Abstract
Multidrug resistance-associated protein 1 (MRP1) belongs to ATP-binding cassette transporters family. The overexpression of MRP1 is predominantly related with the failure of chemo-radiotherapy in various tumors. However, its possible role in hypertrophic scar (HS) is hardly investigated. Here we showed that the mRNA level and protein expression of MRP1 were higher in HS and HS derived fibroblasts (HSFs) than that in normal skin (NS) and NS derived fibroblasts (NSFs). Immunohistochemistry and immunofluorescence showed that the percentage of positive cells was higher in HS and HSFs. Meanwhile, the co-localization of MRP1 and α-SMA was stronger in HS. MRP1 knockdown in HSFs provoked a significant reduction in the protein expressions of collagen 3 and α-SMA in vitro. Moreover, MRP1 siRNA transfection could decrease the deposition of collagen in cultured tissues ex vivo and inhibit the scar formation in rabbit ear scar model in vivo. H&E staining and Masson trichrome staining revealed thinner and more orderly arranged collagen fiber in the MRP1 siRNA transfection group. The appearance of scar was improved as well. All these results indicate that MRP1 plays an important role in the formation of HS, MRP1 knockdown could be a potential method to reduce the accumulation of collagen and to improve the abnormal deposition of extracellular matrix in HS, which indicates that down-regulation of MRP1 has the potential therapeutic effect in the treatment and prophylaxis of HS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypertrophic scar (HS) resulting from burns, trauma and operation is a common fibrotic disease, often leads to aesthetical and functional obstacles, causing psychological and physical problems (Brown et al. 2008; Bayat et al. 2003). After injury, fibroblasts are activated and migrate into the wound and then synthesize matrix proteins. It is widely accepted that the major characteristics of fibrosis are the excessive abnormal deposition and the metabolism disorder of collagen-based extracellular matrix proteins, mainly including collagen 1 (Col1), collagen 3 (Col3) (Zhang et al. 2013). The transformation of fibroblasts to myofibroblasts during scar formation is reflected by α-SMA. Clinical therapy is currently unsatisfying, and the specific mechanism of scar formation remains unclear.
ATP-binding cassette (ABC) transporters are a family of transmembrane proteins that transport a wide variety of substrates across biological membranes in an energy dependent manner (Dean et al. 2001). MRP1 (ABCC1), a 190kD transmembrane protein, is one of 13 polytopic proteins that comprise the ‘C’ subfamily of ABC transport proteins (Yabuuchi et al. 2002; Cole 2014). MRP1 has close association with the effect of chemo-radiotherapy in many types of cancers (Deeley et al. 2006; Li et al. 2010; Bagnoli et al. 2013; Hlaváč et al. 2013). It is also found to express in cutaneous disorder such as psoriasis and melanoma. However, there are seldom reports in HS. In view of the close structural homology and possible functional overlap between cystic fibrosis transmembrane conductance regulator (CFTR) and MRP1, MRP1 was investigated as potentially relevant factors in cystic fibrosis (CF) pathophysiology (Riordan et al. 1989). It might participate in formation of CF phenotype and might be a novel pharmacotherapy target of CF (Hurbain et al. 2003). It is reasonable for us to hypothesis that MRP1 may play an important role in the formation of HS.
Here, we found that MRP1 was overexpressed in HS and HSFs. MRP1 knockdown could reduce the expression of Col3, α-SMA in vitro and decrease the deposition of collagen as well as improve the formation of HS in vivo. All these results indicate that MRP1 plays the important role in HS formation. Our study suggests valuable insights into the molecular mechanism on HS formation and provides possible evidence of the therapeutic effect of MRP1 on HS.
Materials and methods
Cell culture
Cell culture was performed as previously reported method (Liu et al. 2012). Briefly, NS and HS were obtained from patients (n = 3) who underwent plastic surgery at Xijing Hospital (Xi’an, China). All specimens were obtained from the Human Subjects Committee of the local institution. Dermal portions were minced and incubated in a solution of collagenase type I (Sigma, 0.1 mg/mL) at 37 °C for 3 h to separate fibroblasts. Fibroblasts were pelleted and grown in Dulbecco modified eagle medium (DMEM, Gibco) supplemented with 10 % fetal calf serum (FCS, Gibco), 100 U/mL penicillin and 100 U/mL streptomycin at 37 °C in a 5 % (v/v) CO2 humidified atmosphere. Cells between the third and fifth sub-passages were used for the following experiments. The cells were seeded into six-well plates (2 × 105 cells per well) in DMEM with 10 % FCS.
