Abstract
The dynamics of populations of short-lived organisms are very patchy, both in space and time. The production of dormant propagules, however, results in an effective increase in generation time. We hypothesize that prolonged dormancy, together with variable regeneration niches, result in integration of temporal variability in community structure. In addition, in aquatic habitats, mechanisms such as sediment focussing can contribute to the integration of spatial variability. We tested the hypothesis that dormant propagule banks integrate spatial and temporal variation in active zooplankton communities. This was done by comparing cladoceran species richness and the community structure of hatchling assemblages retrieved from propagule bank samples collected on a single occasion with assemblages encountered in active community samples covering spatial variation (littoral and pelagic zone), diel (day and night), intra-year (May–October) and inter-year variation (1996–2000). The egg bank community structure differed significantly from the active community structure, but the dissimilarity decreased as spatial and temporal variation was better covered by the active community samples. Furthermore, the identification of all fully grown hatchlings (n=214) yielded an equally high number of species (n=22) to that occurring in all active community samples together (a total of 1,730 individuals were analysed). We conclude that the analysis of dormant propagules may form a cost-efficient alternative tool to the analysis of active community samples for an integrated assessment of cladoceran communities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biological communities tend to be highly variable in time and space. This is particularly the case for taxa which have a short generation time (Pinnel-Alloul 1995; Baur et al. 1996; Vinson and Hawkins 1998). However, accumulations of dormant stages (e.g. diapausing spores, seeds, eggs and cysts) have the potential to integrate this variation (Hairston 1996). It has been shown that dormant propagule banks (DPBs) may be important in determining the rate of evolutionary processes (Hairston and De Stasio 1988), and may strongly contribute to the long-term coexistence of competing species or genotypes (storage effect hypothesis; Chesson 1983; Ellner and Hairston 1994; Cáceres 1998). In addition to these important ecological and evolutionary implications, DPBs may also provide important opportunities from a methodological point of view. Firstly, DPBs contain an interesting archive of information on the species composition and the recent evolutionary history of the populations (Cousyn et al. 2001; Jeppesen et al. 2001; Brendonck and De Meester 2003) and on the chemical environment and trophic structure (Anderson and Battarbee 1994; Jeppesen et al. 2001). Secondly, DPBs may also be very useful for the characterisation of active communities (ACs), given that they integrate temporal and spatial variation at an ecologically relevant scale. As such, they may provide a more integrated picture of species composition and richness of the community under study than an analysis of snapshot samples of the AC (Brendonck and De Meester 2003). The interpretation of dormant assemblages is, however, not free of problems. Studies on terrestrial plant communities have shown that species may strongly differ in their seed production (Silvertown et al. 2002) and that seed banks typically show high spatial heterogeneity (Geertsema and Sprangers 2002; Wiles and Schweizer 2002). These characteristics may interfere with a straightforward interpretation of the composition of dormant propagule assemblages. In addition, not all plant and animal species can be correctly identified on the basis of propagule morphology. For most taxa, hatching of propagules is required to enable species-level identifications. Species- or even population-specific differences in hatching requirements and hatching success may therefore bias the structure of the hatched community.
Like plants, many freshwater organisms produce long-lived, resistant dormant propagules. The generation time of freshwater zooplankton is generally very short. Their populations tend to be very dynamic and, consequently, the seasonal (Sommer et al. 1986) and inter-annual variation (Arnott et al. 1999; Grover 1999) in the composition of zooplankton communities is often substantial. In addition, zooplankton populations often exhibit diel vertical (De Meester et al. 1999) or horizontal migration (Lauridsen and Lodge 1996; Burks et al. 2002) in response to both biotic (e.g. fish predation pressure) and abiotic (e.g. UV radiation) factors.
Many zooplankton taxa produce dormant propagules in order to cope with the uncertainty of their habitat. Only a fraction of these long-lived diapausing stages hatches in the subsequent growing season. The hatching fraction varies between systems, depending on the predictability of the chance for successful recruitment [bet-hedging strategy: Cohen (1966); Philippi and Seger (1989)]. Sasaki and Ellner (1995) worked out the nature of bet-hedging in plankton with constant hatching probability of dormant eggs. Hairston and Cáceres (1996) empirically showed that, in microcrustaceans, the duration of diapause covaries negatively with the length of the reproductive life stage (but see Ellner et al. 1998).
