Abstract
Floodplains show a high biodiversity due to their spatial heterogeneity and temporal variability, which are governed by environmental dynamics resulting from the flood pulse. We evaluated the importance of this driving force, the flood pulse, in the structuring of environmental gradients that influence species diversity in a neotropical floodplain. Gamma (γ) and alpha (α) zooplankton diversities were higher in the year with a typical flood pulse (2010), indicating that flood dynamics contributed to high diversity component values. We found significant relationships between α- and β-diversity and local environmental gradients, indicating that in years with a flood pulse, environmental filters might be the dominant mechanisms that structure the zooplankton community. Additive partitioning of γ-diversity showed that even in 2000 with atypical flood conditions, zooplankton diversities showed non-random patterns of spatial distribution and temporal variation in the floodplain. Our results indicate that the driving force of a floodplain can determine the spatial distribution of α- and β-diversity of aquatic communities owing to its primary effect on environmental filters. Therefore, if human activities that influence this driving force, such as water regulation, affect those environmental filters, floodplain biodiversity may decline.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A central aim of ecology is to understand patterns and mechanisms of the spatial distribution of species diversity at local, regional and global scales (Andrewartha and Birch 1954; Ricklefs 1987; Cornell and Lawton 1992; Gaston 2000; Scheiner and Willig 2011). Among the theories proposed to explain patterns of species diversity, the niche theory (Hutchinson 1957) stands out as the basis for many causal mechanisms, because it includes a complex set of abiotic and biotic interactions that define the ranges of species (Chase 2011), on the underlying assumption that species differ ecologically, and their spatial variation is a consequence of their responses to environmental gradients (Siepielski and McPeek 2013). Under these circumstances, habitat heterogeneity increases species diversity due to a higher availability of niches. Several studies have noted the positive association between habitat heterogeneity and species diversity in terrestrial (MacArthur and MacArthur 1961; Pianka 1966; Cramer and Willig 2002) and aquatic (Palmer et al. 1997; Maia-Barbosa et al. 2008) environments. Temporal variation in the environment, however, is also important in explaining patterns in species diversity. In addition, recent human-induced processes of environmental change have raised concerns about the future of biodiversity (Smith and May 1993; Pimm et al. 1995; Luck and Daily 2003; Sax 2003), especially if this change affects the natural patterns of environmental variation (Palmer et al. 1997; Simões et al. 2013).
An important step towards unravelling the drivers of regional diversity is, therefore, to describe the importance of spatial and temporal variation (Tylianakis and Klein 2005). Firstly, regional diversity (γ-diversity, which is the total diversity in a given region) can be split into local diversity (α-diversity, corresponding to the number of species belonging to a local community) and turnover (β-diversity, resulting from the difference in species richness between localities) (Whittaker 1972). Thus, temporal variation in diversity can be tracked on multiple levels. This two-step process provides a deeper comprehension of patterns and mechanisms that govern diversity, particularly if local and regional scales interact over time to influence diversity.
For several reasons, floodplains can be considered model systems for understanding the relationships between local and regional diversity (Cottenie and De Meester 2005). The first reason is that floodplains have a high level of diversity, which is frequently attributed to spatial heterogeneity and temporal variability that interact with the driving force, i.e., the flood pulse (Junk et al. 1989; Opperman and Luster 2010). Secondly, the spatial distribution of aquatic biotopes within floodplains can also be viewed as a hierarchical unit (nested groups) that follow a marked seasonal variability (Tockner et al. 1999; Thomaz et al. 2007). Thirdly, environmental fluctuation resulting from the flood pulse occurs as a natural process that governs the temporal dynamics of the populations (Junk et al. 1989; Neiff 1990), and is affected by human activity, e.g., the regulation of water flow (Tockner et al. 2002; Agostinho et al. 2004). Thus, biotic and abiotic components are constantly changing both on spatial and temporal scales (Junk et al. 1989; Neiff 1990), making relevant the investigation of how space, time, and environmental change affect diversity.
The present study examined the importance of the flood pulse on environmental gradients that influence the zooplankton diversity in a neotropical floodplain. To this end, the following hypotheses were formulated: (H1) floodplains governed by the flood pulse demonstrate higher α-, β- and γ-diversity in years when a flood pulse occurs, by promoting spatial and temporal variability of environmental filters. In this way, propositions supported by the niche theory can explain H1, leading to the second hypothesis: (H2) environmental gradients influence α- and β-diversity when a typical flood pulse occurs. Finally, we tested the hypothesis (H3) that the relative importance of α- and β-diversity in determining γ-diversity also differs between years with and without typical flood pulses because of the presence of the flood pulse within the system.
