Abstract
We have recently shown that Latent transforming growth factor-beta-1 binding protein-2 (LTBP-2) has a single high-affinity binding site for fibroblast growth factor-2 (FGF-2) and that LTBP-2 blocks FGF-2 induced cell proliferation. Both proteins showed strong co-localisation within keloid skin from a single patient. In the current study, using confocal microscopy, we have investigated the distribution of the two proteins in normal and fibrotic skin samples including normal scar tissue, hypertrophic scars and keloids from multiple patients. Consistently, little staining for either protein was detected in normal adult skin and normal scar samples but extensive co-localisation of the two proteins was observed in multiple examples of hypertrophic scars and keloids. LTBP-2 and FGF-2 were co-localised to fine fibrous elements within the extracellular matrix identified as elastic fibres by immunostaining with anti-fibrillin-1 and anti-elastin antibodies. Furthermore, qPCR analysis of RNA samples from multiple patients confirmed dramatically increased expression of LTBP-2 and FGF-2, similar TGF-beta 1, in hypertrophic scar compared to normal skin and scar tissue. Overall the results suggest that elevated LTBP-2 may bind and sequester FGF-2 on elastic fibres in fibrotic tissues and modulate FGF-2’s influence on the repair and healing processes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fibrotic disorders are a major cause of morbidity and mortality worldwide since fibrosis is central to a range of severe life-threatening human diseases across multiple organ systems including kidney, liver, lung, skin and cardiovascular systems (Wynn 2004). In the United States, fibrotic disorders have been estimated to contribute to 45 % of all-cause mortalities (Wynn 2004) and annual incidences of new cases of pulmonary fibrosis and fibrotic kidney disease have been estimated as 270/million/year (Raghu et al. 2006) and 63/million/year respectively (Rastegar and Kashgarian 1998). Despite advances in our understanding of the pathogenesis of fibrotic processes and the development of therapeutic agents that target known pro-fibrotic factors (Akhurst and Hata 2012), effective anti-scarring therapies and potential therapeutic targets are limited, and the vast majority of studies and clinical trials have failed (Brown and Wells 2008).
A persistent finding among all the fibrotic diseases in humans is the disruption of tissue architecture and progressive loss of organ function (Thannickal et al. 2014). Fibrosis is characterized by excessive, abnormal accumulation of extracellular matrix (ECM), particularly collagen fibres, following injury or repeated insult to a tissue or organ (Kissin and Korn 2003; de Vega et al. 2009). The mechanism of fibrosis is complex and poorly understood. Generally, following injury, damaged tissues undergo regeneration and repair whereby insulted epithelial or endothelial cells are replaced by activated fibroblasts that produce excess ECM components including collagens type I and type III, fibronectin and hyaluronic acid, resulting in matrix overgrowth and scarring (Wynn 2007). Persistently elevated TGF-β levels are a key element for progressive and idiopathic forms of pulmonary fibrosis; renal interstitial fibrosis; liver fibrosis; cardiac fibrosis in hypertrophy and heart failure; development of aberrant scars and scleroderma (Wynn 2008; Goodwin and Jenkins 2009; Pohlers et al. 2009). When inflammation subsides during the healing process, matrix synthesis in the post-inflammatory stages of fibrogenesis continues, stimulated by overexpression and aberrant TGF−β activity which results in massive progressive deposition of fibrous matrix (Strutz and Neilson 2003). In cultured human keloid fibroblasts, TGF-β up-regulates the expression of collagen type 1 and fibronectin with an approximately 25-fold increase compared to normal fibroblasts (Daian et al. 2003). Another important growth factor involved in fibrotic disorders is fibroblast growth factor-2 (FGF-2), a mesenchyme-derived growth factor that displays migratory, mitogenic, and morphogenic properties and has functions in organ development, angiogenesis, organ regeneration, tissues remodelling and wound healing (Ono et al. 2007).
FGF-2 appears to have anti-fibrotic functions (Ortega et al. 1998; Ono et al. 2007; Xie et al. 2008; Eto et al. 2012; Shi et al. 2013). FGF-2 knockout mice exhibit a significant delay in the rate of healing of full-thickness excisional skin wounds (Ortega et al. 1998). In chronic wounds including hypertrophic scars (HTS) and keloids, post-operative administration of FGF-2 inhibits widening of the lesions without any serious side-effects (Ono et al. 2007). In the rabbit ear model of wound healing, scars treated with FGF-2 show down-regulation of TGF-β-induced collagen production and increased levels of collagen-specific matrix metalloproteinase-1, compared with control scars (Xie et al. 2008; Eto et al. 2012; Shi et al. 2013).
Recently, we have identified a very strong interaction of FGF-2 with the matrix protein, latent TGF-β-1 binding protein-2 (LTBP-2) with the binding site in a central cluster of six tandem epidermal growth factor (EGF)-like repeats (Menz et al. 2015). The function of LTBP-2 is unclear and it differs from other members of the LTBP family since it does not bind to TGF-β (Saharinen and Keski-Oja 2000). Previous in situ hybridisation and immunohistochemical studies showed that LTBP-2 was tightly associated with fibrillin–microfibrils and elastic fibres in a variety of adult tissues such as lung, heart, maternal decidua, liver, spleen and skeletal muscles (Moren et al. 1994; Gibson et al. 1995). We have also demonstrated that exogenous LTBP-2 inhibits elastinogenesis in cultured ear cartilage chondrocytes indicating LTBP-2 may have a negative regulatory role during elastinogenesis (Sideek et al. 2014).
In vitro studies found that LTBP-2 competes with LTBP-1 for binding to fibrillin-1, suggesting that LTBP-2 may indirectly modulate storage and activation of the TGF-β/LTBP-1 complex (LLC) (Hirani et al. 2007). An early study revealed that LTBP-2 mRNA and protein levels increased following tissues damage but the exact mechanism of how this occurs is unknown (Shi and Massague 2003). In addition, patients with LTBP-2 mutations develop a recessive form of Weill–Marchesani syndrome (WMS) which is characterized by brachydactyly, short stature, ectopia lentis and thick fibrotic skin suggestive of aberrant TGF-β signalling (Haji-Seyed-Javadi et al. 2012). LTBP-2 null humans develop primary congenital glaucoma from changes in the elastic fibre-rich ocular trabecular meshwork and ciliary processes (Ali et al. 2009). However some patients also have varied Marfan Syndrome-like phenotypes including abnormal bone over-growth, arachnodactyly, osteopenia, aortic stenosis, and aortic valve problems (Ali et al. 2009). Our recent studies have shown that exogenous LTBP-2 was able to inhibit FGF-2-induced cell proliferation and that both proteins co-localised in keloid tissue from one patient (Menz et al. 2015). These findings suggest that LTBP-2 could act as a tissue store for FGF-2 released in fibrotic conditions and thus modulate FGF-2’s influence in the repair process. The current study has investigated the expression and distribution of LTBP-2 and FGF-2 in fibrotic tissues from a range of patients and has found that both proteins consistently showed strong co-localisation in a range of fibrotic HTS and keloid skin samples.