Although there is also mortality due to senescence, predation and parasitism, as well as dispersal out of the ecosystem (Cáceres and Hairston 1998), the DPB accumulates, and is expected to partially integrate inter-annual variation. Spatial heterogeneity is also believed to be reduced at the level of the habitat by sediment focussing and sediment resuspension by wind and bioturbation (Jeppesen et al. 2001). Even though temporal integration does occur, it is often at a biologically relevant scale, so that the vertical structure of a DPB still reflects the historical changes of a given habitat at a resolution of only a few years (e.g. Weider et al. 1997; Cousyn et al. 2001).
There is some evidence that DPBs contain a relatively high taxon richness compared to samples from the AC in species-poor aquatic habitats (May 1986; Havel et al. 2000; Crispim and Watanabe 2001; Duggan et al. 2002). However, up to now no studies have systematically assessed to what extent aquatic habitat DPBs integrate the spatial and temporal variability of zooplankton communities, and whether this integration is biased in terms of species composition. In the present study, we determine the extent to which DPBs integrate variation in the taxon composition of the cladoceran community of a small shallow pond over time and space. We compare cladoceran species richness and community structure of single-date DPB samples with different sets of samples of the AC, that capture to a variable degree the spatial (pelagic/littoral) and temporal (diel, seasonal and inter-annual) variation in this community.
Materials and methods
Model system
Maten 13 (M13) is a shallow pond (average depth 1 m; surface area 2.9 ha) situated in the nature reserve ‘De Maten’, Genk, Belgium. De Maten comprises a system of 35 neighbouring interconnected ponds that differ strongly from each other with respect to their ecological characteristics and zooplankton species composition (Cottenie et al. 2001). During summer, large parts of M13 are covered by a diverse macrophyte flora (e.g. Potamogeton sp., Polygonum amphibium, Utricularia vulgaris and Chara globularis var. globularis; W. Romme, personal communition). M13 shows all the characteristics of a typical clear-water shallow water body (Jeppesen et al. 1997; Scheffer 1998).
Collection and analysis of samples
On 10 April 2000, the DPB of M13 was sampled using a plexi-glass sediment corer (tube of 140×5.2 cm) at four stations in the pelagic zone and eight stations in the littoral zone. In the littoral zone, four sediment samples were collected from the upwind side and another four from the downwind side of the pond. Wind may affect the horizontal distribution of dormant propagules, as many of them float on the surface for days or weeks after deposition. Only the top 3 cm of each core were retained, so that only eggs deposited in recent years were incorporated. Sedimentation rate data obtained for a shallow pond of the same size as M13 in Denmark indicate that resting eggs buried 3 cm deep are approximately 4 years old (Schroll 2002). We do not expect much variation in dormant egg age between littoral and corresponding pelagic sediment layers as M13 has no steep slopes. Samples were wrapped in aluminium foil and stored at 4°C. Samples were kept separately. After an 8-month resting period, one 50-g subsample was taken from each sediment sample. The dormant propagules in these subsamples were isolated by means of the sugar centrifugation technique developed by Onbé (1978) and modified by Marcus (1990). Isolated dormant eggs were transferred to 12 2-l aquaria (one for each subsample), filled with diluted ADAM medium (conductivity: 200 μs/cm; Kluttgen et al. 1994) and placed in an incubator that simulated summer conditions (20°C; 16 h light per day). Over a period of 36 days, the 12 aquaria were checked every 3 days for hatched individuals. We recovered 106 hatchlings from the downwind littoral samples of the DPB (DPBLIT+; codes are explained in Table 1), 72 from the upwind littoral samples (DPBLIT−) and 36 from the pelagic samples (DPBPEL). The total DPB assemblage, covering hatchlings from all three zones, thus contained 214 individuals (DPB).
During five consecutive years (1996–2000), the AC of M13 was sampled once a year in July at four randomly selected pelagic stations. During the growing season of 2000 (May–October) the zooplankton community was monitored in more detail, involving monthly sampling at eight randomly selected stations (four pelagic and four littoral stations; Table 1). On 26 July 2000, the pelagic zone was sampled during both the day and night at four locations. In addition, on the same date, the littoral zone was sampled at four locations during the day. On all sampling occasions, depth-integrated samples were collected using a quantitative sampling device (1996–1999: 12-l Schindler Patalas trap, samples taken at two depths; 2000: 6-l tube sampler, tube of 140×7.5 cm). Cladocerans were concentrated by filtration over a 64-μm mesh. With the aim of integrating to a varying degree temporal and spatial variation in zooplankton composition and richness, three composite samples were created. One sample (INTRAYEAR) integrated intra-year variation and was created by pooling standardised fractions of the six monthly samples from the year 2000. Another composite sample (INTERYEAR) integrated inter-annual variation and was created by pooling standardised fractions of the July samples from the years 1996–2000. Finally, a composite sample was created by combining standardised subsamples of all samples taken from the AC (INTEGRATED). In these three composite samples, all original samples were represented by fractions corresponding to equal volumes of pond water.
