Abstract
Hypertrophic scar (HS) is a fibro-proliferative disorder which is characterized by excessive deposition of collagen and accumulative activity of myofibroblasts. Increasing evidences have demonstrated miRNAs play a pivotal role in the pathogenesis of HS. MiR-192 is closely associated with renal fibrosis, but its effect on HS formation and skin fibrosis remains unknown. In the study, we presented that miR-192 was up-regulated in HS and HS derived fibroblasts (HSFs) compared to normal skin (NS) and NS derived fibroblasts (NSFs), accompanied by the reduction of smad interacting protein 1 (SIP1) expression and the increase of Col1, Col3 and α-SMA levels. Furthermore, we confirmed SIP1 was a direct target of miR-192 by using luciferase reporter assays. Meanwhile, the overexpression of miR-192 increased the levels of Col1, Col3 and α-SMA. The synthesis of collagen and more positive α-SMA staining were also observed in bleomycin-induced dermal fibrosis model of BALB/c mice treated with subcutaneous miR-192 mimics injection, whereas the inhibition of miR-192 decreased the expression of Col1, Col3 and α-SMA. Moreover, SIP1 siRNA could enhance the levels of Col1, Col3 and α-SMA, showing that the effect of knockdown SIP1 was similar to miR-192 mimics, and the phenomenon manifested miR-192 regulated HS fibrosis by targeting SIP1. Together, our results indicated that miR-192 was a critical factor of HS formation and facilitated skin fibrosis by targeting directly SIP1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypertrophic scar (HS), as a serious skin fibrotic disease, has always been a major problem for clinical treatment. HS usually occurs in 30–72% of patients after skin trauma or severe burn injury (Niessen et al. 1999; Tyack et al. 2012) and leads to a significant impairment of the patients’ quality of life because of functional limitations or aesthetic disfigurement (Brown et al. 2008). However, there are few effective and specific therapeutic approaches in clinics, and the underlying mechanism remains poorly understood. HS is characterized by excessive deposition of extracellular matrix (ECM) and activity of skin myofibroblasts, mainly shown by the expression of Col1, Col3 and α-SMA. Emerging evidences have demonstrated the important role of miRNAs in HS formation (Ong et al. 2007; Li and He 2014; Zhou et al. 2015).
MicroRNA (miRNA) is short, non-coding RNAs of approximately 20–22 nucleotides, it can negatively regulate target gene expressions by binding to 3′-untranslated regions (UTR) of their messenger RNAs, leading to the degradation or translational suppression of target gene (Ambros 2004; Fabian and Sonenberg 2010). Bioinformatics analyses have suggested that more than 50% of all genes are regulated by miRNAs (Witkos et al. 2011). Recent studies show that the aberrant expression of miRNAs are associated with fibrosis disease, such as the heart, lung, liver and kidney fibrosis (Jiang et al. 2010; Kato et al. 2007; Lv et al. 2013). MiR-192 is located on human chromosome 11q13.1 and transcribed as a cluster with miR-194 (Rosa–Rosa et al. 2009; Hino et al. 2008). Inhibiting miR-192 ameliorates renal fibrosis in diabetic nephropathy (Putta et al. 2012). However, the precise biological function of miR-192 and its molecular mechanism in hypertrophic scar has not been elucidated.
Smad interacting protein 1 (SIP1, also known as zinc finger E-box binding homeobox 2, ZEB2), which is a 1214-amino acid, 140 kDa protein encoded by the ZFHX1B gene at chromosome 2q22 that consists of 10 exons (Garavelli et al. 2003; Gibbs and Singleton 2006), has been acted as a DNA-binding transcriptional repressor by directly binding to 5′-CACCT sequences located in various gene promoters (Grunsven et al. 2003; Mejlvang et al. 2007). Many literatures regarding SIP1 focused on its role in driving the transition from epithelial to mesenchymal phenotypes (EMT), particularly during cancer progression. SIP1 expression was associated with aggressive tumor properties and a poor prognosis (Rosivatz et al. 2002; Imamichi et al. 2007). We and other previous studies had revealed that SIP1 negatively regulated Col1 expression by binding to E-box elements in the far upstream region of its promoter (Zhang et al. 2011; Kato et al. 2009), leading to the deposition of ECM and the formation of scar. Increasing evidences indicated the expression of SIP1 was under the control of miRNAs, but the regulatory relationship between miR-192 and SIP1 in the scar formation remained unknown.