RT-PCR
RNA was isolated with Trizol Reagent (Invitrogen) according to the manufacturer’s instructions (Zhang et al. 2011). Synthesis of cDNA was conducted according to the Prime Script RT Kit protocol (Takara). Real-time PCR was performed using the Bio-Rad IQ5 Real-Time System and was carried out using a SYBR Premix Ex Taq kit (Takara) in a final volume of 20 µL for PCR. The primer sequence used were as follows: MRP1 Sense: 5′-GTGATGGCGATGAAGACCAAGA-3′, anti-sense: 5′-GCCAGCTCCCAGGCATAAAG-3′; β-actin Sense: 5′-TGGCACCCAGCACAATGAA-3′, anti-sense: 5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′. RT-PCR were performed in triplicate. The mRNA level were normalized to those of the housekeeping gene encoding β-actin.
Western blot
50 μg lysates were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to PVDF membranes (Millipore). After blocking with 5 % nonfat milk, the membranes were incubated with human anti-Col1 (1:1000, Abcam), anti-Col3 (1:1000, Abcam), anti-α-SMA (1:1000, Abcam), β-actin antibodies (1:500, Santa Cruz) overnight at 4 °C, next day the membranes were incubated with horseradish peroxidase conjugated secondary antibodies (1:3000, Boster) 37 °C for 1 h. Proteins were visualized by enhanced chemiluminescence system using FluorChem FC (Alpha Innotech).
Immunofluorescence
NSFs and HSFs were fixed with 4 % paraformaldehyde in PBS for 30 min, washing four times with PBS, cells were permeated with 0.1 % Triton-X100 in PBS for 10 min at room temperature and then blocked with 1 % bovine serum albumin at 37 °C for 1 h. The samples were incubated at 4 °C overnight with anti-MRP1 (1:100, Abcam). After washing with PBS, the samples were incubated at 37 °C for 1 h with either FITC-488 or CY3-555 (1:100, Invitrogen). Then the samples were stained with 4′, 6′-diamidino-2-phenylindole (DAPI) for 30 min. The coverslips were then mounted on to glass slides and viewed with Olympus Box-Type Photofluorography Unit Model FSX100.
Immunohistochemistry
NS and HS were fixed in 10 % buffered formalin solution. The paraffin embedded sections with 5 μm thick were dewaxed and microwave thermal remediation was made by using citrate antigen retrieval solution, endogenous peroxidase activity was quenched with 3 % hydrogen peroxide for 15 min, and then blocked non-specific binding by normal goat serum for 30 min. The sections were incubated overnight at 4 °C with anti-MRP1 (1:100, Abcam). The next day, the secondary antibody (IgG-HRP) was incubated at 37 °C for 1 h by application instruction of PV6000 Histostain TM Kit (ZSJQ, China). Finally, after diaminobenzidine staining, they were counterstained with hematoxylin. An isotype matched IgG was used as negative control. Images were captured using an Olympus FSX100 fluorescence microscope.
SiRNA transfection
MRP1 siRNA were transfected with lipofectamine™2000 (Invitrogen) in HSFs following the manufacturer’s protocol. HSFs were transfected with 100 nM non-targeting control siRNA (scramble siRNA) or special MRP1 siRNA. About 24 h thereafter, transfection mixture was removed and replaced with fresh DMEM, and HSFs were cultured for another 48 h before the termination, the samples were harvested to use for western blot and RT-PCR. SiRNA sequence Sense: 5′-GGAAGAAGGAAUGCGCCAATT-3′, anti-sense: 5′-UUGGCGCAUUCCUUCUU CCTT-3′; Sense: 5′-GCAGACCUCUUCUACUCUUTT-3′, anti-sense: 5′-AAGAGUAGAAGAGGUCUGCTT-3′; Sense: 5′-CCUACUUCCUCAUGAGCUUTT-3′, anti-sense: 5′-AAGCUCAUGAGGAAGUAGGCT-3′.