For all samples from the AC (DAYPEL, NIGHTPEL, DAYLIT and composite samples), at least 220 cladoceran individuals were identified. This number exceeded the total number of hatchlings obtained and identified from the DPB samples (214 individuals). DPB hatchlings and AC individuals were identified to species level using Flößner’s (2000) identification key.
Statistical analysis
A one-way ANOVA was applied to test for differences between pond zones (e.g. upwind littoral, downwind littoral and pelagic) with respect to the number of hatched zooplankton individuals (following logarithmic transformation) in the DPB samples. Numbers of hatchlings were logarithmically transformed prior to analysis to obtain a normal distribution (Kolmogorov Smirnov test). The assumption of homogeneity of variances was checked using the Bartlett’s test.
The composition of the hatchling assemblages obtained from samples collected in different zones (DPBLIT−, DPBLIT+ and DPBPEL) was compared at the genus level. This allowed all hatchlings to be included (some hatchlings died before identification to species level could be made) and thus increased the statistical power of the analyses. From the hatchling assemblage data (percentage composition of genera, square-root transformed), Bray-Curtis (BC) similarity coefficients were calculated for all pairwise combinations of sediment samples. The resulting similarity matrix was used to perform a one-way analysis of similarities (ANOSIM), testing for differences in taxonomic composition between hatchling assemblages. Analyses of similarity percentages (SIMPER) detected the genera that contributed most to the observed differences between assemblages.
The community composition of the AC assemblages could not be analysed statistically using ANOSIM as there were no replicate samples available. Instead, we used a loglinear analysis to test for differences in taxonomic composition between the total hatchling assemblage and AC samples. Two assemblages were considered taxonomically different if inclusion of the interaction effect between the factors ‘assemblage’ (two levels: DPB and AC) and ‘genus’ (14 levels) resulted in a significant improvement in fit of the model explaining the number of hatchlings per genus in assemblages. Loglinear analysis was not applied to test for differences in community structure between different sets of AC samples, given the lack of independence between them.
Given that our DPB samples were taken before the AC samples were collected in 2000, our data cannot be used to assess to what extent community composition in the DPB reflects that of the AC of recent years, unless one assumes that the seasonal abundance of zooplankton taxa in 2000 reflects that of previous years.
Species richness was compared between samples and combinations of samples after rarefaction to 200 individuals (H200). Error bars for species accumulation curves were obtained using resampling software (EstimateS: Colwell 1997).
Bray-Curtis similarity index calculations, ANOSIM and SIMPER analyses were done using the computer program Primer 5.2.2 (Clarke and Warwick 1994). Parametric statistics were computed in Statistica 6.0 (Statsoft 2003)
Results
The number of individuals that hatched after incubation tended to be higher in the sediment samples from the littoral zone than in those from the pelagic zone. On average, 3.7 times more cladocerans hatched from sediment samples from the downwind littoral zone than from sediment samples from the pelagic zone (Fig. 1; ANOVA: F=6.8, P<0.02, Scheffé post-hoc: PDPBPEL, DPBLIT+<0.02). The number of hatchlings retrieved from samples taken in the upwind littoral zone tended to be intermediate (Scheffé post-hoc: PDPBLIT-, DPBPEL<0.05; PDPBLIT-,DPBLIT+>0.05).
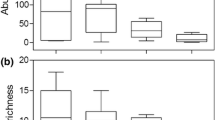
a Taxonomic composition of the hatchling and active community assemblages. b Lower bars give the corresponding hatchling abundance [no. hatchlings × (100 g sediment)−1] or the concentration of cladocerans in the water column (no. indivs. l−1). Note the difference in scale for water column and sediment density. Error bars (hatchling assemblages) give 2×SE. Codes are explained in Table 1.
The upwind and downwind littoral sediments gave rise to relatively similar hatchling assemblages (ANOSIM: R=0.13, P>0.05; Fig. 1). ANOSIM analysis contrasting the eight littoral hatchling assemblages with the four pelagic hatchling assemblages indicated a different taxonomic composition of the DPB in the littoral and the pelagic zones (ANOSIM: R=0.45, P<0.008). The dissimilarity between hatchling assemblages from littoral and pelagic sediments was mainly due to differences in the relative abundance of Daphnia, Chydorus/Alonella, Acroperus and Bosmina (contribution to total between zone dissimilarity as determined by SIMPER analysis: 14.9, 14.8, 10.7 and 10.4%, respectively). Pelagic genera, like Daphnia and Bosmina, hatched in higher proportions from pelagic sediments, whereas macrophyte-associated genera, like Acroperus and Chydorus/Alonella, were better represented in hatchling assemblages recovered from littoral sediments (Fig. 1). Identification of all individuals hatched from the DPBPEL samples (n=36) yielded fewer species than identification of an equal number of individuals retrieved from littoral DPB samples (H36DPBLIT = 13.3 sp. > H36DPBPEL = 10.0 sp.).
