated the microfaunal egg bank communities in slackwater habitats of an Australian floodplain river, and compared them with the communities occurring in nearby floodplain wetlands. This was achieved by taking replicate sediment cores from paired examples of each habitat and later incubating the resting stages within these sediment cores. Results from the study indicated that the egg bank communities in each habitat differed in both composition and structure, with only 12 of the 31 taxa recorded being common to both habitat types. This suggests that in addition to supporting microfaunal persistence in the main channel, riverine egg bank communities represent an important source of microfaunal diversity together with floodplain egg bank communities in river–floodplain systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dormancy has long been accepted as a critical adaptation of microfauna for surviving in variable environments (Hutchinson, 1967; Wiggins et al., 1980). Most microfauna enter dormancy via the production of resting eggs or cysts, while some can enter dormancy as juveniles or adults (Hairston & Cáceres, 1996; Brendonck & De Meester, 2003). The accumulation of resting stages in the sediments of aquatic ecosystems over time leads to the formation of a potentially long-lived resting stage community (or ‘egg bank’) that is inherently linked to the active (water column) microfaunal community (Cáceres & Hairston, 1998; Gyllström & Hansson, 2004). This egg bank not only supports the persistence of microfaunal communities through unfavourable conditions, but can also play an important role in influencing active population and community dynamics, seasonal succession, zoogeographic patterns, and the evolution of populations (De Stasio, 1990; Hairston et al., 2000).
Numerous studies have investigated the egg bank communities in floodplain habitats and have found them to be highly diverse and abundant (e.g. Havel et al., 2000; Shiel et al., 2001). Such communities are thought to be important in supporting the persistence, productivity and diversity of microfaunal communities in river–floodplain ecosystems (Boulton & Lloyd, 1992; Havel et al., 2000). In comparison, the importance of egg bank communities in riverine habitats is much less clear (Ning, 2008). A number of studies have acknowledged the occurrence of microfaunal egg bank communities in rivers (Lair, 2006; Nielsen & Watson, 2008); however, very few have empirically investigated their abundance and composition (but see el Moghraby, 1977). The scarcity of such studies is probably largely due to the perception that egg bank communities are seldom abundant in rivers, and/or that dormancy is of little value in the ecology of riverine microfauna (Ning, 2008). Nevertheless, results from the few empirical riverine investigations that have been undertaken thus far indicate that egg bank communities do occur in the main channel (el Moghraby, 1977; Ning et al., 2008, 2010a) and suggest that they may be of particular importance in areas of inshore retention, or ‘slackwater’ habitats (Ning et al., 2008, 2010a), where microfauna recruit and reproduce (Schiemer et al., 2001; Nielsen et al., 2005)
The occurrence of an egg bank community in main channel sediments has a number of important implications for the ecology of river–floodplain microfauna. Firstly, it suggests that riverine microfauna are potentially capable of persisting through catastrophic events without supplementation from floodplain populations (Ning, 2008). Secondly, emergence from these dormant egg banks may also be important in influencing the population dynamics of taxa that persist throughout the water column year-round, and/or the community dynamics of active assemblages in places/times of low environmental stress (De Stasio, 1990; Hairston et al., 2000). Indeed, if egg bank communities present in the sediments of riverine habitats are taxonomically distinct from those in the sediments of floodplain habitats, then riverine egg bank communities may represent a similarly important source of microfaunal species richness along with floodplain egg bank communities in river–floodplain systems. Alternatively, if these egg bank communities are taxonomically similar, then riverine egg bank communities may be more influential in supporting microfaunal persistence or productivity, rather than in acting as a source of diversity.
This study compared the similarity of egg bank communities in slackwater sediments with those in floodplain wetland sediments as a fundamental step towards understanding the importance of riverine egg bank communities in the ecology of microfauna in river–floodplain systems. Specifically, we tested the hypothesis that river slackwaters support egg bank communities that are taxonomically distinct from those in floodplain wetlands, and hence, that the former play a similarly important role along with floodplain communities in providing a source of microfaunal diversity within river–floodplain systems. The communities were compared in both late spring and summer to determine whether their similarity changed over time in association with physico-chemical conditions and potentially, egg bank dynamics.