Materials and methods
Human tissue samples
Samples of normal human skin, normal scar tissue, HTS and keloid were obtained from the Calvary Hospital and Royal Adelaide Hospital with ethics approval from the Human Research Ethics Committee (11-CHREC-F007) and University of Adelaide (#H-16-2001) in accordance with the Declaration of Helsinski principles. Written consent was obtained from patients undergoing surgery to obtain excised tissue. Following ethics approval protocols de-identified samples were collected from male and female patients, ranging in age from 18 to 39 from various body sites including: arm, leg, back and ear. Skin and scar samples were bisected and fixed in 10 % (v/v) neutral buffered formalin for histology/immunofluorescence or frozen in liquid nitrogen for biochemical analysis.
Antibodies
Rabbit anti-[human LTBP-2 peptide] antibody 3504 has been described previously (Hirani et al. 2007). Human monoclonal FGF-2 antibody (#610871) for immunohistochemistry was supplied by BD labs. Anti-fibrillin-1 antibody MAB1919 was purchased from Millipore Australia Ltd, Klisyth, Victoria. Mouse anti-human elastin monoclonal antibody BA4 was purchased from Sigma-Aldrich, St. Louis, MO. Immunoblotting to check for antibody cross-reactivity was performed as described previously (Menz et al. 2015).
Immunohistochemistry
Cell culture
Human foreskin fibroblasts (HFF) cells were cultured in DMEM for 21 days at an initial density of 4 × 105/ml on microscope chamber slides (Lab-Tek NALGE NUNC). The cell layers were incubated with 3 % para-formaldehyde in PBS for 10 min and fixed with cold acetone and methanol for 1 min and rehydrated in PBS for 5 min. The rehydrated sections were then incubated with 50 mM of dithiothreitol (DTT) made up in 2 M GuHCl/50 mM Tris (100 µl/chamber) for 5 min followed by addition of 1 M iodoacetamide (100 µl/chamber) and incubation in the dark for 5 min. The cell layer was washed with TBS and blocked with 0.2 ml 1 % ovalbumin in TBS for 15 min. After blocking, the cells were incubated overnight at 4 °C with affinity purified rabbit anti-LTBP-2 antibody 3504 and mouse monoclonal anti-FGF-2 antibody. Control sections were incubated with matched concentrations of rabbit IgG and mouse IgG. Sections were washed with TBS and incubated with the appropriate secondary antibody conjugated to fluorescein isothiocyanate (FITC) for 60 min at room temperature in the dark. Sections were washed and mounted in 90 % glycerol/10 % TBS/methiolate containing anti-fade reagent p-phenylenediamine (Sigma-Aldrich, St. Louis, MO) prior to laser confocal microscopy. Labelled sections were viewed on a Leicia SP5 spectral scanning confocal microscope using a 488 nm laser with emission settings at 496–533 nm.
Tissue sections
Paraffin-embedded tissue sections from normal skin, normal scar, HTS and keloid were dewaxed in xylene for 30 min and dehydrated through a series of ethanol solutions (100–30 %) followed by water and finally PBS. The sections were then placed in 15 % target retrieval solution [Dako; S1700] in a decloaking chamber (Biocare Medical) for 60 min with initial temperature 90 °C, dropping to 65 °C (Kopecki et al. 2013). After washing with PBS, the sections were incubated with trypsin (0.025 % w/v in PBS) at 37 °C for 3 min and then blocked with 3 % normal goat serum for 30 min. After washing in PBS, the tissues were incubated overnight at 4 °C with primary antibodies or matched concentrations of rabbit and mouse IgG as negative controls. For verification of specific staining patterns, non-specific binding was determined by omitting primary or secondary antibodies Sections were washed with PBS and incubated for 1 h with a 1:200 dilution of appropriate secondary antibody (goat anti-rabbit IgG antibody conjugated to fluor Alexa 488 or goat anti-mouse IgG antibody conjugated to Alexa 594 [Life Technologies]. DAPI, (4′,6-diamidino-2-phenylindole dihydrochloride) [Sigma;D9542] (1 ng/µl) was added 20 s prior to mounting the sections with Dako fluorescence mounting medium and sealing under coverslip. The slides were examined sequentially using the Leica TCS SP5 microscope with the 488 nm laser for Alexa 488 (emission window 496–533 nm) and the 561 nm laser (emission window 596–763 nm) for Alexa 594. For quantitation, 3 random areas (each 0.038 mm2) per section (total of 16 sections) were analysed for fluorescence intensity using the AnalySIS software package (Soft Imaging System GmbH, Munster Germany).
