Abstract
The aim of this study was to assess the leaf aqueous extract composition of Serjania marginata and the effects of its inclusion on the diet of Nile tilapia (Oreochromis niloticus), with respect to the activity of digestive enzymes and enzymes associated with the metabolism of the liver and intestine and liver histopathology. Fish (initial mean weight, 54.36 ± 17.04 g) were divided into groups: fasting (without feeding), control (commercial feed), and treatment (commercial feed with leaf aqueous extract of Serjania marginata), and in each aquarium, there were five individuals and the fish fed ad libitum for 15 days. Treatment fish had ingested on average 224.3 mg of extract/kg of fish/day. In the extract analysis by mass spectrometry, quercitrin, isoquercitrin, A-type proanthocyanidin trimer, and quinic acid were identified. In the enzymatic activity, fish from the treatment group showed higher level of alkaline phosphatase, while the hepatotoxic markers (AST and ALT) and levels of lipase, amylase, and nonspecific protease did not differ (p > 0.05). In liver histopathological analysis, it was observed that fish from the treatment showed normal structure, while abnormalities were associated with control (fibrosis, loss of cordonal architecture, vacuolated hepatocytes with nucleus displaced to the periphery) and fasting (reduction in hepatocyte size and sinusoidal space). The intestine histopathology evidenced that the extract favored the development of goblet cells and intestinal fold height. The results indicated that the leaf aqueous extract of S. marginata assists in the structural maintenance of the liver and intestine and stimulates intestinal alkaline phosphatase production in Nile tilapia, suggesting that the identified compounds act on the liver and intestine, showing hepatoprotective effects and stimulating intestinal digestion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fish is currently the most widely produced animal protein, especially farmed fish, which had a 60% increase in production between 2007 and 2017, representing the greatest growth between commodities of protein sources (OECD/FAO 2017). However, aquaculture is affected by several factors, such as cultivation systems, climate change, water quality, and nutrition, which affect fish physiology and may cause loss of homeostasis and reduce productive performance (FAO 2018; Karapanagiotidis et al. 2018; Yuan et al. 2018). Studies have been performed incorporating plant ingredients in fish feed in order to promote benefits to the animal (Manaf et al. 2017; Reverter et al. 2014; Quesada et al. 2013; Azaza et al. 2009; Emre et al. 2008; Mandal and Ghosh 2010a, 2010b). However, the effects of plant extracts on aquatic organisms are underexploited, being relevant to understand how plants can affect fish health, as a preliminary step for large-scale use in aquaculture (Stratev et al. 2018; Awad and Awaad 2017; Reverter et al. 2014).
Medicinal plants and their derivatives are rich in many active principles that feature a variety of biological activities (Awad and Awaad 2017). Among several medicinal plants, the species of the genus Serjania (Sapindaceae) stand out, occurring in tropical and subtropical regions (Moreira et al. 2013) and featuring a wide distribution in Brazil, especially in the Pantanal and Cerrado biomes (Souza and Lorenzi 2013; Moreira et al. 2013). Crude extracts from different parts of the plant, such as stem, leaves, and root, of species of this genus exhibit biological activities such as anti-inflammatory (Gomig et al. 2008), antimicrobial (Cardoso et al. 2013; Lima et al. 2006), gastroprotective (Arruda et al. 2009; Silva et al. 2012), antioxidant (David et al. 2007), and antispasmodic (Silva et al. 2017). However, to date, the effect of genus Serjania plants in fish was reported only as ictiotoxic (Andrade et al. 2015; Petrere Jr. 1989; Teixeira et al. 1984). This toxicity was proven for Serjania lethalis and Serjania caracasana that feature saponins called “serjanosides,” which kill fish by asphyxia (Teixeira et al. 1984; Aragão and Valle 1973; Cordeiro and Valle 1975). On the other hand, studies related to leaf hydroethanolic extract of Serjania marginata indicated gastroprotective (Périco et al. 2015) and antioxidant activities (Heredia-Vieira et al. 2015).
Diet formulated with plant extracts contain compounds that can lead to an improvement of the digestive and/or immune system of fish (Hamed and El-Sayed 2018; Du et al. 2018; Hassaan et al. 2018; Gbadamosi et al. 2016; Gabriel et al. 2015; Verma and Nath 2015; Shivashri et al. 2013), which feature adaptability of digestive processes, such as the following: enzymatic and profile secretion, absorption, and transport of nutrients. In this context, the morphological study reveals the performance of the digestive process, absorption, and metabolism (Lundstedt 2003), besides being a selective barrier allowing nutrient absorption and eliminating many toxic substances (Liquori et al. 2007) and a developer of different adaptations of digestive tract in function of diet offered (Evans et al. 2005). Therefore, histological and enzymatic studies are essential for decision-making regarding the kind of food that can be recommended on fish feeding (Romarheim et al. 2008).
The present study was performed considering the potential displayed by the biological activities of other species of the genus Serjania and the gastroprotective and antioxidant activities identified for the leaf extract of Serjania marginata and due to lack of information regarding the effect of the use of this species on aquatic organisms. The aim of this study was to assess the leaf aqueous extract composition of Serjania marginata and the effects of its inclusion on the diet of Nile tilapia, in relation to digestive enzyme content and hepatic metabolism and intestine and liver histopathology.
Materials and methods
Plant material
Leaves of S. marginata Casar. were collected in the Cerrado (Dourados, Mato Grosso do Sul, Brazil) (21° 59′ 41.8 South latitude, 55° 19′ 24.9″ West longitude, and 429 m altitude. Identification was performed by Dr. Arnildo Pott, and an exsiccate was deposited (number 41054) at the CGMS - Herbarium of the Fundação Universidade Federal do Mato Grosso do Sul.