In the study, we demonstrated that miR-192 was significantly up-regulated in HS and HS derived fibroblasts (HSFs) compared to normal skin (NS) and NS derived fibroblasts (NSFs). The expression of SIP1 was inversely associated with miR-192. Furthermore, luciferase reporter assay showed that SIP1 was a direct target of miR-192, which was confirmed by western blotting and qRT-PCR in HSFs transfected with miR-192 mimics or inhibitors. Besides, overexpression of miR-192 increased the levels of Col1, Col3 and α-SMA, and anti-miR-192 reduced the synthesis of collagen and the trans-differentiation of fibroblasts to myofibroblasts in HSFs. Meanwhile, the fibro-proliferative effect of miR-192 mimics was detected in the bleomycin-induced fibrosis model of BALB/c mice. Moreover, knockdown SIP1 using siRNA resulted in the inhibition of collagen and α-SMA expression, which was similar to the effect of miR-192 mimics. These results indicated that miR-192 played an important role in the formation of HS, and the effect was achieved by targeting SIP1.
Methods and materials
Patients and ethics approval
The hypertrophic scar and matched normal skin tissues were obtained from patients (mean age 30 years, n = 6) who underwent surgical excision in Xijing Hospital (Xi’an, China). Before surgery, all patients were informed the purpose and procedures of this study and agreed to offer their excised tissues. Written consent was obtained from all participants involved in this study. Diagnosis was confirmed by routine pathological examination. All protocols were performed after obtaining the approval of the ethics committee of Xijing Hospital affiliated with Fourth Military Medical University.
Animals
Five- to six-week-old male BALB/c mice were purchased from the Experimental Animal Center of the Fourth Military Medical University. The animal experimental procedures and ethical approval were performed in strict accordance with the Institutional Animal Care and Use Committee of the Fourth Military Medical University (Xi’an, China).
Cell culture
Human HSFs and matched NSFs were isolated as previously reported (Liu et al. 2012). Briefly, the dermal portions were minced and cultured by tissue block explant to fibroblasts. Cells were incubated with Dulbecco’s modified Eagle medium (DMEM; GIBCO, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FCS; GIBCO), 100 U/ml penicillin, and 100 μg/ml streptomycin at 37 °C in a 5% CO2 humidified atmosphere. HSFs and NSFs between the third and fifth passages were used for the following experiments. Cells were seeded in six-well plates at a density of 2 × 105/well; HSFs were starved for 12–16 h in the serum-free medium when achieved 70–80% confluent, and then transfected with miR-192 mimics (50 nM), inhibitors (50 nM) or negative control (50 nM) using lipofectamine 2000 (Invitrogen, USA) according to the manufacturer’s instructions. The mRNA and protein analysis were used to assess the expression of the fibrotic markers (Col1, Col3, and α-SMA).
Real-time quantitative polymerase chain reaction (qRT-PCR)
qRT-PCR was performed as previously reported (Zhang et al. 2011). Total RNA was extracted with Trizol. The primer pairs (human) used for gene amplification from the cDNA template were as follows: Col1: forward 5′-GAGGGCAACAGCAGGTTCACTTA-3′ and reverse 5′-TCAGCACCACCGATGTCCA-3′; Col3: forward 5′-CCACGGAAACACTGGTGGAC-3′ and reverse 5′-GCCAGCTGCACATCAAGGAC-3′; α-SMA: forward 5′-GACAATGGCTCTGGGCTCTGTAA-3′ and reverse 5′-TGTGCTTCGTCACCCACGTA-3′; SIP1: forward 5′-CCCTTCTGCGACATAAATACGA-3′ and reverse 5′-TGTGATTCATGTGCTGCGAGT-3′; glyceraldehyde 3-phosphate dehydrogenase (GAPDH): forward 5′-GCACCGTCAAGCTGAGAAC-3′ and reverse 5′-TGGTGAAGACGCCAGTGGA-3′. For miRNA, 800 ng RNA was transcribed for cDNA with a reverse transcription kit and miRNA-specific primers supplied by Clontech (Mir-X™ miRNA First-Strand Synthesis). qRT-PCR was performed with miScript SYBR green PCR kit and U6 was used as an normalized control. Human -miR-192: 5′-GACCTATGAATTGACAGCC-3′; U6: 5′-CTCGCTTCGGCAGCACA-3′; Universal reverse primer: 5′-GTGCAGGGTCCGAGGT-3′. Human-miR-192 mimics, human-miR-192 inhibitors, mouse-miR-192 mimics and their corresponding negative controls were purchased from QIAGEN. SIP1 siRNA: 5′-GGAGCAGGUAAUCGCAAGU-3′. The results from three independent reactions were used to determine the relative expression levels of the target genes, which were normalized against the expression of GAPDH or U6.
Western blot analysis
The protein concentration was determined by using a BCA Protein Assay Kit (Beyotime). Briefly, cell lysates containing equal amounts of protein (50 μg) were separated in 10% sodium dodecyl sulfate polyacrylamide gels and transferred onto polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA) at 100 V for 40–100 min. The membranes were blocked with 5% non-fat milk in tris-buffered saline/0.5% Tween-20 (TBST) at room temperature for 3–6 h, and then incubated at 4 °C overnight with anti-Col1 (Rabbit, 1:1000; Abcam, Cambridge, UK), anti-Col3 (Rabbit, 1:3000; Abcam), anti-α-SMA (Rabbit, 1:1000; Abcam), anti-SIP1 (rabbit, 1:250; Abcam, Cambridge, UK) and anti-β-actin (Goat, 1:500; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) antibodies. The next day, the membrane was washed with TBST and then incubated with horseradish peroxidase-conjugated secondary antibodies (1:3000; Boster) for 1 h at room temperature. Then, the ECL reagents kit (Millipore, Billerica, MA, USA) was performed. The intensity of protein on the membranes was analyzed by Image J software and normalized to β-actin expression.