Quantitative polymerase chain reaction
Total RNA was extracted from frozen normal and fibrotic tissues with an UltraClear Tissue and Cells RNA Isolation Kit according to the manufacturer’s instruction (Mo Bio Laboratories, Inc., Carlsbad; CA, USA). RNA concentration was determined at 260 nm using the NanoDrop 2000C spectrophotometer (Thermo Fisher Scientific, USA). cDNA was prepared from reverse transcribed mRNA using the SuperScript III Reverse Transcriptase Synthesis System according to the manufacturer’s instruction (Invitrogen, Mount Waverley, Vic., Australia). Briefly, to each 50 µl RNA sample, 12.5 µl of random hexamers (100 µg/ml) and 12.5 µl of 10 mM dNTP mix (10 mM each of dATP, dGTP, dCTP, dTTP) were added. The samples were incubated at 65 °C for 5 min prior to placing on ice for at least 60 s. The sample was made up to a volume of 100 µl in 1 × First-strand buffer, 0.01 M DTT, and 100U of Superscript III. The samples were incubated at room temperature for 5 min, then at 50 °C for 2 h. The Superscript was inactivated by heating to 70 °C for 15 min, and the resultant cDNA was stored at −80 °C. Real-time PCR was then performed using the fluorescent dye SYBR green. Each 25 µl reaction contained 400 nM of each primer and template DNA (up to 1 µg) in SYBR Green Master Mix (#11733-038, Invitrogen, Mount Waverley, VIC, Australia). Real-time PCR was performed on a Corbett Rotorgene 6000 (Qiagen) to measure the mRNA expression of LTBP-2, FGF-2, TGF-β and RNAPolII control. Primer sequences used were as follows: LTBP-2 forward primer 5′-GGGCACCGCACCACCTACACG-3′, and reverse primer 5′-TCATCACACTCATTCACATCTACG-3′; FGF-2 forward primer 5′-GCGGGAGGCTGGTGGGTGTG-3′, and reverse primer 5′-CAAGGCGGGCAGCGTGGTG-3′; TGF-β forward primer 5′-CTCCGAGAAGCGGTACCTGAAC-3′, and reverse primer 5′-CACTTGCAGTGTGTTATCCCT; RNAPolII forward primer 5′- AGGGGCTAACAATGGACACC-3′, and reverse primer 5′-CCGAAGATAAGGGGAACTACT-3′. The primers were all obtained from GeneWorks, Adelaide, Australia. The PCR protocol was a hot start at 95 °C for 10 min, followed by 40 cycles of 95 °C for 20 s, 60 °C for 20 s, and 72 °C for 45 s, with fluorescence measurement at the end of each 72 °C amplification step. A melt curve was performed after 40 cycles to confirm the correct number of PCR products and screen for any contamination. Results were analysed using the 2(-ΔΔC(T)) method as described by Livak and Schmittgen (2001). Briefly, the mRNA expression in each sample was determined by comparing the cycle threshold (Ct) for the gene of interest and the Ct for RNAPolII.
Statistical analysis
All data are expressed as mean ± SD. The quantitative immunostaining and qPCR were statistically analysed by ‘Student’s’ t test using GraphPad Prism 6 software. A p value ≤0.05 was regarded as statistically significant.
Results
LTBP-2 and FGF-2 do not co-localize in fibroblast cell culture
Our previous in vitro studies found that LTBP-2 has a single binding site for FGF-2 and is able to inhibit FGF-2 induced proliferation (Menz et al. 2015). To determine if the interaction of LTBP-2 and FGF-2 could have biological importance in vivo, we first compared immunolocalisation patterns in human skin fibroblasts. Human foreskin fibroblast (HFF) cells were cultured for 21 days and stained with LTBP-2 and FGF-2 antibodies. LTBP-2 was strongly detected in the fibrous meshwork of the extracellular matrix (Fig. 1a, b) while the rabbit IgG controls showed minimal background staining (Fig. 1c). FGF-2 revealed a different staining pattern to LTBP-2 and was mainly localised intracellularly (Fig. 1d, e) while mouse IgG controls showed minimal non-specific staining (Fig. 1f). These findings are consistent with previous studies that suggest FGF-2 is stored intracellularly and only secreted from cells following tissue insult to become strongly bound to the GAG side-chains of HSPGs in the interstitial matrix and basement membranes (Kardami et al. 2007; Schultz and Wysocki 2009). Since the staining patterns of the two antibodies did not overlap, no co-localisation between LTBP-2 and FGF-2 was evident in the fibroblast cultures.
Immunofluorescence staining for LTBP-2 and FGF-2 in human foreskin fibroblast (HFF) cultures. HFFs were cultured for 21 days and the cell layer was fixed then incubated with antibodies to LTBP-2 or FGF-2 or with control IgG. Primary antibody was detected using an appropriate secondary antibody conjugated to fluorescein isothiocyanate (FITC) prior to analysis on the Leicia SP5 spectral scanning confocal microscope (see “Materials and methods” section). a, b Anti-[human LTBP-2 peptide] antibody 3504 (2.08 µg/ml), d, e anti-[human FGF-2] antibody (2.5 µg/ml), c rabbit IgG control (2.08 µg/ml); f mouse IgG control (2.5 µg/ml). Magnification: a and d Bar = 250 µM; b, c, e and f Bar = 100 µM
LTBP-2 and FGF-2 show extensive co-localization in fibrotic skin lesions
Since the fibroblast cultures proved not to be a suitable model for studying the potential interaction of LTBP-2 and FGF-2, immunohistochemical analysis was undertaken in human fibrotic skin samples. To determine the distribution patterns of LTBP-2 and FGF-2, sections were prepared and immunostained with dual combinations of anti-(LTBP-2) antibody (Fig. 2a, f, k, p) and anti-(FGF-2) antibody (Fig. 2b, g, l, q).
LTBP-2 and FGF-2 co-localize in fibrotic skin lesions. Tissue sections from normal adult skin (a–e), mature scar (f–j) HTS (k–p) and keloid (q–v), were incubated with antibodies to LTBP-2 and FGF-2 and analysed by confocal microscopy as described in “Materials and methods”. Tissue from 4 patients in each group was examined and a representative example from each group is shown. Normal skin; cranial skin, male 6 y.o.; Normal scar, abdominal skin (surgery) male 1 y.o.: HTS; hand (burn injury) female 27 y.o.: keloid; ear lobe (ear piercing), male 18 y.o. a, f, k, and q polyclonal anti-[human LTBP-2 peptide] antibody 3504 (2.08 µg/ml) detected with anti-rabbit IgG antibody conjugated to fluor Alexa 488; b, g, l and r monoclonal anti-[human FGF-2] antibody (2.5 µg/ml) detected with anti-mouse IgG antibody conjugated to Alexa 594; c (a) and (b) merged; h (f) and (g) merged; m (k), (l) and (n) merged; (s), (q), (r) and (t) merged; d, i, o and u rabbit IgG control (2.08 µg/ml); e, j, p and v mouse IgG control (2.5 µg/ml). n and t DAPI (1 ng/µl). Magnification: Bar = 25 µM
The staining patterns for both LTBP-2 and FGF-2 were dull and weak in normal skin tissues indicating only low levels of the two proteins were present (Fig. 2a, b). In normal scar (Fig. 2f, g), stronger staining intensity for both proteins was observed compared to normal skin. Interestingly, similar staining patterns for LTBP-2 and FGF-2 were found on irregular fibrous networks within the tissue matrix. The regions of co-localisation were confirmed from the merged images (Fig. 2c, h), visualized as yellow–orange. Examination of control sections showed negligible non-specific mouse and rabbit IgG staining (Fig. 2d, e, i and j), thus confirming that the staining patterns for LTBP-2 and FGF-2 were specific. In HTS and keloid samples there was extensive staining for LTBP-2 (green) (Fig. 2k, q) and FGF-2 (red) (Fig. 2l, r) with similar widespread, fibrous distributions. The merged images showed strong co-localisation visualised as yellow staining (Fig. 2m, s). There was also some intracellular staining adjacent to nuclei visualised by the DAPI staining (Fig. 2n, t). Again, control sections treated with mouse or rabbit IgG showed negligible background signals (Fig. 2n, o, s, t). Additional controls were performed to ensure that the two primary antibodies were showing specific staining patterns. Firstly immunoblotting of purified LTBP-2 and FGF-2 with anti-LTBP-2 and anti-FGF-2 antibodies showed no cross-staining of the proteins. Moreover additional confocal controls included incubation of sections with one primary antibody together with both secondary antibodies. For each primary antibody, signal was only detected in the correct channel. This eliminated any possibility of the secondary antibodies cross-reacting with the reciprocal primary or secondary antibody, or of signal bleed—through between the two detection channels (Supplementary data in Online Resource 1).