Preparation and chemical analysis of S. marginata leaf aqueous extract
The leaves were oven-dried (37 ± 2 °C) for 48 h and ground (Wiley-type mill, with a 2-mm mesh sieve). The aqueous extract was obtained by maceration, using 20 mL of distilled water for every 2 g of grounded dry matter. The material remained in the water for 24 h, at room temperature (25 °C). Then, the extract was filtered and lyophilized. Solid-phase extraction (SPE) cartridges (Strata C18-E, Phenomenex) were activated with methanol. The extract sample (5.0 mg) was solubilized in the least possible amount of mobile phase and subsequently subjected to SPE with methanol (2 mL). The extract was analyzed by direct injection (FIA-ESI-IT-MS in negative mode—mass spectrometer LTQ XL equipped with an APCI ionization source and linear ion-trap analyzer) to obtain the mass spectra in negative mode [M–H]−, as described by Moreira et al. (2019). The substances identified in this study also were identified in aqueous extract of S. marginata by Moreira et al. (2019).
Animals
The in vivo assay was performed with juvenile Nile tilapia (54.36 ± 17.04 g), acclimated to the environment for 15 days. Fish were divided into 15 experimental units (20 L water) containing five fish, totaling 25 fish per treatment in recirculating system with photoperiod of 12 h of light. Water quality was monitored during the assay (conductivity, pH 7.95, 5.70 ± 0.2 mg L−1 for dissolved oxygen, water temperature 26.27 ± 1.20 °C, 150 mg/L total hardness as CaCO3). Fish were fed a commercial feed (32% crude protein and 4275 kcal kg−1) acquired in local market, for a period of 15 days ad libitum twice a day. The assay has been approved by the Ethics Committee for Animals Use of Centro Universitário da Grande Dourados (protocol number 003/14).
Experimental design of hepatic and gastroprotective activity on Nile tilapia
A completely randomized design was used to evaluate the effects of diet on hepatic and gastroprotective activity of Nile tilapia with three diets and 15 replicates. The juvenile Nile tilapia were divided into three diet groups (n = 25): (i) kept without feeding (fasting), (ii) fed with commercial feed (control), and (iii) received feed supplemented with leaf aqueous extract of S. marginata (5000 mg kg−1) (treatment). The extract (5.5 g) was incorporated in fish feed (1 kg) after dilution in water:ethanol (1:1), with subsequent evaporation of ethanol. The diets were offered twice a day, up to satiety. At the end of the experiment, fish fed with treatment diet had ingested the dosage of 224.3 mg (extract) kg−1 (fish) day−1, defined based on evidence of protective activity of ethanolic extract of this plant on mice (Périco et al. 2015). The fish tolerance was daily measured by survival to the feeding protocols. On the 15th day, fish were euthanasiated and sampling was done between 09:30 and 10:30 h. The liver and intestine were removed for the assessment of hepatic and gastroprotective activity of S. marginata leaf aqueous extract, in Nile tilapia, with histopathological and enzymatic analyses. The collected organs for assays of enzymatic activity from nine samples per treatment from distinct fish were stored at − 70 °C until analysis.
Enzymatic analysis
The alkaline phosphatase activity analysis was performed by colorimetry, and the activity of other enzymes was performed by spectrophotometry. Determination of enzymatic activity was taken using semi-automatic spectrophotometer (BioPlus S-200). The specific assay conditions for each enzyme were as follows.
Liver tissue (100 mg of each fish) was homogenized with 20 mM sodium phosphate buffer and 10 mM Tris (glycerol v/v, pH 7.0) in Potter–Elvehjem homogenizer for the analysis of metabolic enzymes alanine aminotransferase (ALT) and aspartate aminotransferase (AST). The homogenate was centrifuged first at 600×g at 4 °C for 3 min, and then, the supernatant was subjected to new centrifugation at 6000×g at 4 °C for 8 min. The supernatant was collected for analysis by spectrophotometry determined at 690 nm (Reitman and Frankel 1957).
Tissue from the anterior portion of the intestine (100 mg of each fish) was used for the activity of digestive enzymes. To determine the pH for the nonspecific proteolytic and amylohydrolytic optimal activity, pH profile was assayed previously over a wide pH range (2, 4, 6, 7, 8, 9, 10, 11, and 12). The homogenization of the intestine tissue was performed in 20 mM sodium phosphate buffer and 10 mM Tris (glycerol v/v, pH 7.0) with a Potter–Elvehjem homogenizer. The homogenate was centrifugated (600×g, 4 °C, 3 min), and the supernatant was subjected to new centrifugation (6000×g, 4 °C, 8 min). The supernatant from the second centrifugation was used as the crude enzyme extract in enzyme activity assays.
Nonspecific proteolytic activity was determined by the casein-hydrolysis method (Walter 1984). A reaction mixture containing an aliquot crude enzyme source and casein at 0.5% (w/v) was incubated for 1 h at 25 °C. The reaction was stopped by addition of 7% trichloroacetic acid (TCA). The mixture was maintained in ice bath for 30 min; the precipitate was removed by centrifugation at 14.400×g for 3 min, and the absorbance of supernatant was measured at 280 nm. Tyrosine solution was used as standard.
Lipase activity determination was adapted from Albro et al. (1985). The reaction mixture containing an aliquot of crude enzyme extract and 0.4 mM ρ-nitrophenyl myristate in 24 mM ammonium bicarbonate (pH 7.8) with 0.5% Triton X-100 was incubated for 30 min at 25 °C. The reaction was interrupted by addition of 25 mM NaOH and transferred to ice bath for 15 min. The optical density was registered at 405 nm.