Ex vivo culture of HS
Ex vivo culture of HS were performed as described previously (Yasuoka et al. 2008). HS tissues from three individuals (n = 3) were cut into 10 × 10 mm sections, and lipofectamine™2000 transfected 100 nM MRP1 siRNA was injected intra-dermally in a 100 μL volume. 6 h after injection, the specimens were cut into 5 × 5 mm thick sections and incubated for an additional 7 days. The tissues were then fixed in 4 % paraformaldehyde. H&E and Masson trichrome staining were performed using standard protocols (Shi et al. 2012; Wong et al. 2011).
Rabbit ear model
We used rabbit ear model of HS in this study as previously described (Kryger et al. 2007; Morris et al. 1997). Six New Zealand white rabbits in each group (n = 6) weighing 2.0–2.5 kg were purchased from the Experimental Animal Center of the Fourth Military Medical University and maintained in separate cages. The animal experiments were approved by the Experimental Animal Committee of the Fourth Military Medical University. The rabbits were anaesthetized by intraperitoneal injection of sodium pentobarbital (30 mg/kg). In a sterile environment, six round wounds, 10 mm in diameter were created on each ear. Epidermis, dermis and perichondrium were completely removed. The wounds were then covered with sterile gauze for 1 day. On postoperative 7 day, 100 nM MRP1 siRNA was injected intra-dermally into the wound. After 3 days, MRP1 siRNA was injected again, scramble siRNA as control. The images were observed every 3 days. The rabbits were killed on the 28th day. The harvested specimens were analyzed by H&E staining and Masson trichrome staining.
Statistics
Quantitative data was expressed as the mean ± SEM. Statistical analysis was performed by student’s t test using a SPSS 17.0 program and p < 0.05 was considered statistically significant.
Results
MRP1 was overexpressed in HS
To explore the expression of MRP1 in HS, we tested the mRNA and protein levels of MRP1 in NS, HS, NSFs and HSFs respectively. The mRNA level in HSFs was higher than that in NSFs (p = 0.04523) (Fig. 1a). We further detected the protein expression of MRP1 which showed four-fold higher in HSFs compared to NSFs (p = 0.00107) (Fig. 1b, c). Immunofluorescence showed there were more positive cells in HSFs than in NSFs (Fig. 1d). Immunohistochemistry showed that MRP1 was localized in the cytoplasm of dermis fibroblasts and highly expressed in HS, the percentage of MRP1 positive cells was increased in HS (Fig. 1g),the protein level of MRP1 was two-fold higher in HS (p = 0.00311) (Fig. 1e, f). Besides, the co-localization of MRP1 and α-SMA was stronger in HS by immunofluorescence (Fig. 1h). Taken together, all these results suggest MRP1 is overexpressed in hypertrophic scar and it may be involved in HS formation.
The expression of MRP1 in NS, HS, NSFs and HSFs, the paired samples from the same individual (n = 3) a mRNA levels in NSFs and HSFs were detected by RT-PCR. b–c Protein expressions in NSFs and HSFs tested by western blot. d Immunofluorescence shown the expressions in NSFs and HSFs, Scale bars 25 µm. e–f Protein expressions in NS and HS detected by western blot. g Immunohistochemistry staining shown the expressions of MRP1 in NS and HS using 5 µm paraffin sections, Scale bars left, 100 µm; right, 50 µm. h The co-localization of MRP1 and α-SMA in NS and HS shown by immunofluorescence, Scale bars 100 µm, MRP1(green), α-SMA(red), DAPI(blue). Error bars represent mean ± SD of n = 3; *p < 0.05; **p < 0.01
MRP1 knockdown decreased the deposition of collagen in HSFs
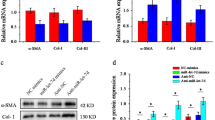
The major characteristics of fibrosis are excessive abnormal deposition of collagen, mainly including Col1, Col3 and the transformation of fibroblasts to myofibroblasts (characterized by α-SMA positive expression). Since the expression of MRP1 was higher in HSFs, it was important to detect whether the high expression of MRP1 was associated with excessive deposition of collagen. We knockdown MRP1 by MRP1 siRNA, the effect of MRP1 knockdown was tested by mRNA level (p = 0.001909) (Fig. 2a) and protein expression (p = 0.04113) (Fig. 2b). Protein expressions of Col3 and α-SMA were down-regulated three-fold and one and a half-fold respectively by MRP1 siRNA in HSFs (p = 0.007705, p = 0.001598), while Col1 level was not changed (p = 0.348414) (Fig. 2b, c). These results indicate that MRP1 knockdown in HSFs could downregulate fibrotic related protein to decrease the deposition of collagen and improve the formation of scar.