Materials and methods
Study site
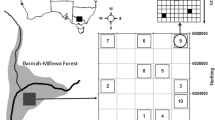
The study was conducted in a lowland section of the Broken River in north-eastern Victoria, Australia. The Broken River rises on the northern-facing slopes of the Great Dividing Range and discharges into the Goulburn River, which is a tributary of the Murray River. It possesses a total length of approximately 180 km, and receives inflows from a number of major tributaries, including Holland’s Creek, Ryan’s Creek and Lima East Creek (Humphries et al., 2006; Ning et al., 2010b).
The lowland section of the river has a bank width of approximately 20–30 m, and possesses a diverse range of habitat types including run sections, deep pools (up to 2–3 m), and shallow slackwaters. Instream structure consists of large amounts of coarse woody debris, as well as variably distributed stands of emergent macrophytes (mainly Phragmites australis). The surrounding floodplain is inundated approximately one in every 4 years, and contains a mosaic of wetlands (King, 2002) This study was carried out at a site (36°30′32″ S 145°56′91″ E) consisting of a 1 km reach, located approximately 7 km north-west of the rural city of Benalla.
Egg bank sampling
Riverine slackwater and floodplain wetland egg bank communities were sampled once on the 12 November, 2005 and once on the 2 February, 2006. Slackwaters were identified as areas within the main channel where either no discernible flow or back eddies occurred (Nielsen et al., 2005). Those sampled ranged between 28 and 120 m2 in area, with average depths from 0.3 to 1.1 m. Wetlands were paired with slackwaters, and identified as nearby waterbodies located on the floodplain and detached from the main channel. These habitats were much more variable in morphometry, with surface areas ranging from approximately 100–3600 m2, and average depths from 0.3 to 0.9 m. All contained water at the time of sampling.
Four sites (i.e. replicate slackwater–wetland pairs) were randomly selected for sampling from a suite of potential sites that were in close proximity to each other, and the same sites were sampled on both sampling occasions. Sampling was undertaken using a 10 cm diameter PVC sediment corer. Four 10 cm diameter core samples were collected from random locations in each habitat-type at each site to account for variation in the distribution of resting stages and the small area of the sediment corer (King, 2004). The corer was inserted to a depth of 5 cm, a depth designed to capture the majority of microfauna resting stages (Herzig, 1985; Cáceres, 1998; Brendonck & De Meester, 2003), and removed with the aid of a trowel. Upon collection, each sediment core was carefully placed into a 2-l plastic container (0.15 × 0.15 × 0.09 m) for subsequent laboratory incubation.
Water physico-chemistry and chlorophyll-a
Water temperature, pH, dissolved oxygen (DO), turbidity and electrical conductivity (EC) were measured once in each slackwater and wetland on each sampling occasion between 1400 and 1600 h, using a Horiba U-10 Water Quality Checker (Horiba Ltd., Kyoto, Japan), and mean daily discharge records for the study period were obtained from a gauging station above Casey Weir (Theiss Engineering), approximately 1 km downstream. Water column chlorophyll-a was also measured once in each slackwater and wetland on every trip as an indicator of algal biomass, and hence a surrogate for food availability for microfauna (Bonecker & Lansac-Tôha, 1996; Kobayashi, 1997). Samples were collected using 1-l opaque plastic jars, put on ice and sheltered from light.
Laboratory sample processing
The sediment core samples were allowed to air dry for 2 weeks in a covered outdoor area to ensure that they were fully dry, and hence, only dormant microfauna remained before re-inundating (Ning et al., 2008, 2010a). Samples were then incubated in a controlled-temperature room using a 12:12 light/dark regime, and a 25°C:10°C day/night air temperature regime following the protocol described in Ning et al. (2008). This temperature regime was used to simulate average maximum and minimum temperatures for the study area in late spring, since this is thought to be the optimum time of year for hatching conditions in temperate regions (Wolf & Carvalho, 1989; Vandekerkhove et al., 2005). In summary, each sediment core sample was inundated with aged tap water and oxygenated using aquarium bubblers. Samples were taken twice weekly over a 28-day period by gently pouring the water from each 2-l container through a 50-μm sieve, with minimum disturbance to the sediment. Twenty-eight days is known to be sufficient for resting stage microfauna to emerge for counting and identification (Boulton & Lloyd, 1992; Nielsen et al., 2007; Ning et al., 2008), and twice weekly sampling minimises the potential for reproduction by any individuals that emerge (Nielsen et al., 2007, 2008). On each occasion, the collected sample was put into a 200-ml storage jar and preserved in 70% ethanol, while the sediment remaining in each incubation container was re-inundated with aged tap water. Samples from each incubation container were combined over the 28-day period so that all of the emergent microfauna from one incubation container were placed into one 200-ml sample jar.