The genus composition of the DPB assemblage deviated strongly from that of the open water daytime community (DAYPEL), which is the community most often sampled in surveys (Fig. 1; Table 2). The similarity in taxonomic composition between the DPB and AC samples increased with increasing integration over time and space of the AC samples. Although there was a remarkably high similarity in taxonomic composition between the total hatchling assemblage and the AC sample realizing the highest integration of variation in space and time (INTEGRATED; Fig. 1), the difference in community structure was still significant (Table 2).
Species richness after the identification of 200 individuals (H200) from single-date AC samples (DAYPEL, DAYLIT and NIGHTPEL) was low compared to H200 of AC samples covering longer sampling periods (INTERYEAR and INTRAYEAR; Fig. 2). Still, the composite AC samples were less species rich than the DPB assemblage created by combining hatchlings from DPB samples taken on a single sampling occasion (Fig. 2). Six cladoceran species were exclusively found in the hatchling assemblages, whereas six other species were only observed in the samples from the AC.
Species accumulation curves for the samples derived from the active cladoceran community of the pond M13 and for the total cladoceran hatchling assemblage recovered from the sediment samples. Mean and error values are calculated using the program EstimateS (Colwell 1997). See Table 1 for an explanation of the codes.
Species richness of littoral AC samples tended to be higher than species richness of open-water AC samples (H200DAYLIT = 11.9 sp. > H200DAYPEL = 7.7 sp.), a result similar to that for the DPB assemblages. Therefore, the difference in species richness between the DPB sample and the pooled AC samples could be partially due to the fact that the contribution of littoral samples in the pooled DPB sample (lit:pel=2:1) was higher than in the AC samples (e.g. INTEGRATED lit:pel=1:13). Spatial variation in species presence is unlikely to be the only reason for the relatively high number of species present in DPB samples. Indeed, identification of all the hatchlings obtained from pelagic DPB samples (H36DPBPEL=10.0 sp.) enabled considerably more species to be determined than identification of an equal number of individuals retrieved from pelagic AC samples (H36DAYPEL=5.1 sp.) and nearly as many species as identification of 36 individuals retrieved from the INTEGRATED sample (H36INTEGRATED=11.7 sp.). The species yield of single-date AC samples (DAYPEL, DAYLIT and NIGHTPEL) was unambiguously lower than the species yield of single-date DPB samples (DPB; Fig. 2).
Discussion
We observed substantial differences in the taxonomic composition of hatchling assemblages derived from sediment samples collected in different lake zones. Macrophyte-associated taxa, such as Acroperus and Chydorus/Alonella, hatched in higher fractions from the littoral than from the open water sediments, whereas open-water sediments yielded a larger proportion of pelagic genera, such as Daphnia and Bosmina. The taxonomic similarity between the pelagic and littoral DPB assemblages (BC=61.2) was only slightly higher than between the pelagic and littoral AC assemblages (BC=55.2). These results suggest that spatial integration is not complete in the DPB of M13 and that the horizontal distribution of dormant propagules in the sediments to some extent reflects the heterogeneity of the AC. This may be typical for shallow macrophyte-covered lakes. The presence of well-developed macrophyte beds in the littoral probably limits the effect of resuspension events and sediment focussing. In deeper or more wind-exposed lakes, the mixing of dormant stages will probably be more effective, due to the higher impact of sediment focussing or wind-mediated resuspension events. Furthermore, the often high pelagic over littoral ratio in large lakes implies that the impact of littoral organisms on the total propagule pool will be low, whereas the mixing of littoral samples with propagules from the pelagic will be more substantial. Our results thus suggest that at least in small, shallow lakes with well-developed macrophyte beds, homogeneous distribution of the DPB cannot be taken for granted, and that spatial heterogeneity should be taken into account in the design of studies aimed at assessing the community structure of the DPB.