The expression of Col1, Col3 and α-SMA in HSFs after MRP1 knockdown. a–b 100 nM MRP1 siRNA and scramble siRNA were transfected with 80 % confluence HSFs, knockdown efficiency of siRNA on mRNA and protein level were detected by RT-PCR and western blot. b–c Protein expressions of Col1, Col3 and α-SMA after MRP1 siRNA transfection were detected by western blot. Error bars represent mean ± SD of n = 3; *p < 0.05; **p < 0.01
MRP1 knockdown relieved the accumulation of collagen in HS tissues
As we found that MRP1 siRNA could reduce the deposition of collagen in vitro, we further detected the influence of MRP1 siRNA on cultured HS ex vivo by directly injecting in subcutaneous tissue. Immunofluorescence and western blot showed that MRP1 was knockdown by MRP1 siRNA (p = 0.046283) (Fig. 3a, b). Proteins extracted from cultured tissues in the MRP1 siRNA group presented decreased expression of Col3 and α-SMA (p = 0.024906, p = 0.045559), there was no statistical difference for Col1 between the scramble siRNA group and the MRP1 siRNA group (p = 0.170233) (Fig. 3b, c). H&E staining and Masson trichrome staining showed that MRP1 siRNA resulted in thinner and orderly arranged collagen fibers compared to the control group, while the appearance of scar was improved (Fig. 3d, e). These results suggest that MRP1 knockdown improves the arrangement of collagen and provides a potential target for the treatment of HS.
Dermal architecture in cultured HS tissues after MRP1 knockdown, HS tissues were obtained from patients (n = 3) who underwent plastic surgery a the expression of MRP1 was detected by immunofluorescence after injected 100 nM siRNA and scramble siRNA into intradermal section. b–c Protein expressions of Col1, Col3 and α-SMA after MRP1 siRNA transfection were detected by western blot. d H&E staining shown the structure of morphology after MRP1 siRNA injection. e Masson trichrome staining shown the deposition, density and arrangement of collagen after MRP1 knockdown. Scale bars left, 100 µm; right, 50 µm. Error bars represent mean ± SD of n = 3; *p < 0.05; **p < 0.01
MRP1 knockdown improved ECM deposition and decreased scarring formation in rabbit ear scar model
To further assess the effect of MRP1 knockdown on scarring formation, cutaneous incision wound models were established on New Zealand white rabbit ear and were treated with MRP1 siRNA by subcutaneous injection. The wound tissues were harvested 4 weeks later. As shown in Fig. 4a, the appearance of rabbit ear scar was obviously improved after MRP1 siRNA treatment, the height index was significantly decreased (Fig. 4b), there were statistical differences between the scramble siRNA group and the MRP1 siRNA group at the 21th and 28th days (p = 0.037725, p = 0.0415581). The efficiency of MRP1 knockdown was tested by immunofluorescence, and fluorescence intensity was less in MRP1 siRNA treatment group (Fig. 4c). H&E and Masson trichrome staining showed that the deposition of collagen was observably improved, which presented thinner and orderly arranged collagen fibers at the 28th day after MRP1 siRNA treatment, while arranged in a disordered manner, with a bulky appearance in the scramble siRNA group (Fig. 4d, e). These results prove that MRP1 knockdown could improve scarring formation in rabbit ear model and further present MRP1 has an anti-fibrosis effect in HS.
Dermal architecture in rabbit ear scar model after MRP1 knockdown, six rabbits in each group (n = 6) a100 nM MRP1 siRNA and scramble siRNA were injected into subcutaneous section on 7th and 9th day after operation, the growth situation of rabbit ear scars presented in different periods. b The heights of rabbit ear scars at different time points were measured. c Immunofluorescence shown the expressions of MRP1 in rabbit ear scar tissues at the 28th day after operation. Scale bars 200 µm. d H&E staining presented structure of rabbit ear scar in the MRP1 siRNA and scramble siRNA treated group. e Masson trichrome staining shown the arrangement of collagen, Scale bars left, 100 µm; right, 50 µm
Discussion
Hypertrophic scar is a common complication of burn injury that can be regarded as a fibroproliferative disorder, which can cause significant problems in growth, movement and esthetics. Healing by fibrosis, instead of regeneration, places a huge burden on public health. Dermal fibroblasts are responsible for synthesizing collagen and other ECM proteins, thus they play a critical role in the wound healing and scarring formation (Russell and Witt 1976; Zhang et al. 2013). After skin injury, fibroblasts transdifferentiate into myofibroblasts, which characterized by increased expression of α-SMA, increased the ability of collagen synthesis and secretion of fibroblasts and facilitate the wound healing. However, the persistence of myofibroblasts after wound healing may result in the formation of hypertrophic scars. Therefore, we usually measure HS by the expression of Col1, Col3 and α-SMA.