Materials and methods
Study area
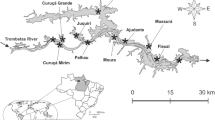
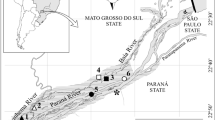
The Upper Paraná River floodplain (22°40′–22°50′S and 53°10′–53°24′W) (Fig. 1) is located in the La Plata River basin (South America). It occupies an area of about 802,150 km2 in Brazilian territory and comprises several aquatic, transitional and terrestrial environments. This floodplain can be divided into three subsystems (Paraná, Baía and Ivinhema), each with a different geology, hydrology and limnology (Roberto et al. 2009; Souza Filho 2009). Recent studies have highlighted the high biodiversity of this region (Agostinho et al. 2004); for example, the zooplankton community (testate amoebae, rotifers and crustaceans) accounts for 541 species (Lansac-Tôha et al. 2009).
Flood pulse as a driving force in the system
On the Upper Paraná River floodplain, the flood pulse shows a typical seasonal dynamic, with floods that usually occur between December and March. However, atypical variations occur because of longer periods of drought or flood, which are influenced by La Niña and El Niño events, respectively. The study years (2000 and 2010) showed different flood dynamics (Fig. 2), indicating that the year 2000 was an atypical dry period in which flood pulse attributes, such as the intensity and amplitude of inundation and connectivity index, were lower than in 2010 (Neiff 1995) (Fig. 2).
Comparison of the flood dynamics in the floodplain of the Upper Paraná River between 2000 and 2010. The reference water level (measured at the Nupélia Advanced Research Base) used to indicate the threshold between potamophase and limnophase was the level at which river water overflowed into the marginal isolated ponds, i.e., 3.5 m
Sampling design for zooplankton and environmental variables
The zooplankton was sampled in two periods, separated by 10 years: 2000 and 2010. In each of these years, samples were obtained every 3 months (in March, June, September, and December) in 12 environments (rivers, secondary channels, backwaters, tributaries, and temporary and permanent lakes) in each of the subsystems, totaling 36 sites (Fig. 1, hierarchical design).
Samples were always obtained in the morning in the limnetic region at a depth of 0.5–1.5 m. Sampling was performed from a moving boat and using a motorised pump and plankton net (68 μm) to filter 600 L of water per sample (standardised sampling effort). Samples were preserved in formaldehyde solution (4 %) buffered with calcium carbonate. Species of testate amoebae, rotifer, cladocera, and copepods were identified to the lowest taxonomic level possible using specific literature (for further details see Lansac-Tôha et al. 2009), and a Sedgewick-Rafter chamber under an optical microscope. The 68 μm mesh can underestimate rotifer (Chick et al. 2010) and testate amoebae abundance but this bias was the same for all samples, leading to a systematic error which may have underestimated the diversity of rotifers and testate amoebae. An accumulation curve (Magurran 2004) was used to determine the diversity of samples. In principle, if stabilisation of the curve is achieved, then additional sampling effort is unnecessary.
Several abiotic measurements and water samples for subsequent laboratory analysis were taken at the same time as the zooplankton samples. The measured parameters were as follows: depth (m), transparency (m; Secchi disc), dissolved oxygen (mg L−1; YSI portable oximeter), temperature (°C; thermometer coupled to the oximeter), pH (Digimed portable potentiometer), electric conductivity (μS cm−1; Digimed portable potentiometer), turbidity (NTU; LaMotte2008© portable turbidimeter), total suspended matter (μg L−1), alkalinity (μEq L−1), and chlorophyll a (μg L−1), total nitrogen (μg L−1), nitrate (μg L−1), ammonium ion (μg L−1), total phosphorus (μg L−1) and phosphate (μg L−1) concentrations. Details of the methods employed for determining limnological variables can be found in Roberto et al. (2009).
Data analysis
To test H1, we first plotted a species accumulation curve to identify the rate of species increase and infer γ-diversity. We then tested for differences in α-diversity between years, months within each year, and between systems within each month using a nested analysis of variance (Sokal and Rohlf 2011). Tukey’s test was performed to analyse significant differences among the nested analyses.
Subsequently, also in relation to H1, temporal and spatial variation in β-diversity was evaluated by mean dissimilarity (Jaccard index) as a measure of overall β-diversity (Anderson et al. 2006). As this procedure is not adapted to incorporate the hierarchical design, we tested the significance using permutations and applied a Bonferroni correction to the multiple comparisons performed between months within years, and between systems within months.