Amylohydrolytic activity was assayed according to Bernfeld (1955). Briefly, a 1.5% starch solution was prepared for use as substrate. The substrate solution, 0.07 M citrate/phosphate buffer and 0.028 M Cl− as enzyme co-factor, was added to the aliquot of enzyme extract. The mixture was incubated at 25 °C for 30 min, and the reaction stopped by addition of 1.0 mL 5% ZnSO4:0.3 N Ba (OH)2 mixture. The precipitate was removed by centrifugation at 11.000×g for 3 min, and free glucose concentration was determined at 690 nm (Park and Johnson 1949).
The alkaline phosphatase activity was determined according to the method of Subramanian et al. (2007). The substrate solution was incubated with 100 mM NH4HCO3 and 1 mM MgCl2, pH 7.8, at 30 °C for 15 min. The 4 mM p-nitrophenol phosphate substrate (50 μL) was added, and the absorbance was measured at 405 nm over a 30-min period at 30 °C.
Unit (U) of enzyme activity was defined as millimole (mmol) of product generated per minute under the measurement conditions described above and expressed per milligram soluble protein (specific activity). Protein concentration was determined according to Bradford (1976) using a Sigma–Aldrich (Química, S.L., Sintra, Portugal) protein assay kit (ref. B6916) with bovine serum albumin as standard.
Histopathological analysis of the liver and intestine
Liver and intestine fragments were immersed in Bouin’s solution for 24 h and subsequently washed in 70% alcohol. After fixation, the fragments were dehydrated in graded ethanol solutions (70%, 80%, 90%, and 96%), diaphonized, and embedded in paraffin with plastic polymer Histosec® (Merck). Microtomy was performed in order to obtain two slices with 5-μm thickness, which were stained by hematoxylin–eosin (HE) and by histochemical method of periodic acid Schiff hematoxylin (PAS-H) (McManus 1948). The microscopic analysis and documentation of the material were performed using a light microscope (Olympus, BX41). First, qualitative histological analyses were done to observe main treatment effects. Based on those observations, quantitative analyses were defined. For the morphometry of the material, seven slices per treatment were selected and seven sections were photographed. Size of the hepatocytes and height of intestinal folds were measured. Intensity of reaction of the goblet cells in the intestine, indicated by PAS-H dyeing (McManus 1948; Ortiz-Delgado et al. 2003), was classified as strong (+ + +), moderate (++), weak (+), or absent (−). Intestinal fold height was classified as short (+), moderate (++), or high (+++) (Hirji and Courtney 1982; Gawlicka et al. 1995; Tengjaroenkul et al. 2000; Moretti et al. 2014).
Statistical procedure
Statistical analyses were performed using RStudio software (version 1.1.423 – © 2009–2018 RStudio, Inc.). Data normality and variance homogeneity of gastroprotective and hepatic parameters were checked by the Shapiro–Wilk test, using BioEstat software (version 5.0). It was performed by an analysis of variance (ANOVA), and when differences were significant (p < 0.05), the means were compared by Tukey’s test. The results were expressed as means ± standard deviation (SD).
Results
Chemical composition of S. marginata leaf aqueous extract
In chemical characterization of aqueous extract of S. marginata performed by mass spectrometry, it was possible to identify substances such as quinic acid, quercitrin, isoquercitrin, and A-type proanthocyanidin trimer (Table 1).
Effect of S. marginata leaf aqueous extract on Nile tilapia liver and intestine enzymatic profile
There was no significant differences in the activities of liver metabolic enzymes, ALT (fasting = 121.9, control = 119.7, and treatment = 124.4 mmol min−1 mg protein−1) and AST (fasting = 112.6, control = 104.0, and treatment = 131.4 mmol min−1 mg protein−1).
Regarding intestine digestive enzymes, it was observed that the activity of nonspecific protease, lipase, and amylase did not differ between treatment and control (Table 2). On the other hand, tilapia fed with diet treatment showed an increase in alkaline phosphatase activity (Table 2), revealing responsive to the presence of S. marginata leaf aqueous extract.
Effect of S. marginata leaf aqueous extract on Nile tilapia histopathological morphology of the liver and intestine
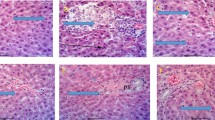
Fish liver histology subjected to fasting showed reduced hepatocyte size with small lipid vacuoles and smaller sinusoidal space (Fig. 1). Fish fed commercial feed showed vacuolized hepatocytes with nucleus displaced to periphery of the cell and increased sinusoidal space. Hepatocellular necrosis has been evidenced, steatosis with discreet inflammatory reaction and septal fibrosis (Fig. 1). Fish fed diet supplemented with S. marginata extract showed hepatocytes with centralized nucleus and cordonal arrangement (Fig. 1).
Liver morphology of Nile tilapia subjected to different diets, × 400. Fasting showed reduced cytoplasmic vacuole (arrowhead); control showed loss of cordonal arrangement of the hepatic cells evidenced (fine trace), nucleus displacement to the periphery of the cell (arrowhead), and vacuolized cells (asterisk); and treatment showed centralized nucleus (asterisk) and cordonal architecture (fine trace.) Fasting, without feeding; control, commercial feed; treatment, commercial feed with S. marginata leaf aqueous extract; PAS, periodic acid Schiff hematoxylin; HE, hematoxylin–eosin
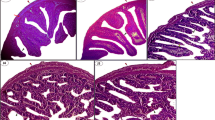
Anterior intestine of Nile tilapia is characterized by four layers: mucosa, submucosa, muscularis, and serosa. The mucosa layer is composed of cylindrical epithelium with brush border and enterocytes interspersed by goblet cells, with the lamina propria containing intraepithelial lymphocytes; the submucosa is formed by cells, collagen fibers, and blood vessels; the muscularis layer is composed of circular smooth muscle fibers and externally to the serosa layer, characterized by loose connective tissue (PAS, positive) and pavement cells (Fig. 2).