Luciferase reporter assay
To confirm that SIP1 was indeed a direct target of miR-192, we obtained luciferase-3′-untranslated region (3′UTR) reporter constructs for ZEB2 mRNA. For the construction of the 3′UTR of ZEB2 reporter plasmid, the 3′UTR segment of ZEB2 was amplified by PCR, then they were sub-cloned into pGL3 luciferase vector, and the plasmid was designated as pGL3-CMV-LUC-H_SIP1-WT or pGL3-CMV-LUC-H_SIP1-MT. The fragment was then inserted into the 3′-end of the firefly luciferase gene of the dual-luciferase miRNA target expression vector. Co-transfections of the wild-type ZEB2 3′UTR, the mutant ZEB2 3′UTR or a non-targeting control RNA with the miR-192 mimics at a final concentration of 50 nM were accomplished with the lipofectamine 2000 transfection reagent, and the samples were harvested after 24 h for luciferase assays performed using a kit (Promega, WI, USA).
Animal model
Five- to six-week-old male BALB/c mice were used to make the bleomycin-induced dermal fibrosis models. Filter-sterilized bleomycin sulfate (Selleck, Catalog No.S12124) (100 μg/injection dissolved in phosphate-buffered saline), PBS, or bleomycin plus miR-192 mimics were administered by daily subcutaneous injections into the shaved backs of mice using a 27-gauge needle. After two weeks, mice were sacrificed and lesional skin tissues were processed for histological analysis. Each experimental group consisted of six mice at least (n = 6).
Histopathology, immunohistochemistry and Masson′s trichrome staining analysis
The skin samples were embedded in paraffin and cut into 4 μm thick sections. One section was used for H&E staining and another was used for Masson’s trichrome staining analysis of collagen fibers.α-smooth muscle actin (α-SMA), a marker for identifying myofibroblasts that play a crucial role in the pathological fibrogenesis, was determined by immunohistochemistry staining. Briefly, sections were dewaxed and endogenous peroxidase activity was quenched with 3% hydrogen peroxide for 15 min, then blocked with normal goat serum for 30 min to eliminate non-specific binding and incubated overnight at 4 °C with primary antibodies against α-SMA (1:100; Abcam). The next day, the sections were treated with a PV6000 Histostain™ kit (ZSGB, Beijing, China) and stained with diaminobenzidine (ZSGB, Beijing, China). Finally, the sections were counterstained with hematoxylin and images acquired with FSX100.
Statistical analysis
All data were analyzed using SPSS17.0 software; every experiment was repeated at least three times, and the data were presented as mean ± standard error of the mean. Statistical analysis was performed by Student′s t tests. p < 0.05 was considered statistically significant.
Results
MiR-192 was highly overexpressed in HS and HSFs
To test the potential function of miR-192 in HS, firstly we examined the expression level of miR-192 in NS, HS and their derived fibroblasts (NSFs and HSFs) by qRT-PCR, as shown in Fig. 1a, b, the expression of miR-192 in HS and HSFs were significantly higher than NS and NSFs, while the levels of ZEB2 inversely decreased in HS and HSFs (Fig. 1c, d), accompanied with the enhanced expression of Col1, Col3 and α-SMA in the same samples (Fig. 1e, f). There were significantly statistical differences between two groups (p < 0.05). The results revealed miR-192 had the fibro-proliferative effect on HS formation, and presented negative correlation with the expression of ZEB2.