Community composition of the hatchling assemblage better resembled the composition of the integrated AC assemblage (BCDPB- INTEGRATED=74.2) than that of any single-date AC sample (e.g. BCDPB- DAYPEL=52.0). This may suggest that propagule banks integrate at least part of the temporal variation in active cladoceran community structure. Probably, BCDPB- INTEGRATED would have been even higher if the integrating AC sample had covered the seasonal variation in all sampling years, and if equal proportions of littoral to pelagic samples had been included for both types of analyses. The remaining dissimilarity (loglinear analysis: P<0.001; Table 2) could also indicate that either the DPB, the INTEGRATED or both assemblages still display a biased picture of the actual community composition. Both the AC and the DPB analysis have their intrinsic limitations for the assessment of community structure of short-lived organisms. Even after combining AC samples taken on many occasions and locations throughout subsequent years, observed community composition might be biased due to relatively low capture efficiency of taxa exhibiting an efficient escape response or taxa attached to substrata, like macrophyte-associated and bottom-dwelling taxa (De Stasio 1993; Wetzel and Likens 2000). Analysis of DPB samples may yield biased estimates of community structure due to taxon-specific propensities to produce dormant propagules, to survive as dormant propagules in the sediment and to emerge (Cáceres 1997). Jankowski and Straile (2003), for instance, showed that it was not possible to faithfully reconstruct the relative abundance of two Daphnia species in Lake Constance through analysis of DPB samples, and they attributed this to species-specific sexual reproductive activity. Assessing which assemblage gives the most accurate view of the average community structure over a number of years remains a challenge.
Analysis of sub-fossils of active stages may provide a direct approach to the study of community structure of short-lived organisms. Sub-fossil analysis is being increasingly appreciated by palaeolimnologists as it allows historical reconstruction of past conditions through analysis of present-day associations between assemblage structure and environment (overview in Battarbee et al. 2000; Jeppesen et al. 2001). This approach most probably integrates the temporal heterogeneity over seasons and some years, like the DPB analysis, whereas it does not yield biased estimates caused by differential induction or termination of dormancy. However, variation in likelihood of preservation among taxa (e.g. soft-shelled versus hard-shelled organisms) and among lakes (e.g. with high versus low microbial activity in the sediment) limits general applicability of sub-fossil analysis. Furthermore, not all taxa can be identified to species level based on the morphology of their remains. In the Cladocera, for example, high resolution identification is only possible for Chydoridae and Bosminidae. For these taxa, a combined analysis of DPB, AC and sub-fossil samples would allow quantification of the biases associated with each approach.
The observation that the DPB of M13 integrates the temporal variation inherent to the AC has important ecological and evolutionary implications. Theoretical models suggest that coexistence of competitors in a temporally fluctuating environment is favoured by the presence of overlapping generations and different regeneration niches (storage effect hypothesis: Chesson 1983). Generational overlap can occur in organisms with a long-lived stage (e.g. resting stages of organisms with short generation time). The long-lived stage allows species to persist during periods of competitive inferiority and consequently poor recruitment. Cáceres (1998) has shown that the presence of a DPB has enabled the coexistence of multiple Daphnia species in Oneida lake. Our observation that resting stages of many species, present at different times in the AC, co-occurred in the upper sediment layer indicates that the storage effect may be important for the persistence and coexistence of many cladoceran populations. The resulting high potential species richness allows an efficient response to selection among taxa (species sorting) and genotypes and, consequently, facilitates a more deterministic response to changes in environmental conditions (Leibold 1996; Cottenie and De Meester 2004).
Large-scale studies on zooplankton species richness often rely on single-date sampling efforts. Arnott et al. (1998) have shown that single samples only detected 50% of the annual macrozooplankton species pool and 33% of the total estimated macrozooplankton species pool in a set of Canadian Shield lakes. Our results suggest that DPB analysis may be a more reliable method for the assessment of cladoceran species richness in aquatic habitats than even an elaborate and repeated sampling of the AC, provided that sediment samples from the pelagic and littoral zone are incorporated in the study. Given the presence of unique AC and DPB species, ideally a combination of DPB and AC samples should be processed. The observation that the DPB provides a better picture of species richness and thus environmental state for a prolonged period may imply that DPB analysis is less well-suited for detecting immediate responses in lakes undergoing marked changes. For this purpose, analysis of AC samples is probably still the most suitable method.