Anterior intestine of Nile tilapia subjected to different diets, × 400. Normal morphology of the anterior portion of intestine. Fish displayed mucosa layer (M) composed of cylindrical epithelium with brush border and enterocytes interspersed by goblet cells (arrowhead) (PAS, positive), with lamina propria (LP) containing intraepithelial lymphocytes. Fasting, without feeding; control, commercial feed; treatment, commercial feed with S. marginata leaf aqueous extract; PAS, periodic acid Schiff hematoxylin; HE, hematoxylin–eosin
It was observed that the intestinal fold height, hepatocyte size (Table 3), and variation of intensity of glycoprotein secretion by goblet cells (Table 4) were directly related to the feeding regime. In the intestine of fish fed diets supplemented with S. marginata, there was an increase in intestinal fold height and stronger intensity of reaction of goblet cells.
Discussion
The present study assessed the hepatic and gastroprotective effect of the aqueous extract of S. marginata on Nile tilapia (Oreochromis niloticus), considering the histopathological and biochemical aspects of the liver and intestine and its possible relationship with the identified compounds in the extract, based on studies of the effects reported in the literature. The isoquercitrin, quercitrin, A-type proanthocyanidin trimer, and quinic acid are parts of the composition of other plants (Chapman Jr and Horvat 1989; Soares 1997; Roessner et al. 2000; Gutzeit et al. 2007; Santos et al. 2011; Aderogba et al. 2013; Roby et al. 2013; Simirgiotis et al. 2013; Dhondge et al. 2015; Heredia-Vieira et al. 2015; Lucon Júnior 2016; Gopi et al. 2016; Salinas-Sánchez et al. 2017) and have several biological activities (Yokota et al. 2013; Arya et al. 2014; Zhai et al. 2014; Heredia-Vieira et al. 2015; Périco et al. 2015; Salinas-Sánchez et al. 2017).
Isoquercitrin was isolated from the pulp of Hippophaë rhamnoides L. (Gutzeit et al. 2007) and from methanolic extract 0.1% HCl of leaves and berries of Luma apiculata (Simirgiotis et al. 2013). Quercitrin was identified as one of the constituents of Kalanchoe pinnata aqueous extract (Lucon Júnior 2016). This compound was also isolated from the methanolic extract fractions of Euphorbia hirta (Gopi et al. 2016), extract aerial parts of Phyllanthus amarus (Soares 1997), and hydromethanolic (20%) leaf extract of Croton menyharthii Pax (Aderogba et al. 2013). The A-type proanthocyanidin trimer was reported as a compound isolated from ethyl acetate extract of Serjania schiedeana (Salinas-Sánchez et al. 2017) and of S. marginata leaf ethanolic extract (Heredia-Vieira et al. 2015). The quinic acid was detected in aqueous and ethanolic extracts of Ilex paraguariensis and leaf methanolic extract of Thymus vulgaris L. (Lamiaceae) and Salvia officinalis L. (Lamiaceae) (Roby et al. 2013), methanolic extract of potato tuber (Roessner et al. 2000), and aqueous and methanolic extract of stem bark of Eucalyptus globulus Labill. (Santos et al. 2011), among others (Dhondge et al. 2015; Chapman Jr and Horvat 1989).
The isoquercitrin and quercitrin have not been evaluated as to effect on fish. However, the aglycone of quercitrin, called quercetin, were not toxic to aquatic animals, as observed for rainbow trout (Oncorhynchus mykiss) (Plakas et al. 1985); it can be absorbed by tilapia and deposited in the same way (aglycone), and maybe, its pharmacological effects are more beneficial (Park et al. 2009). Park et al. (2009) detected the presence of quercetin in tilapia, when fed diets based on cotton seeds, which, however, contain quercetin (0.8%), with the majority in glycated form (Dabrowski and Lee 2001; Piccinelli et al. 2007). In addition, hypocholesterolemic and antioxidant effects of quercetin were evidenced, as well as increasing immune function and maintenance of physiological homeostasis of flounder (Paralichthys olivaceus) (Shin et al. 2010) and immunostimulant in rainbow trout (Awad et al. 2013). Zhai et al. (2014), studying the effect of quercetin on growth parameters and serum lipid level of tilapia, found that the final mean of body weight, specific growth rate, and condition factor were greater in fish that received diet with quercetin and the crude lipid level in the fish body was reduced by supplementation of quercetin in this study.
The proanthocyanidins from grape seeds (GSPs) may provide beneficial effects on growth and body composition of tilapia fingerlings, under diet of 200 mg kg−1, but the chemical configuration of compounds was not clarified (Zhai et al. 2014). According to the authors, promoting growth by GSPs may have been a result of increased activity of digestive enzymes and immune and antioxidant capabilities, verified in animals (Zhai et al. 2014). A wide range of biological activities has been reported to the class of the A-type proanthocyanidins (Heredia-Vieira et al. 2015; Périco et al. 2015; Zhai et al. 2014; Yokota et al. 2013; Kresty et al. 2008; Buzzini et al. 2007; Howell 2007; Howell et al. 2005; Cos et al. 2004; Yamakoshi et al. 2002; Foo et al. 2000a, b), encouraging the search for new analogues (Ferreira et al. 2005; Porter 1994). The A-type proanthocyanidins demonstrated anti-inflammatory activity in mice (Salinas-Sánchez et al. 2017) and antioxidant (Heredia-Vieira et al. 2015) and gastroprotective (Périco et al. 2015) activities. The activity of A- and B-type proanthocyanidins on digestive enzymes of mice was studied by Yokota et al. (2013), who observed that these compounds inhibit pancreatic lipase, showing a specificity of enzymatic inhibition depending on the proportion of bond types A and B, as well as the degree of polymerization. The dimeric forms of proanthocyanidins are found in the bloodstream, and some products of hydrolyte of superior oligomers and polymers are presumably absorbed by the intestinal membrane, and then, the absorbed forms can display several physiological and biological functions in vivo (Yamakoshi et al. 2002).