Human MRP1 is encoded by ABCC1, located on chromosome 16p13.12. Cole et al. discovered a non-P-gp mediated MDR mechanism in the human lung cancer cell line H69AR in 1992 (Cole et al. 1992). In the past decades, MRP1’s relevance in human neoplasm has been firmly established and it is still considerable to be pre-clinical and clinical interest. The presence of MRP1 in human skin has been verified in earlier studies (Baron et al. 2001). MRP1 is mainly localized in plasma membrane in many tumor cells, it also has been described in other cellular localizations such as lysosomes, cytoplasmic vesicle and secretory vesicles putatively derived from the Golgi apparatus. Our results presented MRP1 was localized in the cytoplasm of dermis and revealed granular accumulation in HS. Up-regulation of MRP1 expression was found in lesional skin taken from patients with psoriasis and lichen planus (Sehgal 1990; Fayyazi et al. 1999), and we found that MRP1 was highly expressed in HS. Elevated mRNA and protein levels had been also observed in HS and HSFs. Besides, the co-localization of MRP1 and α-SMA was stronger in HS. These results suggest overexpression of MRP1 is closely associated with HS, it may participate in the trans-differentiation of fibroblasts to myofibroblasts.
We had presented MRP1 was highly expressed in HS, it also had been reported MRP1 was related with cystic fibrosis (Riordan et al. 1989). The recent research showed MRP1 mRNA expression was up-regulated in liver fibrosis, which decreased liver distribution of entecavir (Xu et al. 2015), the drug transport mechanism in liver fibrosis was associated with the activation of farnesoid X receptor (Chen et al. 2015; Meng et al. 2014). We also found that MRP1 siRNA could significantly decrease the expressions of Col3 and α-SMA protein in HSFs and HS tissues, while there was no difference for Col1 level. Although Col1 is the most abundant of all in HS fibrosing tissues, as a regulator of HS, MRP1 can be just for special effect for Col 3 and α-SMA. MRP1 knockdown could change the proportion of collagen to improve the formation of scarring, the ratio of Col1/Col3 was inclined to normal. The proliferative activity of HSFs was inhibited. Piñeiro (Piñeiro et al. 2011) also found MRP1 knockdown in human prostate cancer cells was associated with decreased proliferation. We further verified the effect of MRP1 knockdown in cultured HS tissues and in the rabbit ear scar model. The outcome revealed that MRP1 knockdown could inhibit the process of scarring formation, which reflected the appearance was improved and the collagen was arranged orderly, which proved that MRP1 knockdown decreased the deposition of collagen to inhibit HS formation, which was beneficial to inhibit fibrosis. These results were in accordance with in MRP1 deficient mice which indicated that there was no gross abnormalities in lungs and other tissues after treatment with the chemotherapeutic (Wijnholds et al. 1997). Therefore, MRP1 is likely to be a candidate factor for changing the abnormal distribution and accumulation of ECM and treating HS.
Taken together, the overexpression of MRP1 was closely related with HS, it may be a regulator to participate in the formation of HS. MRP1 siRNA could decrease the deposition of collagen and improve scarring formation, which provides a possible therapy target for HS and has an important theoretical and clinical meaning. But it is still unknown that which pathway MRP1 participate in and how they interact with each other. The specific mechanism still needs further research.