a–b The qRT-PCR analysis of miR-192 in human normal skin, hypertrophic scar tissues and their derived primary fibroblasts (NS, HS and NSFs, HSFs). c–d The mRNA expression of ZEB2 in NS, HS and NSFs, HSFs. e–f In the same samples, the qRT-PCR analysis for the levels of Col1, Col3 and α-SMA, graph represented the expression of aforementioned molecules relative to that of GAPDH. The data were shown as mean ± SEM (*p < 0.05; **p < 0.01)
ZEB2 was a direct target of miR-192
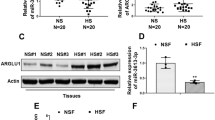
In the next step, we performed the following experiments to confirm that ZEB2 was indeed a target of miR-192. Using three algorithms (miRanda, TargetScan and Diana-microT), ZEB2 was thought to be a theoretical target gene of miR-192 (the predicted binding sites for miR-192 were shown in Fig. 2a). Thus, the 3′-UTR of ZEB2 was cloned into the pGL3-CMV-vector. The luciferase reporter activity of WT-ZEB2-3′UTR was significantly inhibited upon transfection with miR-192 mimics; Furthermore, site-directed mutagenesis of the reporter containing the 3′-UTR of the ZEB2 gene was performed, and the regulatory effect of miR-192 mimic was abolished in the mutant MT-ZEB2-3′UTR reporter (Fig. 2b). Besides, ZEB2, the predicted target of miR-192 by bioinformatics analysis, was confirmed by qRT-PCR (Fig. 2c, d) and western blotting (Fig. 2e, f). MiR-192 mimics, inhibitors and the corresponding negative controls were transfected into HSFs with lipofectamine 2000 (Invitrogen). The mRNA level showed that miR-192 mimics down-regulated the expression of SIP1, whereas miR-192 inhibitors increased the level of SIP1 (Fig. 2c, d). There were statistical differences between miR-192 mimics, inhibitors and their matched control groups (p < 0.01); and the protein expression verified the result (p < 0.05) (Fig. 2e, f). Our data demonstrated miR-192 directly targeted SIP1 in HS.
a The sequence alignment showed that ZEB2 (region at base pairs 956–962 in the 3′UTR) harbored a binding site for miR-192. Solid lines depicted matched base pairing between the wild-type SIP1 3′UTR and miR-192, while dots represented mismatched bases between the mutant SIP1 3′UTR and miR-192. b Luciferase activity was significantly decreased upon miR-192 overexpression in case of ZEB2 wild-type 3′UTR, but was not affected by mutant 3′UTR, indicating that miR-192 directly targeted ZEB2. c HSFs were transfected with miR-192 mimics or miR-192 inhibitors, respectively. The overexpression or inhibition of miR-192 was measured by qRT-PCR. d miR-192 mimics decreased the expression of ZEB2 and anti-miR-192 increased its mRNA level. e miR-192 mimics down-regulated the expression of SIP1 and anti-miR-192 up-regulated its protein level. Data represented mean ± SD of triplicate values from a representative experiment performed at least three times (*p < 0.05; **p < 0.01)
Overexpression of miR-192 promoted the synthesis of collagen and the accumulation of myofibroblasts
Since we had found miR-192 was overexpressed in HS, the following experiment was performed to measure the effect of miR-192 on the deposition of collagen and the activity of myofibroblasts. The results revealed miR-192 mimics significantly increased the expression of Col1, Col3 and α-SMA by qRT-PCR (Fig. 3a), whereas anti-miR-192 down-regulated the levels of the aforementioned molecules (Fig. 3b). The phenomenon was further confirmed by western blotting (Fig. 3c, d) and demonstrated miR-192-driven pro-fibrotic effects in HS formation. There were statistical differences between miR-192 mimics, inhibitors and their matched control groups (p < 0.05).
a miR-192 mimics increased the expression of Col1, Col3 and α-SMA compared to negative control by qRT-PCR. b Anti-miR-192 reduced the mRNA levels of Col1, Col3 and α-SMA. c Immunoblot analysis for the protein expression of Col1, Col3 and α-SMA in HSFs transfected with miR-192 mimics or inhibitors, graph showed the relative band density to β-actin. d Quantitative analysis was shown by histogram. Every experiment was repeated for three times at least. The data were shown as mean ± SEM (*p < 0.05; **p < 0.01)
Knockdown SIP1 could increase the expression of Col1, Col3 and α-SMA, the effect was similar to miR-192 mimics
We had proved miR-192 targeted directly SIP1 to down-regulate its expression, and miR-192 facilitated the synthesis of collagen and the trans-differentiation of fibroblasts to myofibroblasts. Therefore, we speculated miR-192 modulated HS fibrosis by targeting SIP1. Cells growth were arrested in the serum-free medium 12 h prior to transfection with siRNA at a final concentration of 100 nM, the result was identified by qRT-PCR (Fig. 4a). Based on this, we tested the effect between miR-192 mimics and SIP1 siRNA, the result revealed SIP1 siRNA had the similar function to miR-192 mimics, presenting the up-regulation of the protein levels of Col1, Col3 and α-SMA (Fig. 4b, c). SIP1 could suppress the synthesis of collagen and the expression of α-SMA. There were statistical differences between miR-192 mimics, SIP1 siRNA and their corresponding negative controls (p < 0.05). The results illustrated miR-192 exerted the fibro-proliferative effect via the repression of SIP1, resulting in the change of ECM deposition and myofibroblasts differentiation.