The effect of local environmental gradients (limnological variables), regardless of spatial autocorrelation on α and β-diversity (H2), was tested with multiple regression analyses and partial redundancy analysis. For multiple regression analysis, the response variable was the α-diversity, and the independent variables were: local environmental gradients, which were summarised by orthogonal axes from a principal component analysis selected according to the Broken-Stick criterion (Jackson 1993); and spatial structure, which was quantified by creating spatial filters based on eigenvector maps (Borcard and Legendre 2002; Griffith and Peres-Neto 2006), to exclude spatial autocorrelation (more details in Supplementary material 1). For each month, we assessed the effects of the environmental gradients and spatial filters on α-diversity by running a multiple regression using a forward elimination (Zar 2010). The assumptions were checked by visual inspection of the residuals.
The relative importance of environmental gradients on β-diversity, regardless of the spatial structure, was investigated using a partial redundancy analysis (RDA; Legendre and Legendre 1998; Legendre et al. 2005). Results were based on adjusted R 2 values, since these are independent of the sample size and number of predictor variables, and also allow for comparisons between the results (Peres-Neto et al. 2006). The significance of the RDA (P < 0.05) was tested by 999 randomisations (Legendre et al. 2011). For the RDA, species composition was transformed using the method of Hellinger (Legendre and Gallagher 2001) prior to analyses. Multicollinearity was explored by computing the variance inflation factors of the variables (VIF), which measure the proportion by which the variance of a regression coefficient is inflated in the presence of other explanatory variables. Environmental variables with a VIF higher than 5 were removed from analyses.
To verify the relative importance of α and β-diversity on spatial and temporal scales in determining the γ-diversity of each year (H3), we performed an additive partitioning of diversity on the hierarchical design (Fig. 1). The first partition level was comprised of the species diversity of each location (α); the second, of the difference in species diversity between locations (β1); the third, of the difference in species diversity between months (β2); and the fourth, of the difference in species diversity between systems (β3). The additive partitioning of diversity is a way of integrating analyses, since it considers the contribution of different levels and sub-levels of diversity, α and β, to the γ-diversity of a given region of interest (Lande 1996). By knowing the relative contribution of each hierarchical partition of diversity to the total diversity (γ), the environmental sampling effort can be minimised, thus assisting in decision-making regarding the prioritisation of areas for conservation (Gering et al. 2003; Ligeiro et al. 2010). There is some controversy regarding the additive or multiplicative partitioning of diversity, but both methods are useful and have proven effective for studies on species diversity (Veech and Crist 2010). We chose the additive partitioning of diversity because the contribution of each component can be represented using the same unit.
All statistical analyses were run using the software R 2.14.1 (R Core Team 2011).
Results
A total of 365 species were identified in the floodplain (see list of species in the Supplementary material 2). In 2000, the γ-diversity was 245 species, and in 2010, 305 species. The species accumulation curve indicated a greater number of species in 2010, and from the seventh location sampled, the γ-diversity in 2010 showed a significant difference from the year 2000 (Fig. 3a).
Mean α-diversity differed between years, months, and systems (Table 1S, Supplementary material 3). The mean α-diversity was higher in 2010 (nested ANOVA, F 2,8 = 66.7, P < 0.001; Fig. 3b, c), when it ranged from 18 to 85 species, whereas in 2000, it ranged from 16 to 73 species. The monthly variation in α-diversity was evident and significant (Tukey’s HSD test < 0.05) only in 2010, with higher values in March and December, except for the Baía system (Fig. 3c). Similar to monthly variation, the α-diversity differed between the systems only in 2010 (nested ANOVA, F 24,258 = 7.8, P < 0.001; Fig. 3c), with greater values in the Baía system and lower values in the Paraná system (Tukey’s HSD test < 0.05).
β-diversity (spatial variation in species composition) did not differ between years (Pseudo-F = 1.76, P = 0.176; Fig. 4a) or between months within each year (2000: Pseudo-F = 0.89, P = 0.448; 2010: Pseudo-F = 1.61, P = 0.177; Fig. 4b). However, β-diversity differed between systems (Fig. 4d) during all months of 2010, with lower spatial variability in the Baía system and higher spatial variability in the Paraná system.
The α-diversity was related to local environmental conditions in 2010 (Table 1). From those local environment gradients selected by multiple regressions, the variables that were consistently important throughout 2010 were transparency, total alkalinity, nitrate, total phosphorus, soluble reactive phosphorus and chlorophyll a concentration (Table 2S, Supplementary material 3).
β-diversity (spatial turnover) was also more strongly correlated with local environmental conditions (environmental gradients) in 2010 than in 2000 (Table 1). All results of the variance partitioning in 2010 showed that β-diversities associated with environmental gradients in a non-random way, even though adjusted R 2 values were lower than 0.1 (Table 1).