The quinic acid was characterized as a pro-metabolite that extends the metabolism of nicotinamide and tryptophan, and also features antioxidant activity in humans (Pero et al. 2009) and inhibition of vascular inflammation (Jang et al. 2017). Arya et al. (2014) found synergistic effects of quercetin and quinic acid, with a significant improvement of hyperglycemia, hyperlipidemia, and insulin resistance in diabetic mice and reduced degeneration of the liver, kidney, and pancreas at histological level. In a study regarding the effect of methanolic extract from leaves of quince (Cydonia oblonga Miller) on catfish (Clarias gariepinus), exposed or not to UV-A radiation, Osman et al. (2010) found that quinic acid was responsible for immune system improvement in function of the significant increase in number of white blood cells and lymphocytes, indicating hemotoxic stress prevention in fish exposed to this type of radiation. The leaf ethanolic extract of quince, in the study performed by Abliz et al. (2014), promoted hypolipidemic and hepatoprotective effects in mice, probably due to its antioxidant capacity, lipoprotein metabolism in the liver, and inhibition of lipogenesis; however, the authors did not relate with the chemical composition of the extract. On the other hand, the use of quinic acid as active component of the extract C-Med 100 of Uncaria tomentosa promoted the same effect in mice (Akesson et al. 2005) than that observed by Osman et al. (2010) on the white blood cells and lymphocytes of catfish.
The effect of plant extracts on aquatic organisms can vary depending on the dose and composition of the extract, and the organism, besides the exposure time. The dosage of proanthocyanidins, quercitrin, and isoquercitrin used in this study was relatively low, because the crude extract (5000 mg kg−1) was employed, and not an isolated substance. Considering the studies regarding the effect of these compounds on fish, Zhai et al. (2014) tested doses of 0, 200, 400, 600, and 800 mg kg−1 of GSPs, stating that the dose of 200 mg kg−1 indicated better results in growth and body composition of tilapias. Zhai and Liu (2013) used doses of 0, 200, 400, 800, and 1600 mg kg−1 of quercetin, observing that the positive effect on tilapia growth occurred from the dose of 200 mg kg−1, which was also responsible for improvement in the level of high-density lipoprotein cholesterol (HDL-C). However, in none of these studies, the assessment of the effect of doses of active compounds between 0 and 200 mg kg−1 has been included. In addition, the assessment of biological effect caused by the action of a compound is more accurate when it is tested on its pure form because the plant extracts can have complex and synergistic behavior due to the presence of different compounds (Chirumbolo 2010). On the other hand, Arya et al. (2014) evaluated the effect of quercitrin and quinic acid at the dose of 50 and 100 mg kg−1 day−1, in the isolated and composed forms, in mice.
The highest level of alkaline phosphatase, greater height of intestinal folds, and greater intensity of reaction of goblet cells observed in the present study, in diet with extract of S. marginata, indicate greater contribution to the digestive processes and use of digesta (Murashita et al. 2018). The intestinal folds increase the intestinal absorption surface and produce a series of enzymes such as alkaline phosphatase, maltase, sucrose, and dipeptidases (Kuz’mina and Gelman 1997; Rust 2002). Intestinal alkaline phosphatase is involved in the absorption of several nutrients, such as lipids, glucose, calcium, and inorganic phosphate (Malagelada et al. 1977; Roubaty and Portmann 1988; Harris 1989; Dupuis et al. 1991; Mahmood et al. 1994; Tengjaroenkul et al. 2000). This enzyme regulates mainly the absorption of fatty acids and acts as an indicator of the absorption capacity of brush border, and the increase in its activity indicates better absorption of fatty acids by enterocytes, maybe in part due to an increase in the microvilli of enterocytes (Hayes and Volkoff 2014). On the other hand, the presence of goblet cells in fish intestine is related to different feeding conditions, protection against bacterial activity (Liu et al. 2009; Ellis 2001) and epithelial defense (laminar flow) against food from the stomach, rich in digestive enzymes and pH sharply acid (Cardoso et al. 2015). Similar effect was observed in intestinal histology of Nile tilapia (Oreochromis niloticus), rainbow trout (Oncorhynchus mykiss Walbaum), and African catfish (Clarias gariepinus) fed diets supplemented with extracts of Echinacea purpurea, Aloe vera (Aloe barbadensis), and craib (Bauhinia strychnifolia), respectively (Rhaman et al. 2018; Heidarieh et al. 2013; Munglue and Dasri 2015).
Despite reports of the ictiotoxic effects of plants belonging to the same group (Teixeira et al. 1984), the toxicity was not observed in the present study with use of the aqueous extract of S. marginata, since the mortality of treated fish was null, and levels of AST and ALT enzymes did not differ between treatment and control in gastroprotective activity assay. The content of liver enzymes (AST and ALT) would indicate damage in hepatocytes, since these enzymes are located in this region of the cell, and its rise is associated with acute hepatic lesions. Changes in the content of these enzymes are signs of hepatic injury suffered by animals exposed to toxic substances (Mirmazloomi et al. 2015), inadequate nutritional balance of the diet, such as protein excess (Abdel-Tawwab et al. 2010), effect of antinutritional factors (Soltan et al. 2008), or type of nutrient source (Ayisi et al. 2018; Ebrahimi et al. 2017), demonstrating enzymatic secretion stimulation and liver overloading. The content of liver enzymes observed in the present study is probably due to the absence or presence in low concentration of ictiotoxic compounds, such as those isolated from S. lethalis, called “serjanosides” A and B (Teixeira et al. 1984).