a The effect of SIP1 knockdown using siRNA was tested by qRT-PCR. b Immunoblot analysis for the protein expression of Col1, Col3 and α-SMA in HSFs transfected with miR-192 mimics, SIP1 siRNA and their corresponding negative controls. c Quantitative analysis was shown by histogram. Every experiment was repeated for three times at least. The data were shown as mean ± SEM (*p < 0.05; **p < 0.01)
MiR-192 mimics facilitated the deposition of collagen and the expression of α-SMA in the bleomycin-induced fibrosis model of BALB/c mice
A major limitation in the progress of scar management is the lack of physiologically relevant human models to explore the pathogenesis of HS formation. In the experiment, we made the bleomycin-induced dermal fibrosis model of BALB/c mice. The mice were divided into three groups: control group (PBS), bleomycin group and bleomycin plus miR-192 mimics group, and they were sacrificed after 2 weeks of subcutaneous injections. H&E and Masson’s trichrome staining were used to measure the structure and arrangement of collagen. The dermal thickness was more obvious in the bleomycin plus miR-192 mimics group (Fig. 5a), which presented tensed, excessive, and disorderly arranged collagen bundles fibers compared to thinner fibers and ordered arrangement in only bleomycin-induced group (Fig. 5c). The control group demonstrated the normal histological structure of BALB/c mice skin. There were more positive-α-SMA staining in the bleomycin plus miR-192 mimics group (Fig. 5e). Relative dermal thickness (defined as the thickness of skin from the top of the granular layer to the junction), collagen content and the percentage of α-SMA-positive cells were analyzed by Image Pro Plus software and shown by histogram (Fig. 5b, d, f). There were significantly statistical differences between bleomycin plus miR-192 mimics, bleomycin and the control groups (p < 0.05). The results revealed miR-192 mimics promoted collagen deposition and myofibroblasts accumulation in the bleomycin-induced fibrosis models of BALB/c mice.
Histological analysis of bleomycin-induced dermal fibrosis of BALB/c mice. a The thickness of the dermal layer in PBS, bleomycin and bleomycin plus miR-192 mimics-treated mice were measured by H&E staining. b The analysis of dermal thickness was shown by histogram. Scale bar: 200, 50 μm (n = 6 mice per group). c Masson’s trichrome staining for labeling collagen fibers blue was used to evaluate the deposition and arrangement of collagen in different groups. Scale bar: 200, 50 μm (n = 6 mice per group). d Representative images were shown and the content of collagen was summarized as a graph. Scale bar: 200, 50 μm. e Immunohistochemistry staining of α-SMA expression in HSFs, Scale bar: 50 μm. f The percentage of positive cells staining was shown by histogram (*p < 0.05; **p < 0.01)
Discussion
The study provides the following major findings. First, miR-192 was overexpressed in HS and HSFs, and inversely associated with the level of ZEB2; Furthermore, we confirmed ZEB2 was indeed a direct target of miR-192, and miR-192 regulated the synthesis of collagen and the differentiation of myofibroblasts in HSFs and the bleomycin-induced BALB/c mice; Moreover, the effect of SIP1 siRNA on HS formation was similar to miR-192 mimics, suggesting that miR-192 facilitated HS fibrosis by targeting SIP1. These results demonstrate that miR-192 is a promising therapeutic target to reduce scar formation.
Hypertrophic scar represents the main clinical challenge to burn and plastic surgeons owing to their high incidence and the lack of an effective treatment strategy (Kathju and Gallo 2012). Fibroblasts play a crucial role in the synthesis of collagen and the differentiation into myofibroblasts. Growing evidence revealed miRNAs could potentially regulate skin fibrosis and have therapeutic significance (Brown and Naldini 2009).
MiR-21 presented the fibro-proliferative effect in HS (Li et al. 2016); MiR-181b regulated decorin production by dermal fibroblasts and might be a potential therapy for HS (Kwan et al. 2015); MiR-145 contributed to HS formation by inducing myofibroblast activity (Gras et al. 2015); our study focused on miR-192, which was involved in multiple types of cancer, including lung, breast, liver, colon, bladder, pancreatic cell carcinoma and diabetic nephropathy (Tazawa et al. 2011; Wang et al. 2012; Parrizas et al. 2015). About the fibrotic disease, inhibiting miR-192 ameliorated renal fibrosis in diabetic nephropathy (Putta et al. 2012); in the study, using patients’ tissue samples and primary fibroblasts, we explored the expression of miR-192 in HS. The results suggested miR-192 was overexpressed in HS and HSFs compared to NS and NSFs, inversely associated with the level of SIP1. There was a positive correlation between miR-192 and the fibrotic proteins (Col1, Col3 and α-SMA), indicating miR-192 was closely related with HS fibrosis.