Results of the additive partitioning of diversity showed that all components of diversity (α, β1, β2 and β3) were significant in explaining γ-diversity. Thus, the spatial distribution and temporal variation in diversity in the floodplain in both years showed a very low probability of occurring at random. This indicates that the hierarchical levels selected were able to contribute significantly towards explaining γ-diversity. The component most significant in explaining γ-diversity was β1, which represented the variation between locations. This was followed by the components β3 and β2, i.e., variation among systems and months, respectively (Fig. 5). The proportion of each component responsible for explaining γ-diversity was similar across years, for instance, β1 explained 53.4 and 54.1 % of γ-diversity 2000 and 2010, respectively. In this way, even under different hydrological situations, the spatial and temporal variation in beta-diversity significantly contributed to the pattern of non-random γ-diversity in the floodplain.
Discussion
Floodplains governed by flood pulses have higher α-, β- and γ-diversity when such forces are present (Hypothesis 1).
The temporal and spatial variation in α-diversity in the year with a typical flood pulse (2010), together with the difference in β-diversity between the systems in this year, support the hypothesis that γ-diversity increases when the flood pulse is present as a driving force in the system. This is due to the interaction between spatial heterogeneity and temporal variability mediated by a typical flood pulse (Ward and Tockner 2001). The increase in γ-diversity stemming from greater α and β-diversity was an expected result (Whittaker 1972; Kraft et al. 2011), but understanding the mechanisms that determine the variations in α- and β-diversity shows how local and/or regional factors contribute to maintaining γ-diversity (Ricklefs 1987). Our results suggest that environmental gradients (when spatial structure was controlled in the analysis), in the year with a typical flood pulse (2010), determined the number of species at the sites and increased the spatial turnover (H2, discussed below). Nevertheless, spatial structure was also occasionally important in α- and β-diversity, indicating spatial links among sites (Table 1). When inundations occurred, they increased the connectivity among biotopes (Agostinho et al. 2004; Thomaz et al. 2007), favouring the exchange of propagules and mediating the dispersion of species (Medley and Havel 2007; Simões et al. 2012). This increase in dispersal capability is expected to increase diversity at local scales (May et al. 2011). Nevertheless, in the year with atypical flood conditions, we suggest that contraction of the aquatic environment and slow current flow limited the dispersal of species across floodplain habitats. While the flood pulse increases the abiotic and biotic similarity in floodplains during inundation phase (Thomaz et al. 2007), different environmental and spatial gradients are formed over time in these systems (see Figure 3 in Thomaz et al. 2007) owing to alternation between seasonally high water (high connectivity, high homogeneity) and low water phases (low connectivity, high heterogeneity).
Thus, the flood pulse ensures the functional trade-off between the inundation phase, when water bodies are connected, and the dry phase, when they are isolated, and contributes to spatial and temporal variability (Neiff 1990). Moreover, according to Gonzalez (2009), the temporal dynamics of abiotic conditions might modify the effect of environmental gradients and the pathways for species dispersal, thereby affecting the patterns of α- and β-diversity.
We suggested that the lower diversity in 2000 was due the absence of a flood pulse, which can result in a stress situation that undermines local biodiversity because the absence of this complex form of disturbance (Rolls et al. 2012; Simões et al. 2013) is unusual within the context of historical patterns and frequencies of natural variability (Hobbs and Huenneke 1992). In floodplains, floods provide disturbances that can offset equilibrium conditions between local and regional processes (Ward and Tockner 2001; Cottenie and De Meester 2005), because floods simultaneously influence abiotic conditions and provide new species from the regional pool. The result of these processes is the maximisation of diversity (given the higher variability of niches), over both time and space (Ward et al. 1999).
Environmental gradients affect α- and β-diversity when flood pulses are present in the system (Hypothesis 2).
Different effects of environmental gradients on the components of α- and β-diversity were observed in the studied years. In 2010, when there was typical flood conditions, the spatial variation in α- and β-diversity was significantly explained by local environmental factors. As predicted by the niche theory (Hutchinson 1957), environmental filters can be the dominant mechanisms structuring aquatic diversity (Schei et al. 2012) in years with floods, as the species experience a range of environmental conditions which modify the extent to which habitat patches are truly discrete (Pedruski and Arnott 2011). In this way, abiotic conditions restrict the number of species (α-diversity) by influencing their spatial distribution (β-diversity). As predicted, the environmental gradient produced a distinct community composition. This demonstrates that β-diversity was related to environmental heterogeneity (Stendera and Johnson 2005; Declerck et al. 2011), which contributed to the increased γ-diversity in 2010. These results corroborated the hypothesis that under typical flood pulses, environmental gradients produced by spatial variability of abiotic conditions are drivers of α- and β-diversity and support the highest level of γ-diversity. In floodplains, local abiotic conditions are the first component to be altered by a flood, and can limit or favour some species (Junk et al. 1989; Neiff 1990; Thomaz et al. 2007). However, abiotic conditions can be spatially structured, making the partition of the main causal mechanisms of diversity more difficult (Legendre 1993). Our RDA showed that abiotic conditions were also spatially structured (data not shown), but our analyses removed these effects.