Microfauna samples were subsampled prior to processing. One millilitre aliquots were successively placed into a Sedgewick-Rafter counting chamber, and identified and counted using darkfield microscopy until 10% of the sample had been analysed. All microfauna were identified to the level of Family or Genus, except for ostracods, which were identified to the level of Class. Taxa were identified using relevant keys in Shiel (1995).
Chlorophyll-a samples were assessed in triplicate using the 90% boiling ethanol method (International Standards Organisation, 1994). Specifically, for each triplicate subsample, 250 ml of water was filtered through a glass fibre GF/C filter, the filter placed into a 10 ml centrifuge tube, 10 ml of 90% AR grade (100%) ethanol added, the tube capped and the sample placed into a water bath between 70 and 75°C for 5 min. The sample was then removed, allowed to cool and refrigerated overnight. On the next day, the filter paper was extracted and the sample centrifuged at 3,000 rpm for 10 min. The sample chlorophyll-a concentration was then analysed using a Varian Cary 1E spectrophotometer (optical adsorption at 665 and 750 nm).
Data analysis
Prior to analysis, microfaunal count data were converted to density values (animals m−2). Multivariate analysis of the community data was undertaken using permutational analysis of variance (PERMANOVA; Anderson, 2001) and non-metric multidimensional scaling (nMDS in PRIMER Version 6, Clarke & Warwick, 2001) to investigate differences in community patterns according to ‘habitat’ and ‘sampling occasion’. All analyses were based on Bray-Curtis similarity matrices, and performed on both square root and presence–absence transformed data to examine patterns for both community structure (i.e. the relative abundance of taxa) and composition (i.e. the presence or absence of taxa), respectively.
Linear mixed-effects ANOVA, with ‘site’ as a random effect and ‘habitat’ (slackwater vs. wetland), and ‘sampling occasion’ as fixed effects, was undertaken to investigate habitat-related and temporal variation in the environmental variables, total microfaunal density, and the densities of three of the most common rotifer and microcrustacean taxa. Datasets were log10 (x + 1) transformed prior to analysis to satisfy the assumptions of analysis of variance. Analyses were undertaken using SPSS Version 15.0 (SPSS, Inc., Chicago, IL, USA).
Taxon richness analyses were undertaken using rarefaction curves (Primer Version 6; Clarke & Warwick, 2001). Rarefaction curves are generated by repeatedly re-sampling the pool of N individuals or N samples randomly, and plotting the mean number of taxa represented by 1, 2,…, N individuals or samples (Gotelli & Colwell, 2001). Such curves allow for comparisons of taxon richness with respect to specific numbers of individuals, and hence, avoid biases associated with comparing potentially different sample sizes (Gotelli & Colwell, 2001).
Results
Environmental variables
Flow was always confined to the channel during the study period and generally declined overall (Fig. 1). EC, turbidity and chlorophyll-a were significantly greater in wetlands than in slackwaters overall, although the inter-habitat variation in EC and turbidity differed over time (Table 1; Fig. 2). In comparison, DO and water temperature were both significantly greater in slackwaters than in wetlands (Table 1; Fig. 2).
Egg bank communities
A total of 31 taxa, comprised of 21 rotifers and 10 microcrustaceans, were found to emerge from the sediments of both habitats during this study (Table 2). The most common rotifer taxa were Bdelloidea (occurred in 94% of samples), Cephalodella spp. (88%) and Lophocaris spp. (50%); while the most common microcrustacean taxa were Ostracoda (56%), Chydoridae (44%) and Daphniidae (25%). Of the 31 taxa, only 12 were common to both slackwaters and wetlands. Conversely, three rotifer and four microcrustacean taxa were found to emerge only from slackwater sediments, while ten rotifer and two microcrustacean taxa were found to emerge only from wetland sediments (Table 2).