It has been proved that the cell factors were related with skin fibrosis and scar formation, such as TGFβ1, FGF2(Sideek et al. 2016), MRP1 (Li et al. 2015). As the important transcriptional regulator of TGFβ/Smad signal pathway, SIP1 was first identified using a yeast two-hybrid system by Verschueren et al. (1999). The function of SIP1 mainly focused on epithelial-mesenchymal transition in cancer, miRNAs (miR-132, miR-205, miR-200 and so on) regulated SIP1 in the process (Lee et al. 2014; Kim et al. 2011). In the next step, we needed to confirm SIP1 was a direct target of miR-192 in HS. Using three algorithms (miRanda, TargetScan and Diana-microT), we got the theoretical binding sites of miR-192 in the predicted target sequences. The luciferase reporter activity of WT-ZEB2-3′UTR was significantly inhibited upon transfection with miR-192 mimic; whereas MT-ZEB2-3′UTR abolished the effect. Then, HSFs were transfected with the miR-192 mimics or inhibitors to further evaluate the regulatory relationship between miR-192 and SIP1, and our data demonstrated that miR-192 mimics down-regulated the expression of SIP1, while the inhibition of miR-192 increased the level of SIP1. The results revealed miR-192 targeted SIP1 to modulate HS.
To further detect the effect of miR-192, HSFs were transfected with the miR-192 mimics or inhibitors to observe the influence of collagen synthesis and the trans-differentiation of fibroblasts to myofibroblasts, the results showed that overexpression of miR-192 enhanced the mRNA and protein expression of Col1, Col3 and α-SMA, and inhibition of miR-192 suppressed the levels of the fibrotic molecules. The effect of miR-192 was further verified in the bleomycin-induced skin fibrosis model of BALB/c mice in vivo. Daily application of bleomycin over a period of 2 weeks lead to histologically a thickened dermis and the deposition of collagen. MiR-192 mimics increased bleomycin-induced skin dermal fibrosis, leading to the up-regulation of collagen content and more positive staining of α-SMA in the lesional skin compared to the control group and only bleomycin-induced group. Above mentioned results demonstrated miR-192 facilitated HS fibrosis.
Since SIP1 was a direct target of miR-192, and overexpression of miR-192 increased the levels of Col1, Col3 and α-SMA, we speculated miR-192 promoted HS fibrosis by targeting SIP1. Knockdown SIP1 using siRNA up-regulated the protein expression of collagen and α-SMA, and the effect of SIP1 siRNA was similar to miR-192 mimics, which suggested miR-192 targeted SIP1 to modulate HS fibrosis. The level of SIP1 was inversely associated with the expression of collagen and the pathological scar formation. In accordance with previous studies, overexpression of miR-192 increased tumor volume in an orthotopic pancreatic cancer mouse model, coupled with the suppression of SIP1 and the elevation of collagen I (Botla et al. 2016). Kato et al. (2007, 2011) reported that down-regulation of SIP1 could be relevant to the pathogenesis of renal fibrosis, and the repression of SIP1 expression on mouse mesangial cells by miR-192 facilitated collagen synthesis, matrix deposition and glomerulosclerosis. Inverse correlation between miR-192 and SIP1 was observed in glomeruli of human early diabetic nephropathy (Deshpande et al. 2013; Krupa et al. 2010).
Based on these results, we conclude that targeting miR-192 may be a potential option to prevent the progression of hypertrophic scar, and miR-192-mediated regulation of SIP1 provides a novel mechanistic basis for the treatment of hypertrophic scar.
Conclusions
The study demonstrated miR-192 significantly facilitated the deposition of collagen, and the trans-differentiation of fibroblasts to myofibroblasts. In addition, the effect of miR-192 on HS fibrosis was achieved by directly targeting SIP1. Our data provide a potential therapeutic strategy for HS treatment, and the anti-fibrotic mechanism of inhibiting miR-192 need to be further elucidated and explored.