Abbreviations
- MRP1:
-
multidrug resistance-associated protein
- HS:
-
Hypertrophic scar
- HSFs:
-
HS derived fibroblasts
- NS:
-
Normal skin
- NSFs:
-
NS derived fibroblasts
- Col1:
-
Collagen 1
- Col3:
-
Collagen 3
- ABC:
-
ATP-binding cassette
- CFTR:
-
Cystic fibrosis transmembrane conductance regulator
- CF:
-
Cystic fibrosis
- DAPI:
-
4′,6′-diamidino-2-phenylindole
- α-SMA:
-
α-smooth muscle actin
References
Bagnoli M, Beretta GL, Gatti L, Pilotti S, Alberti P, Tarantino E, Barbareschi M, Canevari S, Mezzanzanica D, Perego P (2013) Clinicopathological impact of ABCC1/MRP1 and ABCC4/MRP4 in epithelial ovarian carcinoma. Biomed Res Int 2013:143202. doi:10.1155/2013/143202
Baron JM, Höller D, Schiffer R, Frankenberg S, Neis M, Merk HF, Jugert FK (2001) Expression of multiple cytochrome p450 enzymes and multidrug resistance-associated transport proteins in human skin keratinocytes. J Investig Dermatol 116(4):541–548
Bayat A, McGrouther DA, Ferguson MW (2003) Skin scarring. BMJ 326(7380):88–92
Brown BC, McKenna SP, Siddhi K, McGrouther DA, Bayat A (2008) The hidden cost of skin scars: quality of life after skin scarring. J Plast Reconstr Aesthet Surg 61(9):1049–1058. doi:10.1016/j.bjps.2008.03.020
Chen X, Meng Q, Wang C, Liu Q, Sun H, Huo X, Sun P, Yang X, Peng J, Liu K (2015) Protective effects of calycosin against CCl4 induced liver injury with activation of FXR and STAT3 in mice. Pharm Res 32(2):538–548. doi:10.1007/s11095-014-1483-3
Cole SP (2014) Targeting multidrug resistance protein 1 (MRP1, ABCC1): past, present, and future. Annu Rev Pharmacol Toxicol 54:95–117. doi:10.1146/annurev-pharmtox-011613-135959
Cole SP, Bhardwaj G, Gerlach JH, Mackie JE, Grant CE, Almquist KC, Stewart AJ, Kurz EU, Duncan AM, Deeley RG (1992) Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science 258(5088):1650–1654
Dean M, Rzhetsky A, Allikmets R (2001) The human ATP-binding cassette (ABC) transporter superfamily. Genome Res 11(7):1156–1166
Deeley RG, Westlake C, Cole SP (2006) Transmembrane transport of endo- and xenobiotics by mammalian ATP-binding cassette multidrug resistance proteins. Physiol Rev 86(3):849–899
Fayyazi A, Schweyer S, Soruri A, Duong LQ, Radzun HJ, Peters J, Parwaresch R, Berger H (1999) T lymphocytes and altered keratinocytes express interferon-gamma and interleukin 6 in lichen planus. Arch Dermatol Res 291(9):485–490
Hlaváč V, Brynychová V, Václavíková R, Ehrlichová M, Vrána D, Pecha V, Koževnikovová R, Trnková M, Gatěk J, Kopperová D, Gut I, Souček P (2013) The expression profile of ATP-binding cassette transporter genes in breast carcinoma. Pharmacogenomics 14(5):515–529. doi:10.2217/pgs.13.26
Hurbain I, Sermet-Gaudelus I, Vallee B, Feuillet MN, Lenoir G, Bernaudin JF, Edelman A, Fajac A (2003) Evaluation of MRP1-5 gene expression in cystic fibrosis patients homozygous for the delta F508 mutation. Pediatr Res 54(5):627–634
Kryger ZB, Sisco M, Roy NK, Lu L, Rosenberg D, Mustoe TA (2007) Temporal expression of the transforming growth factor-Beta pathway in the rabbit ear model of wound healing and scarring. J Am Coll Surg 205(1):78–88
Li J, Li ZN, Yu LC, Bao QL, Wu JR, Shi SB, Li XQ (2010) Association of expression of MRP1, BCRP, LRP and ERCC1 with outcome of patients with locally advanced non-small cell lung cancer who received neoadjuvant chemotherapy. Lung Cancer 69(1):116–122. doi:10.1016/j.lungcan.2009.09.013
Liu J, Wang Y, Pan Q, Su Y, Zhang Z, Han J, Zhu X, Tang C, Hu D (2012) Wnt/beta-catenin pathway forms a negative feedback loop during TGF-beta1 induced human normal skin fibroblast-to-myofibroblast transition. J Dermatol Sci 65(1):38–49. doi:10.1016/j.jdermsci.2011.09.012