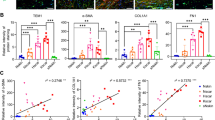
Quantitation of the relative immunofluorescence signals in normal and fibrotic sections was performed. For each category, a section from four different patients was stained, imaged and quantified (Fig. 3). All image processing and analyses were performed using the AnalySIS software package (Soft Imaging System GmbH, Munster Germany) which was kept consistent between images. Three random areas (each 0.038 mm2) were analysed per section, therefore for each category at least 12 areas were analysed. Representative confocal immunofluorescence images in Fig. 3 showed approximately fourfold increases in signal for both LTBP-2 and FGF-2 in HTS and keloid samples compared to normal skin. Signals in normal scar were only slightly higher than in normal skin. Overall, the overlay analysis of dual-labelled sections with LTBP-2 and FGF-2 indicated there is an extensive co-distribution of both proteins in HTS and keloid sections.
Quantitative immunofluorescence analysis of LTBP-2 and FGF-2 in normal skin, mature scar, hypertrophic scar and keloid tissue. The relative fluorescence intensities were quantitated using the Analysis Software package (Soft-Imaging System, Munster, Germany) sampled from 3 random areas (each 0.038 mm2) per section from each of 4 patients per group. LTBP-2 (white columns), FGF-2 (black columns), rabbit IgG control (cross-hatched) and mouse IgG controls (grid) in sections of normal skin, normal scar, HTS and keloids. Values expressed relative to the background signal (=1 unit). Mean values ± SD of 12 determinations are shown
LTBP-2 shows similar distribution with elastin and fibrillin-1 in fibrotic skin
Since LTBP-2 has been documented to be associated with elastic fibres, HTS and keloid tissues were also dual stained for LTBP-2 and fibrillin-1 or elastin (Fig. 4). LTBP-2 showed extensive co-localization with fibrillin-1 and elastin in both tissue types (Fig. 4d, h, l, p). All three proteins showed staining of irregular fibres in the matrix between cells in both HTS and keloid samples. Control sections showed a negligible signal (data not shown). These findings are consistent with previous studies showing abundant association of LTBP-2 with fibrillin microfibrils in developing human foetal aorta and other tissues (Gibson et al. 1995). Since LTBP-2 showed almost complete co-localisation with elastin, the results point to LTBP-2 being predominantly associated with elastic fibres rather than elastin-free fibrillin–microfibril bundles abundant in normal skin. Thus it is apparent that both LTBP-2 and FGF-2 have association with fibrillin-1-microfibrils of elastic fibres in fibrotic skin lesions.
LTBP-2 co-localizes with fibrillin-1 and elastin in fibrotic skin. To identify the fibrous material immunostaining for LTBP-2 and FGF-2 in Fig. 2, HTS (a–h) and keloid (i–p) tissues were also co-stained with LTBP-2 for fibrillin-1 and elastin by confocal microscopy. a, e, i and m Anti-LTBP-2 antibody 3504 (2.08 µg/ml) detected with anti-rabbit IgG antibody conjugated to fluor Alexa 488; b and j monoclonal anti-elastin antibody BA4 (0.96 µg/ml) detected with anti-mouse IgG antibody conjugated to Alexa 594; f and n monoclonal anti-[fibrillin-1] antibody detected with anti-mouse IgG antibody conjugated to Alexa 594; c, g, k and o DAPI nuclear stain. d, (a), (b) and (c) merged; h, (e), (f) and (g) merged; l, (i), (j) and (k) merged, p, (m), (n) and (o) merged. Magnification: Bar = 10 µM
Both LTBP-2 and FGF-2 mRNA expression levels are greatly elevated in hypertrophic scar
The relative expression of LTBP-2 and FGF-2 was compared to that of TGF-β in normal and fibrotic skin samples using real-time quantitative PCR (Fig. 5). The mRNA was harvested from the tissues and reverse transcribed into cDNA. Quantitative PCR was performed using primers specific to LTBP-2, FGF-2 and TGF-β, as well as primers for the housekeeping gene RNAPolII. The mRNA levels for LTBP-2 and FGF-2 were elevated drastically in HTS (12- and 7-fold increases respectively) (black columns) compared to normal skin (cross-hatched). The mRNA levels of LTBP-2 and FGF-2 significantly increased in normal scars (white columns), however the increase is much less than that for HTS. In addition, mRNA levels of TGF-β were also elevated in HTS samples.