The liver histopathological analysis confirms the modulatory action of hepatic structure in relation to the food supplied with leaf aqueous extract of S. marginata. The difference observed in the level of hepatocyte vacuolation between control and treatment indicates that the extract of S. marginata possibly showed a modulator effect of lipid metabolism. Hepatocyte vacuolation is generally considered as a temporary storage of lipids, particularly when the level of absorption exceeds the capacity of lipoprotein synthesis (Castro et al. 2016; Figueiredo-Silva et al. 2005). The benefit of the use of plant extracts for liver protection was reported in several studies (Hamed and El-Sayed 2018; Du et al. 2018; Hassaan et al. 2018; Gbadamosi et al. 2016; Gabriel et al. 2015; Verma and Nath 2015; Shivashri et al. 2013). The liver assimilates and stores nutrients; produces bile; keeps body homeostasis by processing of carbohydrates, proteins, lipids, and vitamins; plays key role in the synthesis of plasma proteins (Genten et al. 2009); and is a central organ in the accumulation and detoxification of contaminants (Al-Yousuf et al. 2000; Köhler 1991). The morphological adaptations in the liver demonstrate the proper functioning of the organ and may reflect the performance of hepatopancreas functionality (Fuentes-Quesada et al. 2018).
The results obtained indicate that the addition of S. marginata leaf aqueous extract in the diet of Nile tilapia helps in digestive function by improving the histopathological aspect of the liver and intestine and stimulation of intestinal alkaline phosphatase production. It is suggested that the extract shows gastroprotective effect, and this can possibly be attributed to the action, isolated or synergistically, of the identified compounds, by antioxidant activities, lipogenesis inhibition, and increase of enzymatic activity. The continuity of studies involving the exposure of fish to different doses of extract and trial duration, in experiments with diverse purposes such as microbiological challenge, hepatotoxicity, performance, and digestibility, should assist in knowledge of the benefits provided by leaf aqueous extract of S. marginata and its possible use in aquaculture.
References
Abdel-Tawwab M, Ahmad MH, Khattab YAE, Shalaby AME (2010) Effect of dietary protein level, initial body weight, and their interaction on the growth, feed utilization, and physiological alterations of Nile tilapia, Oreochromis niloticus (L.). Aquaculture 298:267–274. https://doi.org/10.1016/j.aquaculture.2009.10.027
Abliz A, Aji Q, Abdusalam E, Sun X, Abdurahman A, Zhou W, Moore N, Umar A (2014) Effect of Cydonia oblonga Mill. leaf extract on serum lipids and liver function in a rat model of hyperlipidaemia. J Ethnopharmacol 151(2):970–974. https://doi.org/10.1016/j.jep.2013.12.010i
Aderogba MA, Ndhlala AR, Rengasamy KRR, Van Staden J (2013) Antimicrobial and selected in vitro enzyme inhibitory effects of leaf extracts, flavonols and indole alkaloids isolated from Croton menyharthii. Molecules 18:12633–12644. https://doi.org/10.3390/molecules181012633
Akesson C, Lindgren H, Pero RW, Leanderson T, Ivars F (2005) Quinic acid is a biologically active component of the Uncaria tomentosa extract C-Med 100. Int Immunopharmacol 5:219–229. https://doi.org/10.1016/j.intimp.2004.09.028
Albro PW, Hall RD, Corbett JT, Schroeder J (1985) Activation of non-specific lipase (EC 3.1.1.) by bile salts. Biochem Biophys Acta 835:477–490. https://doi.org/10.1016/0005-2760(85)90117-1
Al-Yousuf MH, El-Shahawi MS, Al-Ghais SM (2000) Trace metals in liver, skin and muscle of Lethrinus lentjan fish species in relation to body length and sex. Sci Total Environ 256:87–94. https://doi.org/10.1016/S0048-9697(99)00363-0
Andrade JN, Costa Neto EM, Brandã H (2015) Using ichthyotoxic plants as bioinsecticide: a literature review. Rev Bras Pl Med Campinas 17(4):649–656. https://doi.org/10.1590/1983-084X/13_105
Aragão JA, Valle JR (1973) Ictiotoxicidade de timbós dos gêneros Serjania, Derris e Tephrosia. Cienc Cult 25:649
Arruda APCCBN, Coelho RG, Honda NK, Ferrazoli C, Pott A, Hiruma-Loma CAJ (2009) Gastroprotective effect of Serjania erecta Radlk (Sapindaceae): involvement of sensory neurons, endogenous nonprotein sulfhydryls, and nitric oxide. Med Food 12:1411–1415. https://doi.org/10.1089/jmf.2008.0269
Arya A, Al-Obaidi MMJ, Shahid N, Noordin MIB, Looi CY, Wong WF, Khaing SL, Mustafa MR (2014) Synergistic effect of quercetin and quinic acid by alleviating structural degeneration in the liver, kidney and pancreas tissues of STZ-induced diabetic rats: a mechanistic study. Food Chem Toxicol 71:183–196. https://doi.org/10.1016/j.fct.2014.06.010
Awad E, Awaad A (2017) Role of medicinal plants on growth performance and immune status in fish, Fish and Shellfish Immunology. Fish Shellfish Immunol 67:40–54. https://doi.org/10.1016/j.fsi.2017.05.034