Eleven rotifer and eight microcrustacean taxa were found to emerge from slackwater sediments (Table 2). Overall, the slackwater community comprised of 83% Rotifera and 17% microcrustacea in terms of abundance. The microcrustacean component was numerically dominated by Ostracoda, followed by Cladocera and Copepoda, respectively (Table 2). In comparison, 18 rotifer and six microcrustacean taxa were found to emerge from wetland sediments (Table 2). Overall, the wetland community comprised of 99% Rotifera and 1% microcrustacea in terms of abundance. The microcrustacean component was numerically dominated by Cladocera, followed by Ostracoda and Copepoda, respectively (Table 2).
Community structure and composition
Total microfaunal community structure and composition both varied significantly according to habitat irrespectively of any differences among sampling occasions (Table 3; Fig. 3). Further investigation indicated that rotifer community structure, microcrustacean community structure and microcrustacean community composition all varied significantly according to habitat independently of any temporal variation (Table 3). In comparison, rotifer community composition varied significantly according to the interaction between habitat and sampling occasion (Table 3).
Two dimensional nMDS solutions for the mean abundance of all microfaunal taxa recorded in both habitats, on both sampling occasions. Ordinations are presented to show community structure (square-root transformed density data) and composition (presence–absence transformed data) (Slackwaters S and Wetlands W)
Density and taxon richness
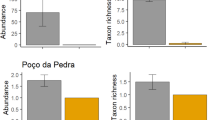
As for total microfaunal community structure and composition, total microfaunal density and rotifer density both varied significantly according to habitat independently of any variation among sampling occasions, and were greater in wetlands than in slackwaters (Table 1; Fig. 4). Further investigation indicated that the common taxa, Bdelloidea, Cephalodella spp. and Daphniidae, were all significantly more abundant in wetlands than in slackwaters (Table 1; Fig. 4). Although a greater overall number of taxa were found to emerge from wetland sediments compared with slackwater sediments, rarefaction analysis indicated that there was no difference in expected taxon richness between habitats when standardised for number of individuals, on either sampling occasion (overlapping standard errors, Fig. 5).
Rarefaction curves showing expected mean taxon richness (±1 standard error) as a function of number of individual microfauna (animals m−2) in slackwaters (S) and wetlands (W) (n = 4). Expected mean taxon richness values for specific numbers of individuals were calculated using rarefaction (PRIMER Version 6; Clarke & Warwick, 2001), and then plotted to produce the curves
Discussion
Despite the potential importance of dormancy in the ecology of riverine microfauna, fundamental knowledge concerning main channel egg bank communities is still scarce compared with that for communities in floodplain habitats. This study followed on from recent work highlighting the occurrence of egg bank communities in river slackwaters (Ning et al., 2008, 2010a), and tested the prediction that slackwater sediments support egg bank communities that are taxonomically distinct from those in floodplain wetland sediments. Findings from the study supported this prediction, suggesting that riverine egg bank communities not only support microfaunal persistence in the main channel, but also perform an important function together with floodplain egg bank communities in acting as a source of microfaunal diversity within river–floodplain systems.
Slackwater and wetland egg bank communities differed in terms of both composition and structure. Fewer than half of all taxa found emerged from the sediments of both habitats, and instead, a high proportion seemed to be habitat-specific in their occurrence. These combined differences suggest that slackwater and wetland habitats support distinct microfaunal communities.
According to Brendonck & De Meester (2003), the structure and composition of an egg bank community in a particular habitat is largely determined by the abiotic and biotic attributes of the habitat, in addition to the population dynamics of its active microfaunal community. Slackwaters are regions of inshore retention that are largely influenced by the abiotic and biotic attributes of the main channel owing to their permanent connection with it (Lancaster & Hildrew, 1993; Schiemer et al., 2001; Ning et al., 2010b). In contrast, floodplain wetlands typically possess abiotic and biotic attributes that are highly independent of the main channel, and are usually much more vulnerable to the effects of drawdown (Thomaz et al., 2007). These inherent differences are likely to have a major influence on factors directly affecting resting stages such as dispersal to and from nearby habitats, sediment movement and mixing, hatching/emergence, predation, parasitism, and degradation (De Stasio, 1989; Cáceres & Hairston, 1998). They would also indirectly affect egg bank dynamics via their influence on active population and community dynamics, and the dormancy induction patterns of specific taxa or individuals (Cáceres & Hairston, 1998; Gyllström & Hansson, 2004). For example, both physico-chemical (water temperature) and food availability (as measured by chlorophyll-a concentration) conditions differed significantly among the slackwaters and wetlands sampled in this study, and such variation may have cued differences in resting stage production for the taxa in each habitat (e.g. Gilbert & Schreiber, 1998; Avery, 2005).