Our DPB analysis recorded, in total, as many species as an elaborate analysis of a set of AC samples covering 5 years of sampling and involving the identification of a total of 1,730 zooplankton individuals, a number about eight times higher than the number of hatchlings identified in the DPB analysis. This is striking, as we only used one incubation condition, and as the response to hatching stimuli is known to vary among species (Fryer 1996). Species specificity in the response to hatching stimuli probably explains why the DPB analysis failed to detect some of the species known to occur in the pond (in total six species: Ceriodaphnia reticulata, Disparalona rostrata, Eurycercus lamellatus, Pleuroxus truncatus, Polyphemus pediculus and Scapholeberis mucronata). Combining different incubation conditions may further increase the effectiveness of the DPB analysis as a tool for assessing species richness. The DPB analysis revealed six species that were not detected in any of the AC samples, including mainly benthic (e.g. Macrothrix rosea) and macrophyte-associated species (e.g. Alona quadrangularis, Megafenestra aurita and Pleuroxus aduncus). These all tend to reside at locations difficult to sample effectively using a plankton sampler.
The advantages of DPB analysis for the assessment of species richness are unlikely to be limited to cladoceran communities, but can probably be applied to a wide variety of aquatic organisms capable of producing long-lived dormant stages, such as many aquatic plants and invertebrates. For instance, two studies that were limited to the documentation of rotifer species lists have demonstrated that single-date sediment samples, collected at multiple sites within the lake, revealed all species detected during multiple-year surveys of the AC (May 1986; Crispim and Watanabe 2001). Duggan et al. (2002) found that single-site DPB analysis failed to detect all rotifer species observed in the AC, but still allowed detection of a much larger number of species than did analysis of one AC sample. These studies, combined with our results, underpin the significant potential of using DPB analysis to assess species richness in aquatic systems. Furthermore, DPB analysis might also be a tool for the assessment of genetic diversity. Indeed, analysis of single-date sediment samples often reveals a high genetic diversity (rotifers: Gómez and Carvalho 2000; Ortells et al. 2000; bryozoans: Freeland et al. 2001), whereas analysis of single-date AC samples sometimes fails to detect numerous genotypes due to the high temporal variability in genetic structure of active populations (e.g. Carvalho and Crisp 1987; Freeland et al. 2001). Development of microsatellite markers makes hatching redundant, and thereby allows a less biased assessment of genetic diversity (Gómez and Carvalho 2000; Cousyn et al. 2001; Gómez et al. 2002).
In conclusion, our results suggest that, in shallow ponds, the DPB poorly integrates the spatial variation in AC structure. The increasing similarity between the hatchling assemblage and AC samples that cover increasing sampling periods suggests temporal variation to be better integrated in the DPB. Our results indicate that DPB analysis can be used as a cost-efficient method for the assessment of species richness, as it requires only one sampling occasion. DPB analysis may, however, yield a biased assessment of community structure due to interspecific variation in propagule production, optimal storage conditions and hatching phenology.
References
Anderson NJ, Battarbee, RW (1994) Aquatic community persistence and variability: a palaeoecological perspective. In: Giller PS, Hildrew AG, Raffaelli D (eds) Aquatic ecology: scale, pattern and processes. Blackwell Scientific Publications, London, pp 233–259
Arnott SE, Magnuson JJ, Yan ND (1998) Crustacean zooplankton species richness: single- and multiple-year estimates. Can J Fish Aquat Sci 55:1573–1582
Arnott SE, Yan ND, Magnuson JJ, Frost TM (1999) Interannual variability and species turnover of crustacean zooplankton in Shield lakes. Can J Fish Aquat Sci 56:162–172
Battarbee RW (2000) Palaeolimnological approaches to climate change, with special regard to the biological record. Quat Sci Rev 19:107–124
Baur B, Joshi J, Schmid B, Hanggi A, Borcard D, Stary J, Pedrolichristen A, Thommen GH, Luka H, Rusterholz HP, Oggier P, Ledergerber S, Erhardt A (1996) Variation in species richness of plants and diverse groups of invertebrates in three calcareous grasslands of the Swiss Jura mountains. Rev Suisse Zool 103:801–833