The expression levels of mRNA for LTBP-2, FGF-2 and TGF-β in fibrotic skin tissues. Total RNA was extracted from snap-frozen normal skin (cross-hatched), mature scars (white columns) and HTS (black columns) from 4 patients in each category, then reverse transcribed into cDNA for quantitation by qPCR as described in “Materials and methods”. The LTBP-2, FGF-2 and TGF-β signals were normalised to the RNAPolII control signal = 1. Mean values ± SD of 12 determinations are shown
Discussion
The key mediator of tissue fibrosis is considered to be the activated fibroblast which produces an accumulation of ECM, mainly collagen, resulting in functional impairment and structural distortion of affected organs and tissues (Krenning et al. 2010). This process is regulated by a number of cytokines and growth factors but primarily by TGF-β (Meng et al. 2015). Another growth factor that contributes to fibrosis is FGF-2, which paradoxically appears to have an anti-fibrotic role in fibrotic skin conditions. The present study investigated the expression and localisation of the FGF-2 binding protein, LTBP-2, in a variety of fibrotic skin conditions. To the best of our knowledge, this is the first study showing consistent upregulation and co-localisation of LTBP-2 with FGF-2 in keloid and HTS, suggesting both proteins may play important interactive roles during tissue repair, fibrosis and scarring.
Our results showed that LTBP-2 and FGF-2 had no overlap in distribution in cultures of normal skin fibroblasts, LTBP-2 showing a fibrous staining pattern in the extracellular matrix and FGF-2 being largely intracellular. The result is consistent with studies showing FGF-2, which lacks a secretion signal (Yu et al. 2007), is stored intracellularly (Chua et al. 2004) and is only released at the time of injury or tissue remodelling (Suga et al. 2009). In normal skin, staining intensity was low for both LTBP-2 and FGF-2 whereas in normal scars slightly more intense signals were detected to dermal fibres suggesting possible direct interaction between LTBP-2 and FGF-2. To pursue this idea further, we investigated fibrotic skin lesions, namely HTS and keloid samples, where expression and extracellular localisation of FGF-2 were anticipated to be greater. Both HTS and keloid tissues represent abnormal wound healing resulting in increased fibrosis, including excessive collagen production by fibroblasts (Aarabi et al. 2007; Verhaegen et al. 2009). Both lesions can be firm, itchy, raised and painful, causing patient physical and psychological distress (Bayat et al. 2003; Bock et al. 2006). The main clinical differences between the two types of lesion are that HTS occurs within 4–8 weeks after surgery or injury and remains confined within the boundaries of the original wound (Aarabi et al. 2007). In contrast, keloid scars develop several months after an initial trauma and the overgrowth of fibrosis extends beyond the boundaries of the original injury (Verhaegen et al. 2009). Aside from clinical features, HTS and keloids also can be distinguished by established histo-pathological criteria. HTS are generally characterized by the presence of nodular structures up-regulated by alpha-smooth muscle actin (α-SMA)-expressing myofibroblasts and fine collagen fibres that run parallel the epithelial surface. However in keloids, (α-SMA)-expressing myofibroblasts are absent and collagen bundles are randomly orientated to the epithelial surface (Bayat et al. 2003; Bock et al. 2006; Aarabi et al. 2007; Verhaegen et al. 2009). In addition, keloids tend to indicate a greater genetic predisposition than HTS (Marneros et al. 2004; Nakashima et al. 2010; Shih and Bayat 2010). Interestingly, we found extensive expression and co-localization of both LTBP-2 and FGF-2 within HTS and keloid skin samples indicating high synthesis rates for both proteins during fibrosis. The close proximity of FGF-2 to LTBP-2 within the fibrotic tissues suggest the two proteins may directly interact during fibrosis and may be involved in mediating wound healing outcomes.
Our data in this study were in agreement with the reports that FGF-2 production is up-regulated during fibrosis and wound healing (Kurita et al. 1992; Floege et al. 1999; Strutz et al. 2000). FGF-2 is a potent mitogen of endothelial cells and fibroblasts, and has the ability to induce angiogenesis following tissue damage (Kardami et al. 2007). Moreover, FGF-2 acts as a survival factor in many models of cell and tissue injury (Bikfalvi et al. 1997). Suga et al. (2009) found that FGF-2 is released at the time of injury and reduces post-injury fibrogenesis. Administration of recombinant FGF-2 to skin wounds inhibits hypertrophy and widening of scars (Ono et al. 2007). Moreover, FGF-2 has been found to accelerate acute and chronic wound healing in both animal models and clinical use (Bikfalvi et al. 1997; Fu et al. 2000; Akita et al. 2008; Tan et al. 2008). In addition, healing of excisional skin wounds is delayed in FGF-2-knockout mice suggesting that FGF-2 is an essential component of wound healing and scar reduction (Ortega et al. 1998). Shi et al. (2013) demonstrated that FGF-2 promoted down-regulation of TGF-β1 activity by inhibiting the SMAD2/SMAD3 signalling system of the TGF-β1/SMAD-dependent pathway. Moreover, FGF-2 significantly reduced the expression of α-smooth muscle actin and increased apoptosis in granulation tissue cells (Eto et al. 2012; Shi et al. 2013). More recently, Kashpur et al. (2013) demonstrated that FGF-2 activates signalling pathways that lead to upregulation of MMP1, the metalloproteinase responsible for cleaving collagen, and improving the healing process by reducing scar tissue formation. FGF-2 treatment also significantly reduces IL-6 levels, a cytokine that is known to increase production of collagen and excessive ECM during fibrosis (Duncan and Berman 1991; Ray et al. 2013).
We have recently reported that LTBP-2 has a single binding site for FGF-2 and that it inhibits FGF-2 induced fibroblast proliferation in vitro. Moreover preliminary investigation of keloid tissue from a single patient showed strong co-localisation of the two proteins in the fibrotic matrix suggesting the two proteins may interact in tissues. The proteins gave a fibrous distribution pattern which also co-stained for fibrillin-1 (Menz et al. 2015). We have previously shown that LTBP-2 has widespread association with fibrillin-1 and co-localizes with elastin-associated microfibrils during development in tissues such as aorta and elastic ligaments (Hirani et al. 2007). We have also demonstrated that exogenous LTBP-2 inhibits elastinogenesis in ear cartilage chondrocyte cultures indicating LTBP-2 may have a negative regulatory role during elastinogenesis (Sideek et al. 2014). The present study showed that in normal skin little LTBP-2 and FGF-2 were evident in the extracellular matrix. However both proteins were greatly upregulated in keloid tissue and consistently stained elastic fibres rather than elastin-free fibrillin–microfibrils in HTS and keloid (Amadeu et al. 2004; Sidgwick and Bayat 2012; Jumper et al. 2015). The results suggest that both LTBP-2 and FGF-2 are associated with the microfibril component of elastic fibres in these lesions. The findings are consistent with studies indicating that elastin is increased in fibrotic lesions whereas total fibrillin-1 (elastin-free plus elastin-associated) is reduced compared to normal skin. Fibrillin-1 gene mutations resulting in disrupted microfibril synthesis cause both Marfan syndrome and WMS and both phenotypes are believed to involve aberrant TGF-β signalling (Faivre et al. 2003; Haji-Seyed-Javadi et al. 2012). Interestingly, LTBP-2 mutations also cause WMS and Marfan-like traits have been observed in LTBP-2 null humans. Since LTBP-2 is the only component of elastic fibres known to bind FGF-2, upregulation of LTBP-2 in fibrotic disorders may influence the activity of FGF-2 in repair and disease processes (Ikeda et al. 2009). FGF-2 can influence TGF-β activity and depending on context, FGF-2 may activate signalling pathways leading to TGF-β elevation or it can inhibit TGF-β induced fibrosis (Phillips et al. 1997; Dhandapani et al. 2007; Shi et al. 2013). The LTBP-2 has also been reported as a pleiotropic tumour suppressor in nasopharyngeal carcinoma and as a marker for pulmonary deaths following acute dyspnea (Chan et al. 2011). A role for LTBP-2 -FGF-2 interactions in WMS and other diseases remains to be investigated.