Conversely, in the year with atypical flood conditions, diversities were not associated with abiotic conditions. This result indicated that local processes independent of abiotic conditions, such as predation, competitive exclusion, or stochastic variation, might have been more important in defining the α-diversity of the community through the local elimination of species (Ricklefs 1987). In years with atypical flood conditions, the reduction in the physical space increases the density of individuals, both competitors and predators and intensifies biotic interactions (Ward et al. 1999; Thomaz et al. 2007; Bonecker et al. 2011; Rolls et al. 2012; Simões et al. 2012). Therefore, it is probable that the smaller influence of local abiotic conditions and regional processes (dispersal, in this case) that influence the diversity, will favour the competitively superior species to the exclusion of less-competitive species (Gonzalez 2009). Unfortunately, no data are currently available with which to test the effect of biotic interactions on α- and β-diversity of zooplankton in our study region. Furthermore, in theory and as predicted by the intermediate disturbance hypothesis (Connell 1978), floods as disturbances might favour species diversity because they reduce the effect of competitive interactions and permit the coexistence of a higher number of species. Experimental studies that aim to investigate this proposed effect of floods would constitute a great advance in ecological models of spatial distribution of the α- and β-diversity of aquatic communities.
The relative importance of α- and β-diversity in determining γ-diversity is different between study years (Hypothesis 3).
The additive partitioning of diversity showed that a similar proportion of α- and β-diversity contributed to γ-diversity in 2000 and 2010. This observation was contrary to our expectation that the relative importance of spatial distribution and temporal variation in α- and β-diversity in determining the γ-diversity would differ between the years in response to the driving force, the flood pulse (absent in 2000 and present in 2010). Although we rejected our hypothesis, these results have an important implication for the ecology of floodplains because they are indicative of the buffering capacity of river-floodplain systems, i.e., their ability to maintain nonrandom patterns of spatial distribution and temporal variation in diversity.
A probable explanation for the occurrence of a similar diversity pattern in years with opposite hydrological characteristics (atypical and typical flood conditions) is that even though the flooded year showed a higher β-diversity among systems, the contribution of each component to the γ-diversity can be maintained as a function of local environmental restrictions. A further explanation might be ecological memory, defined as the ability of past states to shape present or future responses of the community due to the regional pool of species (Padisák 1992). Ecological memory can be represented by the pool of species present in the sediment, which becomes viable when environmental conditions are favourable (Hairston et al. 1995). For example, egg bank communities provide a source of microfaunal diversity within river-floodplain systems (Ning and Nielsen 2011). In the Upper Paraná River floodplain, Palazzo et al. (2008) found resting eggs of species which are rare in the plankton, which hatched under experimental conditions.
Considering the natural temporal changes of biota in floodplain systems (Neiff 1990), a greater contribution of β2-diversity (monthly variation in diversity) to γ-diversity was expected in 2010 (the year with typical flood conditions) than in 2000, because the temporal dynamics of floods affect several features of the zooplankton communities in floodplains (Medley and Havel 2007; Bonecker et al. 2011; Simões et al. 2012) and should increase the temporal turnover of communities (Melo et al. 2011). Understanding how diversity changes over time, however, remains a challenge (Korhonen et al. 2010; Magurran and Henderson 2010). We also expected a greater relative contribution of the spatial β1 and β3-diversity components to γ-diversity in 2000, because atypical flood conditions should increase the effect of local processes and lead to variation in the spatial distribution of communities (Ward et al. 1999; Thomaz et al. 2007). These findings indicate that spatial distribution of zooplankton in the upper Paraná River floodplain depends on non-random patterns.
The greatest contribution to γ-diversity was provided by β1-diversity, indicating that species turnover among sites was the strongest source of variation in the γ-diversity of zooplankton. Thus, conservation strategies should include several habitats to maintain the natural variability of the ecosystem (Stendera and Johnson 2005). However, because all hierarchical levels of diversity were significant in explaining regional diversity in our study, we recommend that conservation efforts also prioritise the maintenance of the floodplain’s natural temporal variation in hydrology. River regulation, overexploitation of natural resources, water pollution, habitat degradation and species invasion are threats to biodiversity because they favour biotic homogenisation (Tockner et al. 2002; Rahel 2002; Agostinho et al. 2004; Dudgeon et al. 2006) to the detriment of the main feature of the floodplains, i.e. their natural variability.