Meng Q, Chen X, Wang C, Liu Q, Sun H, Sun P, Peng J, Liu K (2014) Alisol B 23-acetate promotes liver regeneration in mice after partial hepatectomy via activating farnesoid X receptor. Biochem Pharmacol 92(2):289–298. doi:10.1016/j.bcp.2014.09.009
Morris DE, Wu L, Zhao LL, Bolton L, Roth SI, Ladin DA, Mustoe TA (1997) Acute and chronic animal models for excessive dermal scarring: quantitative studies. Plast Reconstr Surg 100(3):674–681
Piñeiro R, Maffucci T, Falasca M (2011) The putative cannabinoid receptor GPR55 defines a novel autocrine loop in cancer cell proliferation. Oncogene 30(2):142–152. doi:10.1038/onc.2010.417
Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL et al (1989) Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 245(4922):1066–1073
Russell JD, Witt WS (1976) Cell size and growth characteristics of cultured fibroblasts isolated from normal and keloid tissue. Plast Reconstr Surg 57(2):207–212
Sehgal PB (1990) Interleukin-6: molecular pathophysiology. J Investig Dermatol 94(6 Suppl):2S–6S
Shi JH, Hu DH, Zhang ZF, Bai XZ, Wang HT, Zhu XX, Su YJ, Tang CW (2012) Reduced expression of microtubule-associated protein 1 light chain 3 in hypertrophic scars. Arch Dermatol Res 304(3):209–215. doi:10.1007/s00403-012-1204-x
Wijnholds J, Evers R, van Leusden MR, Mol CA, Zaman GJ, Mayer U, Beijnen JH, van der Valk M, Krimpenfort P, Borst P (1997) Increased sensitivity to anticancer drugs and decreased inflammatory response in mice lacking the multidrug resistance-associated protein. Nat Med 3(11):1275–1279
Wong VW, Rustad KC, Akaishi S, Sorkin M, Glotzbach JP, Januszyk M, Nelson ER, Levi K, Paterno J, Vial IN, Kuang AA, Longaker MT, Gurtner GC (2011) Focal adhesion kinase links mechanical force to skin fibrosis via inflammatory signaling. Nat Med 18(1):148–152. doi:10.1038/nm.2574
Xu Q, Wang C, Liu Q, Meng Q, Sun H, Peng J, Sun P, Huo X, Liu K (2015) Decreased liver distribution of entecavir is related to down-regulation of Oat2/Oct1 and up-regulation of Mrp1/2/3/5 in rat liver fibrosis. Eur J Pharm Sci 71:73–79. doi:10.1016/j.ejps.2015.02.010
Yabuuchi H, Si Takayanagi, Yoshinaga K, Taniguchi N, Aburatani H, Ishikawa T (2002) ABCC13, an unusual truncated ABC transporter, is highly expressed in fetal human liver. Biochem Biophys Res Commun 299(3):410–417
Yasuoka H, Larregina AT, Yamaguchi Y, Feghali-Bostwick CA (2008) Human skin culture as an ex vivo model for assessing the fibrotic effects of insulin-like growth factor binding proteins. Open Rheumatol J 2:17–22. doi:10.2174/1874312900802010017
Zhang ZF, Zhang YG, Hu DH, Shi JH, Liu JQ, Zhao ZT, Wang HT, Bai XZ, Cai WX, Zhu HY, Tang CW (2011) Smad interacting protein 1 as a regulator of skin fibrosis in pathological scars. Burns 37(4):665–672. doi:10.1016/j.burns.2010.12.001
Zhang Q, Tao K, Huang W, Tian Y, Liu X (2013) Elevated expression of pleiotrophin in human hypertrophic scars. J Mol Histol 44(1):91–96. doi:10.1007/s10735-012-9453-8
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant Nos. 81272098 and 81171811) and a 2012 Grant-in-Aid for Scientific Research Prom Xijing Hospital (Grant No.XJZT12D01).
Conflict of interest
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Yan Li, Longlong Yang and Zhao Zheng contributed equally to this study.
Rights and permissions
About this article
Cite this article
Li, Y., Yang, L., Zheng, Z. et al. MRP1 knockdown down-regulates the deposition of collagen and leads to a reduced hypertrophic scar fibrosis. J Mol Hist 46, 357–364 (2015). https://doi.org/10.1007/s10735-015-9629-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-015-9629-0