In addition to the variation in microfaunal community composition and structure, wetland communities were both more abundant and possessed a greater overall number of taxa than slackwater communities, especially in terms of the Rotifera. This particular finding may not be all that surprising, given that floodplain habitats typically support a much greater productivity of active microfauna compared with main channel habitats (e.g. Hein et al., 1999; Grosholz & Gallo, 2006). Further to the relatively high number of active microfauna in wetlands (e.g. Hein et al., 1999; Grosholz & Gallo, 2006), it is possible that wetland microfauna produce proportionately more resting stages (i.e. per taxon or individual) in order to cope with the typically greater risk of desiccation associated with their habitats (e.g. García-Roger et al., 2006). Conversely, egg banks in the upper sediments of the main channel may possess a relatively low number of dormant microfauna due to regular scouring events associated with changes in discharge (Ning et al., 2008). In any case, however, the greater overall number of taxa found in wetland samples compared with in slackwater samples seems to have simply been an artefact of the significantly higher number of individuals in wetlands, since there was no difference in each habitat’s expected taxon richness when standardised for number of individuals.
Despite the significant difference in the density of rotifers emerging from slackwater and wetland sediments, rotifers were numerically dominant over microcrustaceans in both habitats. Several studies on active microfaunal dynamics in temperate Australian river (Shiel et al., 1982; Nielsen et al., 2005; Ning et al., 2010b) and floodplain (Tan & Shiel, 1993; Nielsen et al., 2002) habitats have reported a similar pattern, and hence, this result may have been largely reflective of a relatively high abundance of active rotifers in both habitats. Nevertheless, it should be acknowledged that the artificial nature of the incubation may have also potentially contributed to an underestimation of the proportion of microcrustaceans in the slackwaters and wetlands sampled. Artificial incubation techniques are widely used for examining resting stage communities (Jankowski & Straile, 2003; Vandekerkhove et al., 2005), since they provide a standardized method for the counting and identification of viable animals in an emerged form. Unfortunately, however, they are limited in their ability to supply species-specific hatching cues for every taxon in a community (Vandekerkhove et al., 2005), and hence, they generate results that may undervalue certain components of the resting stage community.
While the role of egg bank communities for the microfauna in floodplain habitats is well known, the role of such communities in rivers is much less clear. This study suggests that rivers support egg bank communities that are taxonomically distinct from those in floodplain habitats, and consequently, that riverine communities may perform a similarly valuable function along with floodplain communities in providing a source of microfaunal diversity within river–floodplain systems.
References
Anderson, M. J., 2001. A new method for non-parametric multivariate analysis of variance. Austral Ecology 26: 32–46.
Avery, D. E., 2005. Induction of embryonic dormancy in the Calanoid copepod Acartia hudsonica: proximal cues and variation among individuals. Journal of Experimental Marine Biology and Ecology 314: 203–214.
Bonecker, C. C. & F. A. Lansac-Tôha, 1996. Community structure of rotifers in two environments of the upper river Parana floodplain (MS)-Brazil. Hydrobiologia 325: 137–150.
Boulton, A. J. & L. N. Lloyd, 1992. Flooding frequency and invertebrate emergence from dry floodplain sediments of the River Murray, Australia. Regulated Rivers: Research and Management 7: 137–151.
Brendonck, L. & L. De Meester, 2003. Egg banks in freshwater zooplankton: evolutionary and ecological archives in the sediment. Hydrobiologia 491: 65–84.
Cáceres, C. E., 1998. Interspecific variation in the abundance, production, and emergence of Daphnia diapausing eggs. Ecology 79: 1699–1710.
Cáceres, C. E. & N. G. Hairston Jr., 1998. Benthic-pelagic coupling in planktonic crustaceans: the role of the benthos. Archiv Fuer Hydrobiologie 52: 163–174.
Clarke, K. & R. M. Warwick, 2001. Change in marine communities: an approach to statistical analysis and interpretation. National Environment Research Council, Plymouth.