Although experts have different opinions as to the effects of natural disturbances on biodiversity (Lepori and Hjerdt 2006), many agree on the positive influence of floods on the biodiversity of floodplains, due to the maximisation of spatial and temporal heterogeneity (Junk et al. 1989; Neiff 1990; Tockner et al. 1999; Agostinho et al. 2004). The results of the additive partitioning of diversity in this study support this statement, since the contributions of α- and β-diversity to the γ-diversity were greater than those expected at random, regardless of the year (atypical or typical flood conditions). Thus, non-random ecological processes influence spatial and temporal patterns of diversity (Gering et al. 2003; Sasaki et al. 2012). However, we propose that the influence of environmental gradients on spatial variation in α- and β-diversity only occurred when the driving force (the flood pulse) did not limit the dispersion of species.
Our study was conducted in one typical and one atypical year of a neotropical floodplain (the Upper Parana River Floodplain) and the results indicated that the driving force of a floodplain can determine the spatial distribution of α- and β-diversity of zooplankton communities through its primary influence on environmental filters and its indirect influence on γ-diversity patterns. Thus, to conserve the flood dynamics is an important conservation strategy because floods increase temporal variability and spatial heterogeneity, thereby increasing γ-diversity. According to Stendera and Johnson (2005), it is not only important to identify specific patterns of species diversity, but also to improve our understanding of the underlying processes that generate the patterns. In our study area, this implies that water regulation by upstream reservoirs should be managed in such a way that maintains the natural functioning of the floodplain environment, thereby mitigating environmental impacts in floodplains that can adversely affect their natural buffering capacity.
References
Agostinho AA, Thomaz SM, Gomes LC (2004) Threats for biodiversity in the floodplain of the Upper Paraná River: effects of hydrological regulation by dams. Int J Ecohydrol Hydrobiol 4:255–268
Anderson MJ, Ellingsen KE, McArdle BH (2006) Multivariate dispersion as a measure of beta diversity. Ecol Let 9:683–693
Andrewartha HG, Birch LC (1954) The distribution and abundance of animals. University of Chicago Press, Chicago
Bonecker CC, Azevedo F, Simões NR (2011) Zooplankton body-size structure and biomass in tropical floodplain lakes: relationship with planktivorous fishes. Acta Limnol Bras 23:217–228
Borcard D, Legendre P (2002) All-scale spatial analysis of ecological data by means of principal coordinates of neighbour matrices. Ecol Model 153:51–68
Chase JM (2011) Ecological niche theory. In: Scheiner S, Willing M (eds) The theory of ecology. The University of Chicago Press, Chicago, pp 93–107
Chick J, Levchuk A, Medley K, Havel JH (2010) Underestimation of rotifer abundance a much greater problem than previously appreciated. Limnol Oceanogr Method 8:79–87
Connell J (1978) Diversity in tropical rain forests and coral reefs. Science 199:1302–1310
Cornell H, Lawton JH (1992) Species interactions, local and regional processes, and limits to the richness of ecological communities: a theoretical perspective. J Anim Ecol 61:1–12
Cottenie K, De Meester L (2005) Local interaction and local dispersion in a zooplankton metacommunity. In: Holyoak M, Leibold MA, Holt RD (eds) Metacommunities: spatial dynamics and ecological communities. The University of Chicago Press, pp 189–211
Cramer M, Willig M (2002) Habitat heterogeneity, habitat associations, and rodent species diversity in a sand-shinnery-oak landscape. J Mammal 83:743–753
Declerck SAJ, Coronel JS, Legendre P, Brendonck L (2011) Scale dependency of processes structuring metacommunities of cladocerans in temporary pools of High-Andes wetlands. Ecography 34:296–305
Dudgeon D, Arthington AH, Gessner MO, Kawabata Z-I, Knowler DJ, Lévêque C, Naiman RJ, Prieur-Richard A-H, Soto D, Stiassny MLJ, Sullivan CA (2006) Freshwater biodiversity: importance, threats, status and conservation challenges. Biol Rev 81:163–182
Gaston KJ (2000) Global patterns in biodiversity. Nature 405:220–227
Gering JONC, Crist TO, Veech JA (2003) Additive partitioning of species diversity across multiple spatial scales : implications for regional conservation of biodiversity. Conserv Biol 17:488–499
Gonzalez A (2009) Metacommunities: spatial community ecology. In: Encyclopedia of life sciences. Wiley, Chichester