Awad E, Austin D, Lyndon AR (2013) Effect of black cumin seed oil (Nigella sativa) and nettle extract (quercetin) on enhancement of immunity in rainbow trout, Oncorhynchus mykiss (Walbaum). Aquaculture 388–391:193–197. https://doi.org/10.1016/j.aquaculture.2013.01.008
Ayisi CL, Zhao J, Wu J-W (2018) Replacement of fish oil with palm oil: effects on growth performance, innate immune response, antioxidant capacity and disease resistance in Nile tilapia (Oreochromis niloticus). PLoS One 13(4):e0196100. https://doi.org/10.1371/journal.pone.0196100
Azaza MS, Mensi F, Kammoun W, Abdelouaheb A, Brini B, Kraïem MM (2009) Nutritional evaluation of waste date fruit as partial substitute for soybean meal in practical diets of juvenile Nile tilapia, Oreochromis niloticus L. Aquac Nutr 15:262–272. https://doi.org/10.1111/j.1365-2095.2008.00591.x
Bastos DHM, Saldanha LA, Catharino RR, Sawaya ACHF, Cunha IBS, Carvalho PO, Eberlin MN (2007) Phenolic antioxidants identified by ESI-MS from yerba maté (Ilex paraguariensis) and green tea (Camelia sinensis) extracts. Molecules 12:423–432. https://doi.org/10.3390/12030423
Bernfeld P (1955) Amylase α and β. In: Colowick SP, Kaplan N (eds) Methods in enzymology. Academic Press, New York
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Buzzini P, Turchetti B, Ieri F, Goretti M, Branda E, Mulinacci N, Romani A (2007) Catechins and proanthocyanidins: naturally occurring O-heterocycles with antimicrobial activity. In: Khan MTH (ed) Topics in heterocyclic chemistry. Springer-Verlag, Berlin: Heidelberg. https://doi.org/10.1007/7081_2007_065
Cardoso CAL, Coelho RG, Honda NK, Pott A, Pavan FR, Leite CQF (2013) Phenolic compounds and antioxidant, antimicrobial and antimycobacterial activities of Serjania erecta Radlk. (Sapindaceae). Braz J Pharm Sci 49:775–782. https://doi.org/10.1590/S1984-82502013000400017
Cardoso NDN, Firmiano EMDS, Gomes ID, Nascimento AAD, Sales A, Araújo FG (2015) Histochemical and immunohistochemical study on endocrine cells (5HT, GAS and SST) of the gastrointestinal tract of a teleost, the characin Astyanax bimaculatus. Acta Histochem 117:595–604. https://doi.org/10.1016/j.acthis.2015.05.007
Castro C, Couto A, Pérez-Jiménez A, Serra CR, Díaz-Rosales P, Fernandes R, Corraze G, Panserat S, Oliva-Teles A (2016) Effects of fish oil replacement by vegetable oil blend on digestive enzymes and tissue histomorphology of European sea bass (Dicentrarchus labrax) juveniles. Fish Physiol Biochem 42:203–217. https://doi.org/10.1007/s10695-015-0130-1
Chapman GW Jr, Horvat RJ (1989) Determination of nonvolatile acids and sugars from fruits and sweet potato extracts by capillary GLC and GLC/MS. J Agric Food Chem 37:947–950. https://doi.org/10.1021/jf00088a026
Chirumbolo S (2010) The role of quercetin, flavonols and flavones in modulating inflammatory cell function. Inflamm Allergy Drug Targets 9(3):263–285. https://doi.org/10.2174/187152810793358741
Cordeiro EA, Valle JR (1975) Ictiotoxicidade comparada da rotenona e do serjanosídeo. Cienc Cult 27:561
Correia H, González-Paramás A, Amaral MT, Santos-Buelga C, Batista MT (2005) Characterisation of polyphenols by HPLC-PADESI/MS and antioxidant activity in Equisetum telmateia. Phytochem Anal 16:380–387
Cos P, Bruyne T, Hermans N, Apers S, Van den Berghe D, Vlietinck AJ (2004) Proanthocyanidins in health care: current and new trends. Curr Med Chem 11:1345–1359. https://doi.org/10.2174/0929867043365288
Dabrowski K, Lee K-J (2001) Quercetin—a new phytochemical in fish diets formulations. Paper presented at the International Triennial Conference & Exposition of Aquaculture, Disney’s Coronado Springs Resort, Lake Buena Vista, Florida, 21–25 January 2001
David JP, Meira M, David JM, Brandão HN, Branco A, Agra MF, Barbosa MRV, Queiroz LP, Giulietti AM (2007) Radical scavenging, antioxidant and cytotoxic activity of Brazilian Caatinga plants. Fitoterapia 78:215–218. https://doi.org/10.1016/j.fitote.2006.11.015
Dhondge SS, Shende PH, Paliwal LJ, Deshmukh DW (2015) Volumetric and acoustic study of aqueous binary mixtures of quinine hydrochloride, guanidine hydrochloride and quinic acid at different temperatures. J Chem Thermodyn 81:34–43. https://doi.org/10.1016/j.jct.2014.09.011
Du J, Jia R, Cao L-P, Ding W, Xu P, Yin G (2018) Effects of Rhizoma alismatis extract on biochemical indices and adipose gene expression in oleic acid-induced hepatocyte injury in Jian carp (Cyprinus carpio var. Jian). Fish Physiol Biochem 44:747–768. https://doi.org/10.1007/s10695-017-0428-2
Dupuis Y, Tardivel S, Porembska Z, Fournier P (1991) Effect of some alkaline phosphatase inhibitors on intestinal calcium transfer. Int J BioChemiPhysics 23:175–180. https://doi.org/10.1016/0020-711X(91)90186-Q
Ebrahimi M, Daeman NH, Chong CM, Karami A, Kumar V, Hoseinifar SH, Romano N (2017) Comparing the effects of different dietary organic acids on the growth, intestinal short-chain fatty acids, and liver histopathology of red hybrid tilapia (Oreochromis sp.) and potential use of these as preservatives. Fish Physiol Biochem 43:1195–1207. https://doi.org/10.1007/s10695-017-0365-0