In our previous study we found LTBP-2 blocked FGF-2-induced cell proliferation indicating LTBP-2 is a negative modulator of FGF-2 activity (Menz et al. 2015). Sinha et al. (2002) reported that LTBP-2 synthesis increased following injury in the arteries of porcine model suggesting role of LTBP-2 in tissue repair process. Moreover, significant elevation of LTBP-2 has been observed in failing heart accompanied by obvious ECM remodelling and fibrosis, suggesting LTBP-2 may directly contribute to the regulation of cardiac ECM and be involved in fibrosis causing heart failure (Bai et al. 2012). The finding was consistent with our present studies where LTBP-2 is highly elevated in the matrix of HTS and keloid tissues. Since FGF-2 exhibits repair/regenerative and anti-fibrotic effects, it is possible that elevated LTBP-2 exacerbates the fibrotic process by binding active FGF-2 in the repairing tissues, modulating FGF-2’s contribution to wound repair. Following tissue injury, multiple FGF-2 molecules are released, by proteases and heparanases, complexed to individual heparan sulfate chains and these complexes bind with cell-surface FGF-receptors causing clustering of the FGFR molecules. This enhances intracellular signalling involving RAS/MAPK, PI3 k/Akt, PLC-γ, and ERK1/2 pathways (Kardami et al. 2007; Yu et al. 2007; Schultz and Wysocki 2009). Interestingly, we have identified multiple high-affinity binding sites for heparin and HSPGs, syndecan-4 and perlecan on LTBP-2 (Parsi et al. 2010), including a site adjacent to the FGF-2 binding site (Menz et al. 2015). Thus it is possible that LTBP-2 may also attract and bind the heparin sulphate-bound FGF-2, affecting the anti-fibrotic effects of FGF-2-heparan sulphate complexes.
Recently a combined study using immunofluorescence and siRNA knockdown technology in cellular and animal models has identified multidrug resistance-associated protein 1 (MRP1) as a novel contributor to the fibrotic process in hypertrophic scar (Li et al. 2015). A similar approach could be useful to define the mechanism of LTBP-2 and FGF-2 interactions in tissues and to determine the extent of their influence on wound healing and fibrosis.
In conclusion, this present study highlights consistent upregulation and co-localization of LTBP-2 and FGF-2 in fibrotic skin lesions from multiple patients. Since LTBP-2 strongly binds and inhibits FGF-2 activity in vitro, our study suggests that LTBP-2 may bind and modulate FGF-2 effects in wound repair and contribute to fibrotic diseases.
References
Aarabi S, Longaker MT, Gurtner GC (2007) Hypertrophic scar formation following burns and trauma: new approaches to treatment. PLoS Med 4:e234. doi:10.1371/journal.pmed.0040234
Akhurst RJ, Hata A (2012) Targeting the TGFbeta signalling pathway in disease. Nat Rev Drug Discov 11:790–811. doi:10.1038/nrd3810
Akita S, Akino K, Imaizumi T, Hirano A (2008) Basic fibroblast growth factor accelerates and improves second-degree burn wound healing. Wound Repair Regen Off Publ Wound Heal Soc Euro Tissue Repair Soc 16:635–641. doi:10.1111/j.1524-475X.2008.00414.x
Ali M et al (2009) Null mutations in LTBP2 cause primary congenital glaucoma. Am J Hum Genet 84:664–671. doi:10.1016/j.ajhg.2009.03.017
Amadeu TP, Braune AS, Porto LC, Desmouliere A, Costa AM (2004) Fibrillin-1 and elastin are differentially expressed in hypertrophic scars and keloids. Wound Repair Regen Off Publ Wound Heal Soc Euro Tissue Repair Soc 12:169–174. doi:10.1111/j.1067-1927.2004.012209.x
Bai Y, Zhang P, Zhang X, Huang J, Hu S, Wei Y (2012) LTBP-2 acts as a novel marker in human heart failure—a preliminary study. Biomark Biochem Indic Expo Resp Susceptibility Chem 17:407–415. doi:10.3109/1354750X.2012.677860
Bayat A, McGrouther DA, Ferguson MW (2003) Skin scarring. BMJ (Clin Res ed) 326:88–92
Bikfalvi A, Klein S, Pintucci G, Rifkin DB (1997) Biological roles of fibroblast growth factor-2. Endocr Rev 18:26–45. doi:10.1210/edrv.18.1.0292
Bock O, Schmid-Ott G, Malewski P, Mrowietz U (2006) Quality of life of patients with keloid and hypertrophic scarring. Arch Dermatol Res 297:433–438. doi:10.1007/s00403-006-0651-7
Brown KK, Wells AU (2008) Recent clinical trials in idiopathic pulmonary fibrosis and the BUILD-1 study. Euro Respir Rev 17:116–122. doi:10.1183/09059180.00010903
Chan SH et al (2011) The ECM protein LTBP-2 is a suppressor of esophageal squamous cell carcinoma tumor formation but higher tumor expression associates with poor patient outcome. Int J Cancer 129:565–573. doi:10.1002/ijc.25698
Chua CC, Rahimi N, Forsten-Williams K, Nugent MA (2004) Heparan sulfate proteoglycans function as receptors for fibroblast growth factor-2 activation of extracellular signal-regulated kinases 1 and 2. Circ Res 94:316–323. doi:10.1161/01.RES.0000112965.70691.AC
Daian T et al (2003) Insulin-like growth factor-I enhances transforming growth factor-[beta]-induced extracellular matrix protein production through the P38/activating transcription factor-2 signaling pathway in keloid fibroblasts. J Investig Dermatol 120:956–962
de Vega S, Iwamoto T, Yamada Y (2009) Fibulins: multiple roles in matrix structures and tissue functions. Cell Mol Life Sci 66:1890–1902. doi:10.1007/s00018-009-8632-6