Abbreviations
- HS:
-
Hypertrophic scar
- HSFs:
-
Hypertrophic scar derived fibroblasts
- NS:
-
Normal skin
- NSFs:
-
Normal skin derived fibroblasts
- SIP1:
-
Smad interacting protein 1
- ECM:
-
Extracellular matrix
- 3′-UTR:
-
3′-Untranslated regions
- α-SMA:
-
α-smooth muscle actin
References
Ambros V (2004) The functions of animal microRNAs. Nature 431:350–355
Botla SK, Savant S, Jandaghi P, Bauer AS, Mucke O, Moskalev EA, Neoptolemos JP, Costello E, Greenhalf W, Scarpa A, Gaida MM, Buchler MW, Strobel O, Hackert T, Giese NA, Augustin HG, Hoheisel JD (2016) Early epigenetic downregulation of microrna-192 expression promotes pancreatic cancer progression. Cancer Res 76:4149–4159
Brown BD, Naldini L (2009). Exploiting and antagonizing microRNA regulation for therapeutic and experimental applications. Nat Rev Genet 10:578–585
Brown BC, McKenna SP, Siddhi K, McGrouther DA, Bayat A (2008) The hidden cost of skin scars: quality of life after skin scarring. J Plast Reconstr Aesthet Surg 61:1049–1058
Deshpande SD, Putta S, Wang M, Lai JY, Bitzer M, Nelson RG, Lanting LL, Kato M, Natarajan R (2013) Transforming growth factor-beta-induced cross talk between p53 and a microRNA in the pathogenesis of diabetic nephropathy. Diabetes 62:3151–3162
Fabian MR, Sonenberg N, Filipowicz W (2010) Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem 79:351–379
Garavelli L, Donadio A, Zanacca C, Banchini G, Della Giustina E, Bertani G, Albertini G, Rossi C Del, Zweier C, Rauch A, Zollino M, Neri G (2003) Hirschsprung disease, mental retardation, characteristic facial features, and mutation in the gene ZFHX1B (SIP1): confirmation of the Mowat–Wilson syndrome. Am J Med Genet A 116A:385–388
Gibbs JR, Singleton A (2006) Application of genome-wide single nucleotide polymorphism typing: simple association and beyond. PLoS Genet 2:e150
Gras C, Ratuszny D, Hadamitzky C, Zhang H, Blasczyk R, Figueiredo C (2015) miR-145 Contributes to hypertrophic scarring of the skin by inducing myofibroblast activity. Mol Med 21:296–304
Hino K, Tsuchiya K, Fukao T, Kiga K, Okamoto R, Kanai T, Watanabe M. (2008). Inducible expression of microRNA-194 is regulated by HNF-1alpha during intestinal epithelial cell differentiation. RNA 14:1433–1442
Imamichi Y, Konig A, Gress T, Menke A (2007) Collagen type I-induced Smad-interacting protein 1 expression downregulates E-cadherin in pancreatic cancer. Oncogene 26:2381–2385
Jiang X, Tsitsiou E, Herrick SE, Lindsay MA (2010) MicroRNAs and the regulation of fibrosis. FEBS J 277:2015–2021
Kathju S, Gallo PH, Satish L (2012) Scarless integumentary wound healing in the mammalian fetus: molecular basis and therapeutic implications. Birth Defects Res C Embryo Today 96:223–236
Kato M, Zhang J, Wang M, Lanting L, Yuan H, Rossi JJ, Natarajan R (2007) MicroRNA-192 in diabetic kidney glomeruli and its function in TGF-beta-induced collagen expression via inhibition of E-box repressors. Proc Natl Acad Sci USA 104:3432–3437
Kato M, Putta S, Wang M, Yuan H, Lanting L, Nair I, Gunn A, Nakagawa Y, Shimano H, Todorov I, Rossi JJ, Natarajan R (2009) TGF-beta activates Akt kinase through a microRNA-dependent amplifying circuit targeting PTEN. Nat Cell Biol 11:881–889
Kato M, Arce L, Wang M, Putta S, Lanting L, Natarajan R (2011) A microRNA circuit mediates transforming growth factor-beta1 autoregulation in renal glomerular mesangial cells. Kidney Int 80:358–368
Kim T, Veronese A, Pichiorri F, Lee TJ, Jeon YJ, Volinia S, Pineau P, Marchio A, Palatini J, Suh SS, Alder H, Liu CG, Dejean A, Croce CM (2011). p53 regulates epithelial–mesenchymal transition through microRNAs targeting ZEB1 and ZEB2. J Exp Med 208:875–883
Krupa A, Jenkins R, Luo DD, Lewis A, Phillips A, Fraser D (2010) Loss of MicroRNA-192 promotes fibrogenesis in diabetic nephropathy. J Am Soc Nephrol 21:438–447
Kwan P, Ding J, Tredget EE (2015) MicroRNA 181b regulates decorin production by dermal fibroblasts and may be a potential therapy for hypertrophic scar. PLoS ONE 10:e0123054
Lee JY, Park MK, Park JH, Lee HJ, Shin DH, Kang Y, Lee CH, Kong G (2014) Loss of the polycomb protein Mel-18 enhances the epithelial–mesenchymal transition by ZEB1 and ZEB2 expression through the downregulation of miR-205 in breast cancer. Oncogene 33:1325–1335
Li P, He QY, Luo CQ (2014) Overexpression of miR-200b inhibits the cell proliferation and promotes apoptosis of human hypertrophic scar fibroblasts in vitro. J Dermatol 41:903–911