Griffith D, Peres-Neto P (2006) Spatial modeling in ecology: the flexibility of eigenfunction spatial analyses. Ecology 87:2603–2613
Hairston N Jr, Van Brunt R, Kearns C (1995) Age and survivorship of diapausing eggs in a sediment egg bank. Ecology 76:1706–1711
Hobbs RJ, Huenneke LF (1992) Disturbance, diversity, and invasion: implications for conservation. Conserv Biol 6:324–337
Hutchinson GE (1957) Concluding remarks. Population studies. Anim Ecol Demogr 22:415–427
Jackson D (1993) Stopping rules in principal components analysis: a comparison of heuristical and statistical approaches. Ecology 74:2204–2214
Junk WJ, Bayley PB, Sparks RE (1989) The flood pulse concept in river-floodplain systems. Can Fish Aquat Sci 106:110–127
Korhonen JJ, Soininen J, Hillebrand H (2010) A quantitative analysis of temporal turnover in aquatic species assemblages across ecosystems. Ecology 91:508–517
Kraft NJB, Comita LS, Chase JM, Sanders NJ, Swenson NG, Crist TO, Stegen JC, Vellend M, Boyle B, Anderson MJ, Cornell HV, Davies KF, Freestone AL, Inouye BD, Harrison SP, Myers JA (2011) Disentangling the drivers of β diversity along latitudinal and elevational gradients. Science (New York, NY) 333:1755–1758
Lande R (1996) Statistics and partitioning of species diversity, and similarity among multiple communities. Oikos 76:5–13
Lansac-Tôha FA, Bonecker CC, Velho LFM, Simões NR, Dias JD, Alves GM, Takahashi EM (2009) Biodiversity of zooplankton communities in the Upper Paraná River floodplain: interannual variation from long-term studies. Braz J Biol 69:539–549
Legendre P (1993) Spatial autocorrelation: trouble or new paradigm? Ecology 74:1659–1673
Legendre P, Gallagher E (2001) Ecologically meaningful transformations for ordination of species data. Oecologia 129:271–280
Legendre P, Legendre L (1998) Numerical ecology. Elsevier Science Ltd, Amsterdam
Legendre P, Borcard D, Peres-Neto P (2005) Analyzing beta diversity: partitioning the spatial variation of community composition data. Ecol Monogr 75:435–450
Legendre P, Oksanen J, Ter Braak CJF (2011) Testing the significance of canonical axes in redundancy analysis. Methods Ecol Evol 2:269–277
Lepori F, Hjerdt N (2006) Disturbance and aquatic biodiversity: reconciling contrasting views. Bioscience 56:809–818
Ligeiro R, Melo AS, Callisto M (2010) Spatial scale and the diversity of macroinvertebrates in a Neotropical catchment. Freshw Biol 55:424–435
Luck G, Daily G (2003) Population diversity and ecosystem services. Tree 18:331–336
MacArthur R, MacArthur J (1961) On bird species diversity. Ecology 42:594–598
Magurran AE (2004) Measuring biological diversity. Blackwell Science, USA
Magurran AE, Henderson PA (2010) Temporal turnover and the maintenance of diversity in ecological assemblages. Biol Sci 365:3611–3620
Maia-Barbosa PM, Peixoto RS, Guimarães A (2008) Zooplankton in littoral waters of a tropical lake: a revisited biodiversity. Braz J Biol 68:1069–1078
May F, Giladi I, Ziv Y (2011) Dispersal and diversity—unifying scale-dependent relationships within the neutral theory. Oikos 121:942–951
Medley K, Havel J (2007) Hydrology and local environmental factors influencing zooplankton communities in floodplain ponds. Wetlands 27:864–872
Melo AS, Schneck F, Hepp LU, Simões NR, Siqueira T, Bini LM (2011) Focusing on variation: methods and applications of the concept of beta diversity in aquatic ecosystems. Acta Limnol Bras 23:318–331
Neiff J (1990) Ideas para la interpretación ecológica del Paraná. Interciencia 15:424–441
Neiff J (1995) Large rivers of South America: toward the new approach. Verh Intern Ver Limnol 26:167–180
Ning N, Nielsen DL (2011) Community structure and composition of microfaunal egg bank assemblages in riverine and floodplain sediments. Hydrobiologia 661:211–221
Opperman J, Luster R (2010) Ecologically functional floodplains: connectivity, flow regime, and scale. J Am Water Res Assoc 46:211–226
Padisák J (1992) Seasonal succession of phytoplankton in a large shallow lake (Balaton, Hungary): a dynamic approach to ecological memory, Its possible role and mechanisms. J Ecol 80:217–230
Palazzo F, Bonecker CC, Fernandes APC (2008) Resting cladoceran eggs and their contribuition to zooplankton diversity in a lagoon of the upper Paraná River floodplain. Lakes Res 13:207–214
Palmer MA, Hakenkamp CC, Nelson-Baker K (1997) Ecological heterogeneity in streams: why variance matters. J N Am Benthol Soc 16:189–202
Pedruski MT, Arnott SE (2011) The effects of habitat connectivity and regional heterogeneity on artificial pond metacommunities. Oecologia 166:221–228