Dhandapani KM, Khan MM, Wade FM, Wakade C, Mahesh VB, Brann DW (2007) Induction of transforming growth factor-beta1 by basic fibroblast growth factor in rat C6 glioma cells and astrocytes is mediated by MEK/ERK signaling and AP-1 activation. J Neurosci Res 85:1033–1045. doi:10.1002/jnr.21182
Duncan MR, Berman B (1991) Stimulation of collagen and glycosaminoglycan production in cultured human adult dermal fibroblasts by recombinant human interleukin 6. J Invest Dermatol 97:686–692
Eto H et al (2012) Therapeutic potential of fibroblast growth factor-2 for hypertrophic scars: upregulation of MMP-1 and HGF expression. Lab Invest 92:214–223. doi:10.1038/labinvest.2011.127
Faivre L et al (2003) In frame fibrillin-1 gene deletion in autosomal dominant Weill–Marchesani syndrome. J Med Genet 40:34–36
Floege J, Hudkins KL, Eitner F, Cui Y, Morrison RS, Schelling MA, Alpers CE (1999) Localization of fibroblast growth factor-2 (basic FGF) and FGF receptor-1 in adult human kidney1. Kidney Int 56:883–897
Fu X, Shen Z, Chen Y, Xie J, Guo Z, Zhang M, Sheng Z (2000) Recombinant bovine basic fibroblast growth factor accelerates wound healing in patients with burns, donor sites and chronic dermal ulcers. Chin Med J 113:367–371
Gibson MA, Hatzinikolas G, Davis EC, Baker E, Sutherland GR, Mecham RP (1995) Bovine latent transforming growth factor beta 1-binding protein 2: molecular cloning, identification of tissue isoforms, and immunolocalization to elastin-associated microfibrils. Mol Cell Biol 15:6932–6942
Goodwin A, Jenkins G (2009) Role of integrin-mediated TGFbeta activation in the pathogenesis of pulmonary fibrosis. Biochem Soc Trans 37:849–854. doi:10.1042/BST0370849
Haji-Seyed-Javadi R et al (2012) LTBP2 mutations cause Weill–Marchesani and Weill–Marchesani-like syndrome and affect disruptions in the extracellular matrix. Hum Mutat 33:1182–1187. doi:10.1002/humu.22105
Hirani R, Hanssen E, Gibson MA (2007) LTBP-2 specifically interacts with the amino-terminal region of fibrillin-1 and competes with LTBP-1 for binding to this microfibrillar protein. Matrix Biol J Int Soc Matrix Biol 26:213–223. doi:10.1016/j.matbio.2006.12.006
Ikeda M et al (2009) Elastic fiber assembly is disrupted by excessive accumulation of chondroitin sulfate in the human dermal fibrotic disease, keloid. Biochem Biophys Res Commun 390:1221–1228. doi:10.1016/j.bbrc.2009.10.125
Jumper N, Paus R, Bayat A (2015) Functional histopathology of keloid disease. Histol Histopathol 30:1033–1057. doi:10.14670/HH-11-624
Kardami E, Detillieux K, Ma X, Jiang Z, Santiago JJ, Jimenez SK, Cattini PA (2007) Fibroblast growth factor-2 and cardioprotection. Heart Fail Rev 12:267–277. doi:10.1007/s10741-007-9027-0
Kashpur O, LaPointe D, Ambady S, Ryder EF, Dominko T (2013) FGF2-induced effects on transcriptome associated with regeneration competence in adult human fibroblasts. BMC Genom 14:656. doi:10.1186/1471-2164-14-656
Kissin EY, Korn JH (2003) Fibrosis in scleroderma. Rheum Dis Clin North Am 29:351–369
Kopecki Z et al (2013) Topically applied flightless I neutralizing antibodies improve healing of blistered skin in a murine model of epidermolysis bullosa acquisita. J Invest Dermatol 133:1008–1016. doi:10.1038/jid.2012.457
Krenning G, Zeisberg EM, Kalluri R (2010) The origin of fibroblasts and mechanism of cardiac fibrosis. J Cell Physiol 225:631–637. doi:10.1002/jcp.22322
Kurita Y, Tsuboi R, Ueki R, Rifkin DB, Ogawa H (1992) Immunohistochemical localization of basic fibroblast growth factor in wound healing sites of mouse skin. Arch Dermatol Res 284:193–197
Li Y et al (2015) MRP1 knockdown down-regulates the deposition of collagen and leads to a reduced hypertrophic scar fibrosis. J Mol Histol 46:357–364. doi:10.1007/s10735-015-9629-0
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods (San Diego, CA) 25:402–408. doi:10.1006/meth.2001.1262
Marneros AG, Norris JE, Watanabe S, Reichenberger E, Olsen BR (2004) Genome scans provide evidence for keloid susceptibility loci on chromosomes 2q23 and 7p11. J Invest Dermatol 122:1126–1132. doi:10.1111/j.0022-202X.2004.22327.x
Meng XM, Tang PM, Li J, Lan HY (2015) TGF-beta/Smad signaling in renal fibrosis. Front Physiol 6:82. doi:10.3389/fphys.2015.00082
Menz C, Parsi MK, Adams JR, Sideek MA, Kopecki Z, Cowin AJ, Gibson MA (2015) LTBP-2 has a single high-affinity binding site for FGF-2 and blocks FGF-2-induced cell proliferation. PLoS ONE 10:e0135577. doi:10.1371/journal.pone.0135577
Moren A et al (1994) Identification and characterization of LTBP-2, a novel latent transforming growth factor-beta-binding protein. J Biol Chem 269:32469–32478
Nakashima M et al (2010) A genome-wide association study identifies four susceptibility loci for keloid in the Japanese population. Nat Genetics 42:768–771. doi:10.1038/ng.645
Ono I, Akasaka Y, Kikuchi R, Sakemoto A, Kamiya T, Yamashita T, Jimbow K (2007) Basic fibroblast growth factor reduces scar formation in acute incisional wounds. Wound Repair Regen Off Publ Wound Heal Soc Euro Tissue Repair Soc 15:617–623. doi:10.1111/j.1524-475X.2007.00293.x