De Stasio, B. T., Jr. 1989. The seedbank of a freshwater crustacean: Copepodology for the plant ecologist. Ecology 70: 1377–1389.
De Stasio, B. T., Jr. 1990. The role of dormancy and emergence patterns in the dynamics of a freshwater zooplankton community. Limnology and Oceanography 35: 1079–1090.
el Moghraby, A. I., 1977. A study on diapause of zooplankton in a tropical river—the Blue Nile. Freshwater Biology 7: 207–212.
García-Roger, E. M., M. J. Carmona & M. Serra, 2006. Patterns in rotifer diapausing egg banks: density and viability. Journal of Experimental Marine Biology and Ecology 336: 198–210.
Gilbert, J. J. & D. K. Schreiber, 1998. Asexual diapause induced by food limitation in the rotifer Synchaeta pectinata. Ecology 79: 1371–1381.
Gotelli, N. J. & R. K. Colwell, 2001. Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecology Letters 4: 379–391.
Grosholz, E. & E. Gallo, 2006. The influence of flood cycle and fish predation on invertebrate production on a restored California floodplain. Hydrobiologia 568: 91–109.
Gyllström, M. & L. A. Hansson, 2004. Dormancy in freshwater zooplankton: induction, termination and the importance of benthic-pelagic coupling. Aquatic Sciences 66: 274–295.
Hairston Jr, N. G. & C. E. Cáceres, 1996. Distribution of crustacean diapause: micro- and macroevolutionary pattern and process. Hydrobiologia 320: 27–44.
Hairston Jr, N. G., A. M. Hansen & W. R. Schaffner, 2000. The effect of diapause emergence on the seasonal dynamics of a zooplankton assemblage. Freshwater Biology 45: 133–145.
Havel, J. E., E. M. Eisenbacher & A. A. Black, 2000. Diversity of crustacean zooplankton in riparian wetlands: colonization and egg banks. Aquatic Ecology 34: 63–76.
Hein, T., C. Baranyi, G. Heiler, C. Holarek, P. Riedler & F. Schiemer, 1999. Hydrology as a major factor determining plankton development in two floodplain segments and the River Danube, Austria. Archiv Fuer Hydrobiologie Supplement 115: 439–452.
Herzig, A., 1985. Resting eggs—a significant stage in the life cycle of crustaceans Leptodora kindtii and Bythotrephes longimanus. Verhandlungen Internationale Vereinigung für Theoretische und Angewandte Limnologie 22: 3088–3098.
Humphries, P., R. A. Cook, A. J. Richardson & L. G. Serafini, 2006. Creating a disturbance: manipulating slackwaters in a lowland river. River Research and Applications 22: 525–542.
Hutchinson, G. E., 1967. A Treatise on Limnology. Volume 2. Introduction to Lake Biology and the Limnoplankton. Wiley Interscience Publishers, New York.
International Standards Organisation, 1994. Water quality—measurement of biochemical parameters, spectrophotometric determination of the chlorophyll-a concentration (ISO 10260: 1992(E)). In ISO Standards Compendium, Environment—Water Quality, Vol. 2, Chemical Methods. International Standards Organisation, France: 308.
Jankowski, T. & D. Straile, 2003. A comparison of egg-bank and long-term plankton dynamics of two Daphnia species, D. hyalina and D. galeata: potentials and limits of reconstruction. Limnology and Oceanography 48: 1948–1955.
King, A. J., 2002. Recruitment ecology of fish in floodplain rivers of the southern Murray Darling Basin, Australia. PhD thesis, Monash University, Melbourne, Australia.
King, A. J., 2004. Density and distribution of potential prey for larval fish in the main channel of a floodplain river: pelagic versus epibenthic meiofauna. River Research and Applications 20: 883–897.
Kobayashi, T., 1997. Associations between environmental variables and zooplankton body mass in a regulated Australian river. Marine and Freshwater Research 48: 523–529.
Lair, N., 2006. A review of regulation mechanisms of metazoan plankton in riverine ecosystems: aquatic habitat versus biota. River Research and Applications 22: 567–593.
Lancaster, J. & A. G. Hildrew, 1993. Characterising in-stream flow refugia. Canadian Journal of Fisheries and Aquatic Sciences 50: 1663–1675.