Peres-Neto P, Legendre P, Dray S, Borcard D (2006) Variation partitioning of species data matrices: estimation and comparison of fractions. Ecology 87:2614–2625
Pianka E (1966) Latitudinal gradients in species diversity: a review of concepts. Am Nat 100:33–46
Pimm S, Russell G, Gittleman J (1995) The future of biodiversity. Science 21:347–350
R Core Team (2011). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org/
Rahel FJ (2002) Homogenization of Freshwater Faunas. Ann Rev Ecol Syst 33:291–315
Ricklefs RE (1987) Community diversity: relative roles of local and regional processes. Science 235:167–171
Roberto MC, Santana NF, Thomaz SM (2009) Limnology in the Upper Paraná River floodplain: large-scale spatial and temporal patterns, and the influence of reservoirs. Braz J Biol 69:717–725
Rolls RJ, Leigh C, Sheldon F (2012) Mechanistic effects of low-flow hydrology on riverine ecosystems: ecological principles and consequences of alteration. Freshw Sci 31(4):1163–1186
Sasaki T, Katabuchi M, Kamiyama C, Shimazaki M, Nakashizuka T, Hikosaka K (2012) Diversity partitioning of moorland plant communities across hierarchical spatial scales. Biodivers Conserv 21:1577–1588
Sax D (2003) Species diversity: from global decreases to local increases. Tree 18:561–566
Schei FH, Blom HH, Gjerde I, Grytnes J-A, Heegaard E, Saetersdal M (2012) Fine-scale distribution and abundance of epiphytic lichens: environmental filtering or local dispersal dynamics? J Veg Sci 23:459–470
Scheiner S, Willig M (2011) A general theory of ecology. In: Scheiner S, Willig M (eds) The theory of ecology. The University of Chicago Press, Chicago, pp 3–18
Siepielski A, McPeek M (2013) Niche versus neutrality in structuring the beta diversity of damselfly assemblages. Freshw Biol 58:758–768
Simões NR, Lansac-Tôha FA, Velho LFM, Bonecker CC (2012) Intra and inter-annual structure of zooplankton communities in floodplain lakes: a long-term ecological research study. Rev Biol Trop 60:1819–1836
Simões NR, Lansac-Tôha FA, Bonecker CC (2013) Drought disturbances increase temporal variability of zooplankton community structure in floodplains. Int Rev Hydrobiol 98:24–33
Smith F, May R (1993) Estimating extinction rates. Nature 364:494–496
Sokal R, Rohlf F (2011) Biometry: the principles and practice of statistics in biological research, 4th edn. W.H. Freeman & Company, New York
Souza Filho E (2009) Evaluation of the Upper Paraná River discharge controlled by reservoirs. Braz J Biol 69:707–716
Stendera S, Johnson R (2005) Additive partitioning of aquatic invertebrate species diversity across multiple spatial scales. Freshw Biol 50:1360–1375
Thomaz SM, Bini LM, Bozelli RL (2007) Floods increase similarity among aquatic habitats in river-floodplain systems. Hydrobiologia 579:1–13
Tockner K, Pennetzodorfer D, Reiner N, Schiemer F, Ward JV (1999) Hydrological connectivity, and the exchange of organic matter and nutrients in a dynamic river-floodplain system (Danube, Austria). Freshw Biol 41:521–535
Tockner K, Ward J, Stanford JA, Schiemer F (2002) Riverine flood plains: present state and future trends. Environ Conserv 29:308–330
Tylianakis J, Klein A (2005) Spatiotemporal variation in the diversity of Hymenoptera across a tropical habitat gradient. Ecology 86:3296–3302
Veech JA, Crist TO (2010) Diversity partitioning without statistical independence of alpha and beta. Ecology 91:1964–1969
Ward J, Tockner K (2001) Biodiversity: towards a unifying theme for river ecology. Freshw Biol 46:807–820
Ward J, Tockner K, Schiemer F (1999) Biodiversity of floodplain river ecosystems: ecotones and connectivity. River Res Appl 15:125–139
Whittaker RH (1972) Evolution and measurement of species diversity. Taxon 21:213–251
Zar JH (2010) Biostatistical analysis. Prentice-Hall, New Jersey
Acknowledgments
The authors thank the Nupélia and the Graduate Program in Continental Aquatic Environments for providing logistical support; PROEX/Capes; PELD (site 6)/CNPq for providing financial support, and CNPq for providing doctoral, post-doctoral, and Research Productivity scholarships. NRS is grateful to CNPq for providing a postdoctoral scholarship. We thank the anonymous reviewers and Catherine Leigh who substantially contributed to the improvement of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Simões, N.R., Dias, J.D., Leal, C.M. et al. Floods control the influence of environmental gradients on the diversity of zooplankton communities in a neotropical floodplain. Aquat Sci 75, 607–617 (2013). https://doi.org/10.1007/s00027-013-0304-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00027-013-0304-9