Brendonck L, De Meester L (2003) Egg banks in freshwater zooplankton: evolutionary and ecological archives in the sediment. In: Van Donk E, Spaak P, Boersma M (eds) Recent developments in fundamental and applied plankton research. Hydrobiologia 491:65–84
Burks RL, Lodge DM, Jeppesen E, Lauridsen TL (2002) Diel horizontal migration of zooplankton: costs and benefits of inhabiting the littoral. Freshw Biol 47:343–365
Cáceres CE (1997) Temporal variation, dormancy, and coexistence: a field test of the storage effect. Proc Natl Acad Sci U S A 94:9171–9175
Cáceres CE (1998) Interspecific variation in the abundance, production, and emergence of Daphnia diapausing eggs. Ecology 79:1699–1710
Cáceres CE, Hairston NG Jr (1998) Benthic-pelagic coupling in planktonic crustaceans: the role of the benthos. Arch Hydrobiol Spec Issues Adv Limnol 52:163–174
Carvalho GR, Crisp DJ (1987) The clonal ecology of Daphnia magna (Crustacea: Cladocera). I. Temporal changes in the clonal structure of a natural population. J Anim Ecol 56:453–468
Chesson PL (1983) Coexistence of competitors in a stochastic environment: the storage effect. In: Freeman HI, Strobeck C (eds) Population biology. Lecture notes in biomathematics. Springer, Berlin Heidelberg New York 77:188–198
Clarke KR, Warwick RM (1994) Change in marine communities: an approach to statistical analysis and interpretation. Plymouth Marine Laboratory, Plymouth, p 144
Cohen D (1966) Optimizing reproduction in a randomly varying environment. J Theor Biol 12:119–129
Colwell RK (1997) EstimateS: statistical estimation of species richness and shared species from samples. Version 5. User’s Guide and application. http://viceroy.eeb.uconn.edu/estimates
Cottenie K, De Meester L (2004) Metacommunity structure: synergy of biotic interactions as selective agents and dispersal as fuel. Ecology 85:114–119
Cousyn C, De Meester L, Colbourne JK, Brendonck L, Verschuren D, Volckaert F (2001) Rapid, local adaptation of zooplankton behavior to changes in predation pressure in the absence of neutral genetic changes. Proc Natl Acad Sci U S A 98:6256–6260
Crispim MC, Watanabe T (2001) What can dry reservoir sediments in a semi-arid region in Brazil tell us about cladocera? Hydrobiologia 442:101–105
De Meester L, Dawidowicz P, van Gool E, Loose CJ (1999). Ecology and evolution of predator-induced behavior of zooplankton: depth selection behavior and diel vertical migration. In: Tollrian R, Harvell CD (eds) The ecology and evolution of inducible defenses. Princeton University Press, Princeton
De Stasio BT Jr (1993) Diel vertical migration by zooplankton: population budgets and the diurnal deficit. Bull Mar Sci 53:44–64
Duggan IC, Green JD, Shiel RJ (2002) Rotifer resting egg densities in lakes of different trophic state, and their assessment using emergence and egg counts. Arch Hydrobiol 153:409–420
Ellner SP, Hairston NG Jr (1994) Role of overlapping generations in maintaining genetic variation in a fluctuating environment. Am Nat 143:403–417
Ellner SP, Hairston NG Jr, Babaï D (1998) Long-term diapause and spreading of risk across the life cycle. Arch Hydrobiol Spec Issues Adv Limnol 52:297–312
Flößner D (2000) Die Haplopoda und Cladocera (ohne Bosminidae) Mitteleuropas. Backhuys Publishers, Leiden, p 428
Freeland JR, Rimmer VK, Okamura B (2001) Genetic changes within freshwater bryozoan populations suggest temporal gene flow from statoblast banks. Limnol Oceanogr 46:1121–1129
Fryer G (1996) Diapause, a potent force in the evolution of freshwater crustaceans. Hydrobiologia 320:1–14
Geertsema W, Sprangers JTCM (2002) Plant distribution patterns related to species characteristics and spatial and temporal habitat heterogeneity in a network of ditch banks. Plant Ecol 162:91–108
Gómez A, Carvalho GR (2000) Sex, parthenogenesis and the genetic structure of rotifers: microsatellite analysis of contemporary and resting egg bank populations. Mol Ecol 9:203–214
Gómez A, Adcock GJ, Lunt DH, Carvalho GR (2002) The interplay between colonisation history and gene flow in passively dispersing zooplankton: microsatellite analysis of rotifer resting egg banks. J Evol Biol 15:158–171
Grover JP (1999) Water fleas on cycles. Nature 402:592–593
Hairston NG Jr (1996) Zooplankton egg banks as biotic reservoirs in changing environments. Limnol Oceanogr 41:1087–1092
Hairston NG Jr, Cáceres CE (1996) Distribution of crustacean diapause: micro- and macroevolutionary pattern and process. Hydrobiologia 320:27–44
Hairston NG Jr, De Stasio BT (1988) Rate of evolution slowed by dormant propagule pool. Nature 336:239–242