Nielsen, D. & G. Watson, 2008. The response of epibenthic rotifers and microcrustacean communities to flow manipulations in lowland rivers. Hydrobiologia 603: 117–128.
Nielsen, D. L., T. J. Hillman, F. J. Smith & R. J. Shiel, 2002. The influence of seasonality and duration of flooding on zooplankton in experimental billabongs. River Research and Applications 18: 227–237.
Nielsen, D. L., M. A. Brock, R. Petrie & K. Crosslé, 2007. The impact of salinity pulses on the emergence of plants and zooplankton from wetland seed and egg banks. Freshwater Biology 52: 784–795.
Nielsen, D. L., G. Watson & R. Petrie, 2005. Microfaunal communities in three lowland rivers under differing flow regimes. Hydrobiologia 543: 101–111.
Ning, N. S. P., 2008. The ecology of the microinvertebrate fauna in a temperate Australian floodplain river. PhD thesis, La Trobe University, Wodonga, Australia.
Ning, N. S. P., D. L. Nielsen, T. J. Hillman & P. J. Suter, 2008. Evaluation of a new technique for characterising resting stage zooplankton assemblages in riverine slackwater habitats and floodplain wetlands. Journal of Plankton Research 30: 415–422.
Ning, N. S. P., D. L. Nielsen, T. J. Hillman & P. J. Suter, 2010a. The influence of planktivorous fish on zooplankton resting-stage communities in riverine slackwater regions. Journal of Plankton Research 32: 411–421.
Ning, N. S. P., D. L. Nielsen, T. J. Hillman & P. J. Suter, 2010b. Microinvertebrate dynamics in riverine slackwater and mid-channel habitats in relation to physico-chemical parameters and food availability. River Research and Applications 26: 279–296.
Schiemer, F., H. Keckeis, W. Reckendorfer & G. Winkler, 2001. The “inshore retention concept” and its significance for large rivers. Algological Studies 135: 509–516.
Shiel, R. J., 1995. A Guide to Identification of Rotifers, Cladocerans and Copepods from Australian Inland Waters. Cooperative Research Centre for Freshwater Ecology, Canberra.
Shiel, R. J., J. D. Green & L. W. Tan, 2001. Microfaunal and resting stage heterogeneity in ephemeral floodplain pools, upper River Murray floodplain. Internationale Vereinigung für Theoretische und Angewandte Limnologie 27: 3738–3741.
Shiel, R. J., K. F. Walker & W. D. Williams, 1982. Plankton of the lower River Murray South Australia. Australian Journal of Marine and Freshwater Research 33: 301–327.
Tan, L. & R. J. Shiel, 1993. Responses of billabong rotifer communities to inundation. Hydrobiologia 255(256): 361–369.
Thomaz, S. M., L. M. Bini & R. L. Bozelli, 2007. Floods increase similarity among aquatic habitats in river–floodplain systems. Hydrobiologia 579: 1–13.
Vandekerkhove, J., S. Declerck, L. Brendonck, J. M. Conde-Porcuna, E. Jeppesen & L. De Meester, 2005. Hatching of cladoceran resting eggs: temperature and photoperiod. Freshwater Biology 50: 96–104.
Wiggins, G. B., R. J. Mackay & I. M. Smith, 1980. Evolutionary and ecological strategies of animals in temporary ponds. Archiv Fuer Hydrobiologie Supplement 58: 97–206.
Wolf, H. G. & G. R. Carvalho, 1989. Resting eggs of lake-Daphnia II. In situ observations on the hatching of eggs and their contribution to population and community structure. Freshwater Biology 22: 471–478.
Acknowledgments
We gratefully acknowledge Dr Terry Hillman and Dr Phil Suter for their advice during the design of this study, as well as Dr Mark Fraser, Dr Susanne Watkins, Garth Watson and Sarah Ning for their constructive comments on the manuscript. This study was supported by an Australian Postgraduate Award scholarship and a CSIRO top-up scholarship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: S.M. Thomaz
Rights and permissions
About this article
Cite this article
Ning, N.S.P., Nielsen, D.L. Community structure and composition of microfaunal egg bank assemblages in riverine and floodplain sediments. Hydrobiologia 661, 211–221 (2011). https://doi.org/10.1007/s10750-010-0525-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-010-0525-z