Li Y, Yang L, Zheng Z, Shi J, Wu X, Guan H, Jia Y, Tao K, Wang H, Han S, Gao J, Zhao B, Su L, D Hu (2015) MRP1 knockdown down-regulates the deposition of collagen and leads to a reduced hypertrophic scar fibrosis. J Mol Histol 46:357–364
Li G, Zhou R, Zhang Q, Jiang B, Wu Q, Wang C (2016) Fibroproliferative effect of microRNA-21 in hypertrophic scar derived fibroblasts. Exp Cell Res 345:93–99
Liu J, Wang Y, Pan Q, Su Y, Zhang Z, Han J, Zhu X, Tang C, Hu D (2012) Wnt/beta-catenin pathway forms a negative feedback loop during TGF-beta1 induced human normal skin fibroblast-to-myofibroblast transition. J Dermatol Sci 65:38–49
Lv LL, Cao YH, Ni HF, Xu M, Liu D, Liu H, Chen PS, Liu BC (2013) MicroRNA-29c in urinary exosome/microvesicle as a biomarker of renal fibrosis. Am J Physiol Renal Physiol 305:F1220–F1227
Mejlvang J, Kriajevska M, Vandewalle C, Chernova T, Sayan AE, Berx G, Mellon JK, Tulchinsky E (2007) Direct repression of cyclin D1 by SIP1 attenuates cell cycle progression in cells undergoing an epithelial mesenchymal transition. Mol Biol Cell 18:4615–4624
Niessen FB, Spauwen PH, Schalkwijk J, Kon M (1999) On the nature of hypertrophic scars and keloids: a review. Plast Reconstr Surg 104:1435–1458
Ong CT, Khoo YT, Tan EK, Mukhopadhyay A, Do DV, Han HC, Lim IJ, Phan TT (2007). Epithelial–mesenchymal interactions in keloid pathogenesis modulate vascular endothelial growth factor expression and secretion. J Pathol 211:95–108
Parrizas M, Brugnara L, Esteban Y, Gonzalez-Franquesa A, Canivell S, Murillo S, Gordillo-Bastidas E, Cusso R, Cadefau JA, Garcia-Roves PM, Servitja JM, Novials A (2015). Circulating miR-192 and miR-193b are markers of prediabetes and are modulated by an exercise intervention. J Clin Endocrinol Metab 100:E407–E415
Putta S, Lanting L, Sun G, Lawson G, Kato M, Natarajan R (2012) Inhibiting microRNA-192 ameliorates renal fibrosis in diabetic nephropathy. J Am Soc Nephrol 23:458–469
Rosa-Rosa JM, Pita G, Gonzalez-Neira A, Milne RL, Fernandez V, Ruivenkamp C, van Asperen CJ, Devilee P, Benitez J (2009) A 7 Mb region within 11q13 may contain a high penetrance gene for breast cancer. Breast Cancer Res Treat 118:151–159
Rosivatz E, Becker I, Specht K, Fricke E, Luber B, Busch R, Hofler H, Becker KF (2002) Differential expression of the epithelial–mesenchymal transition regulators snail, SIP1, and twist in gastric cancer. Am J Pathol 161:1881–1891
Sideek MA, Teia A, Kopecki Z, Cowin AJ, Gibson MA (2016) Co-localization of LTBP-2 with FGF-2 in fibrotic human keloid and hypertrophic scar. J Mol Histol 47:35–45
Tazawa H, Kagawa S, Fujiwara T (2011) MicroRNAs as potential target gene in cancer gene therapy of gastrointestinal tumors. Expert Opin Biol Ther 11:145–155
Tyack Z, Simons M, Spinks A, Wasiak J (2012) A systematic review of the quality of burn scar rating scales for clinical and research use. Burns 38:6–18
van Grunsven LA, Michiels C, Van de Putte T, Nelles L, Wuytens G, Verschueren K, Huylebroeck D (2003) Interaction between Smad-interacting protein-1 and the corepressor C-terminal binding protein is dispensable for transcriptional repression of E-cadherin. J Biol Chem 278:26135–26145
Verschueren K, Remacle JE, Collart C, Kraft H, Baker BS, Tylzanowski P, Nelles L, Wuytens G, Su MT, Bodmer R, Smith JC, Huylebroeck D (1999) SIP1, a novel zinc finger/homeodomain repressor, interacts with Smad proteins and binds to 5′-CACCT sequences in candidate target genes. J Biol Chem 274:20489–20498
Wang XW, Heegaard NH, Orum H (2012) MicroRNAs in liver disease. Gastroenterology 142:1431–1443
Witkos TM, Koscianska E, Krzyzosiak WJ (2011). Practical aspects of microRNA target prediction. Curr Mol Med 11:93–109
Zhang ZF, Zhang YG, Hu DH, Shi JH, Liu JQ, Zhao ZT, Wang HT, Bai XZ, Cai WX, Zhu HY, Tang CW (2011). Smad interacting protein 1 as a regulator of skin fibrosis in pathological scars. Burns 37:665–672
Zhou R, Zhang Q, Zhang Y, Fu S, Wang C (2015) Aberrant miR-21 and miR-200b expression and its pro-fibrotic potential in hypertrophic scars. Exp Cell Res 339:360–366
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Nos. 81171811, 81372069, 81571914 and 81272098).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Additional information
Yan Li, Julei Zhang and Wei Zhang are co-first authors.
Rights and permissions
About this article
Cite this article
Li, Y., Zhang, J., Zhang, W. et al. MicroRNA-192 regulates hypertrophic scar fibrosis by targeting SIP1. J Mol Hist 48, 357–366 (2017). https://doi.org/10.1007/s10735-017-9734-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-017-9734-3