Havel JE, Eisenbacher EM, Black AA (2000) Diversity of crustacean zooplankton in riparian wetlands: colonization and egg banks. Aquat Ecol 34:63–76
Jankowski T, Straile D (2003) A comparison of egg-bank and long-term plankton dynamics of two Daphnia species, D. hyalina and D. galeata: potentials and limits of reconstruction. Limnol Oceanogr 48:1948–1955
Jeppesen E, Søndergaard M, Søndergaard M, Christoffersen K (eds) (1997) The structuring role of submerged macrophytes in lakes. Ecological studies 131. Springer, Berlin Heidelberg New York, p 423
Jeppesen E, Leavitt P, De Meester L, Jensen JP (2001) Functional ecology and palaeolimnology: using cladoceran remains to reconstruct anthropogenic impact. Trends Ecol Evol 16:191–198
Kluttgen B, Dulmer U, Engels M, Ratte HT (1994) ADAM, an artificial fresh-water for the culture of zooplankton. Water Res 28:743–746
Lauridsen TL, Lodge DM (1996) Avoidance by Daphnia magna of fish and macrophytes: chemical cues and predator-mediated use of macrophyte habitat. Limnol Oceanogr 41:794–798
Leibold MA (1996) A graphical model of keystone predators in food webs: trophic regulation of abundance, incidence, and diversity patterns in communities. Am Nat 147:784–812
Marcus NH (1990) Calanoid copepod, cladoceran, and rotifer eggs in sea-bottom sediments of northern Californian coastal waters: identification, occurrence and hatching. Mar Biol 105:413–418
May L (1986) Rotifer sampling—a complete species list from one visit. Hydrobiologia 134:117–120
Onbé T (1978) Sugar flotation method for the sorting the resting eggs of marine cladocerans and copepods from sea-bottom sediment. Bull Jpn Soc Sci Fish 44:1411
Ortells R, Snell TW, Gómez A, Serra M (2000) Patterns of genetic differentiation in resting egg banks of a rotifer species complex in Spain. Arch Hydrobiol 149:529–551
Philippi T, Seger J (1989) Hedging one’s evolutionary bets, revisited. Trends Ecol Evol 4:41–44
Pinnel-Alloul B (1995) Spatial heterogeneity as a multiscale characteristic of zooplankton community. Hydrobiologia 301:17–42
Sasaki A, Ellner SP (1995) The evolutionarily stable phenotype distribution in a random environment. Evolution 49:337–350
Scheffer M (1998) Ecology of shallow lakes. Chapman and Hall, London, p 357
Schroll H (2002) Indicators of the long-term eutrophication of a Danish lake (Karlso), and water pollution management. J Transdiscipl Environ Studies 1:1–10
Silvertown J, McConway KL, Hughes Z, Biss P, Macnair M, Lutman P (2002) Ecological and genetic correlates of long-term population trends in the park grass experiment. Am Nat 160:409–420
Sommer U, Gliwicz ZM, Lampert W, Duncan A (1986) The PEG-model of seasonal succession of planktonic events in fresh waters. Arch Hydrobiol 106:433–471
Statsoft Inc (2003) Electronic statistics textbook. OK: Statsoft, Tulsa. http://www.statsoft.com/textbook/stathome.html
Vinson MR, Hawkins CP (1998) Biodiversity of stream insects: variation at local, basin, and regional scales. Annu Rev Entomol 43:271–293
Weider LJ, Lampert W, Wessels M, Colbourne JK, Limburg P (1997) Long-term genetic shifts in a microcrustacean egg bank associated with anthropogenic changes in the Lake Constance ecosystem. Proc R Soc Lond B Biol Sci 264:1613–1618
Wetzel R, Likens G (2000) Limnological Analysis, 3rd edn. Springer, Berlin Heidelberg New York
Wiles L, Schweizer E (2002) Spatial dependence of weed seed banks and strategies for sampling. Weed Sci 50:595–606
Acknowledgements
We thank Natuurpunt v.z.w., and especially warden Willy Peumans, for giving us access to the nature reserve De Maten and for their co-operation. We thank the many people who helped during the sampling campaign, especially Karl Cottenie and Eddy Holsters. We are grateful to Joost Raeymakers for help with statistical analysis. This study was supported by the EU-funded project BIOMAN (Biomanipulation and Human Impact in Shallow Lakes, EVK2-CT-1999-00046) and by the Danish Natural Science Research Council research project “Consequences of weather and climate changes for marine and freshwater ecosystems. Conceptual and operational forecasting of the aquatic environment” (CONWOY).
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s00442-005-0020-9.
Rights and permissions
About this article
Cite this article
Vandekerkhove, J., Declerck, S., Jeppesen, E. et al. Dormant propagule banks integrate spatio-temporal heterogeneity in cladoceran communities. Oecologia 142, 109–116 (2005). https://doi.org/10.1007/s00442-004-1711-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-004-1711-3