Ortega S, Ittmann M, Tsang SH, Ehrlich M, Basilico C (1998) Neuronal defects and delayed wound healing in mice lacking fibroblast growth factor 2. Proc Natl Acad Sci USA 95:5672–5677
Parsi MK, Adams JR, Whitelock J, Gibson MA (2010) LTBP-2 has multiple heparin/heparan sulfate binding sites. Matrix Biol J Int Soc Matrix Biol 29:393–401. doi:10.1016/j.matbio.2010.03.005
Phillips AO, Topley N, Morrisey K, Williams JD, Steadman R (1997) Basic fibroblast growth factor stimulates the release of preformed transforming growth factor beta 1 from human proximal tubular cells in the absence of de novo gene transcription or mRNA translation. Lab Investig J Tech Methods Pathol 76:591–600
Pohlers D et al (2009) TGF-beta and fibrosis in different organs—molecular pathway imprints. Biochim Biophys Acta 1792:746–756. doi:10.1016/j.bbadis.2009.06.004
Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G (2006) Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 174:810–816. doi:10.1164/rccm.200602-163OC
Rastegar A, Kashgarian M (1998) The clinical spectrum of tubulointerstitial nephritis. Kidney Int 54:313–327. doi:10.1046/j.1523-1755.1998.00001.x
Ray S, Ju X, Sun H, Finnerty CC, Herndon DN, Brasier AR (2013) The IL-6 trans-signaling-STAT3 pathway mediates ECM and cellular proliferation in fibroblasts from hypertrophic scar. J Invest Dermatol 133:1212–1220. doi:10.1038/jid.2012.499
Saharinen J, Keski-Oja J (2000) Specific sequence motif of 8-Cys repeats of TGF-beta binding proteins, LTBPs, creates a hydrophobic interaction surface for binding of small latent TGF-beta. Mol Biol Cell 11:2691–2704
Schultz GS, Wysocki A (2009) Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen 17:153–162. doi:10.1111/j.1524-475X.2009.00466.x
Shi Y, Massague J (2003) Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 113:685–700
Shi HX et al (2013) The anti-scar effects of basic fibroblast growth factor on the wound repair in vitro and in vivo. PLoS ONE 8:e59966. doi:10.1371/journal.pone.0059966
Shih B, Bayat A (2010) Genetics of keloid scarring. Arch Dermatol Res 302:319–339. doi:10.1007/s00403-009-1014-y
Sideek MA, Menz C, Parsi MK, Gibson MA (2014) LTBP-2 competes with tropoelastin for binding to fibulin-5 and heparin, and is a negative modulator of elastinogenesis. Matrix Biol J Int Soc Matrix Biol 34:114–123. doi:10.1016/j.matbio.2013.10.007
Sidgwick GP, Bayat A (2012) Extracellular matrix molecules implicated in hypertrophic and keloid scarring. J Euro Acad Dermatol Venereol 26:141–152. doi:10.1111/j.1468-3083.2011.04200.x
Sinha S, Heagerty AM, Shuttleworth CA, Kielty CM (2002) Expression of latent TGF-beta binding proteins and association with TGF-beta 1 and fibrillin-1 following arterial injury. Cardiovasc Res 53:971–983
Strutz F, Neilson EG (2003) New insights into mechanisms of fibrosis in immune renal injury. Springer Semin Immunopathol 24:459–476. doi:10.1007/s00281-003-0123-5
Strutz F, Zeisberg M, Hemmerlein B, Sattler B, Hummel K, Becker V, Muller GA (2000) Basic fibroblast growth factor expression is increased in human renal fibrogenesis and may mediate autocrine fibroblast proliferation. Kidney Int 57:1521–1538
Suga H et al. (2009) IFATS collection: fibroblast growth factor-2-induced hepatocyte growth factor secretion by adipose-derived stromal cells inhibits postinjury fibrogenesis through a c-Jun N-terminal kinase-dependent mechanism. Stem Cells (Dayton, Ohio) 27:238–249. doi:10.1634/stemcells.2008-0261
Tan Y et al (2008) Comparison of the therapeutic effects recombinant human acidic and basic fibroblast growth factors in wound healing in diabetic patients. J Health Sci 54:432–440. doi:10.1248/jhs.54.432
Thannickal VJ, Zhou Y, Gaggar A, Duncan SR (2014) Fibrosis: ultimate and proximate causes. J Clin Invest 124:4673–4677. doi:10.1172/JCI74368
Verhaegen PD, van Zuijlen PP, Pennings NM, van Marle J, Niessen FB, van der Horst CM, Middelkoop E (2009) Differences in collagen architecture between keloid, hypertrophic scar, normotrophic scar, and normal skin: an objective histopathological analysis. Wound Repair Regen Off Publ Wound Heal Soc Euro Tissue Repair Soc 17:649–656. doi:10.1111/j.1524-475X.2009.00533.x
Wynn TA (2004) Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol 4:583–594. doi:10.1038/nri1412
Wynn TA (2007) Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest 117:524–529. doi:10.1172/JCI31487
Wynn TA (2008) Cellular and molecular mechanisms of fibrosis. J Pathol 214:199–210. doi:10.1002/path.2277
Xie JL et al (2008) Basic fibroblast growth factor (bFGF) alleviates the scar of the rabbit ear model in wound healing. Wound Repair Regen Off Publ Wound Heal Soc Euro Tissue Repair Soc 16:576–581. doi:10.1111/j.1524-475X.2008.00405.x
Yu PJ, Ferrari G, Galloway AC, Mignatti P, Pintucci G (2007) Basic fibroblast growth factor (FGF-2): the high molecular weight forms come of age. J Cell Biochem 100:1100–1108. doi:10.1002/jcb.21116
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sideek, M.A., Teia, A., Kopecki, Z. et al. Co-localization of LTBP-2 with FGF-2 in fibrotic human keloid and hypertrophic scar. J Mol Hist 47, 35–45 (2016). https://doi.org/10.1007/s10735-015-9645-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-015-9645-0