Ellis AE (2001) Innate host defense mechanisms of fish against viruses and bacteria. Dev Comp Immunol 25:827–839. https://doi.org/10.1016/S0145-305X(01)00038-6
Emre Y, Sevgili H, Sanli M (2008) A preliminary study on the utilization of hazelnut meal as a substitute for fish meal in diets of European sea bass (Dicentrarchus labrax L.). Aquac Res 39:324–328
Evans JJ, Pasnik DJ, Peres H, Lim C, Klesius PH (2005) No apparent differences in intestinal histology of channel catfish (Ictalurus punctatus) fed heat-treated and non-heat-treated raw soybean meal. Aquac Nutr 11:123–129. https://doi.org/10.1111/j.1365-2095.2004.00329.x
FAO (2018) Impacts of climate change on fisheries and aquaculture: synthesis of current knowledge, adaptation and mitigation options. FAO Fisheries and Aquaculture Technical Paper 627. www.fao.org/3/I9705EN/i9705en.pdf. Accessed 04 Nov 2018
Ferreira D, Slade D, Marais JPJ (2005) Flavans and proanthocyanidins. In: Andersen M, Markham KR (eds) Flavonoids: chemistry, biochemistry and applications. CRC press, Taylor & Francis Group, Boca Raton, FL. Chapter 11, p 553
Figueiredo-Silva A, Rocha E, Dias J, Silva P, Rema P, Gomes E, Valente LMP (2005) Partial replacement of fish oil by soybean oil on lipid distribution and liver histology in European sea bass (Dicentrarchus labrax) and rainbow trout (Oncorhynchus mykiss) juveniles. Aquac Nutr 11:147–155. https://doi.org/10.1111/j.1365-2095.2004.00337.x
Foo LY, Lu Y, Howell AB, Vorsa N (2000a) The structure of cranberry proanthocyanidins which inhibit adherence of uropathogenic P-fimbriated Escherichia coli in vitro. Phytochemistry 54(2):173–181. https://doi.org/10.1016/S0031-9422(99)00573-7
Foo LY, Lu Y, Howell AB, Vorsa N (2000b) A-type proanthocyanidin trimers from cranberry that inhibit adherence of uropathogenic P-fimbriated Escherichia coli. J Nat Prod 63:1225–1228. https://doi.org/10.1021/np000128u
Fuentes-Quesada JP, Viana MT, Rombenso AN, Guerrero-Rentería Y, Nomura-Solísc M, Gomez-Called V, Lazoa JP, Mata-Sotrese JA (2018) Enteritis induction by soybean meal in Totoaba macdonaldi diets: effects on growth performance, digestive capacity, immune response and distal intestine integrity. Aquaculture 495:78–89. https://doi.org/10.1016/j.aquaculture.2018.05.025
Gabriel NN, Qiang J, Ma XY, He J, Xu P, Liu K (2015) Dietary Aloe vera improves plasma lipid profile, antioxidant, and hepatoprotective enzyme activities in GIFT-tilapia (Oreochromis niloticus) after Streptococcus iniae challenge. Fish Physiol Biochem 41:1321–1332. https://doi.org/10.1007/s10695-015-0088-z
Gawlicka A, Teh S, Hung SSO, Hinton E, de Li, Noüe J (1995) Histological and histochemical changes in the digestive tract of white sturgeon larvae during ontogeny. Fish Physiol Biochem 14:357–371. https://doi.org/10.1007/BF00003374
Gbadamosi OK, Fasakin AE, Adebayo OT (2016) Hepatoprotective and stress - reducing effects of dietary Moringa oleifera extract against Aeromonas hydrophila infections and transportation-induced stress in Nile tilapia, Oreochromis niloticus (Linnaeus 1757) fingerlings. Int J Environ Agric Res 2(7) Retrieved from www.ijoear.com/Paper-July-2016/IJOEAR-JUL-2016-25.pdf. Accessed 22 Oct 2018
Genten F, Terwinghe E, Danguy A (2009) Digestive system. In: Genten F, Terwinghe E, Danguy E (eds) Atlas of fish histology. Science Publishers, Enfield, NH
Gomig F, Pietrovski EF, Guedes A, Dalmarco EM, Calderari MT, Guimarães CL, Pinheiro RM, Cabrini DA, Otuki MFJ (2008) Topical anti-inflammatory activity of Serjania erecta Radlk (Sapindaceae) extracts. Ethnopharmacol 118:220–224. https://doi.org/10.1016/j.jep.2008.03.017
Gopi K, Anbarasu K, Renu K, Jayanthi S, Vishwanath BS, Jayaraman G (2016) Quercetin-3-O-rhamnoside from Euphorbia hirta against snake venom induced toxicity. Biochim Biophys Acta Gen Subj 1860(7):1528–1540. https://doi.org/10.1016/j.bbagen.2016.03.031
Gutzeit D, Wray V, Winterhalter P, Jerz G (2007) Preparative isolation and purification of flavonoids and protocatechuic acid from sea buckthorn juice concentrate (Hippophae rhamnoides) by high-speed counter-current chromatography. Chromatographia 65(1–2):1–7. https://doi.org/10.1365/s10337-006-0105-6
Hamed H, El-Sayed YS (2018) Antioxidant activities of Moringa oleifera leaf extract against pendimethalin-induced oxidative stress and genotoxicity in Nile tilapia, Oreochromis niloticus (L.). Fish Physiol Biochem:1–12. https://doi.org/10.1007/s10695-018-0535-8
Harris H (1989) The human alkaline phosphatases: what we know and what we don’t know. Clin Chim Acta 186:133–150. https://doi.org/10.1016/0009-8981(90)90031-M
Hassaan MS, Mahmoud SA, Jarmolowicz S, El-Haroun ER, Mohammady EY, Davies SJ (2018) Effects of dietary baker’s yeast extract on the growth, blood indices and histology of Nile tilapia (Oreochromis niloticus L.) fingerlings. Aquac Nutr:1–9. https://doi.org/10.1111/anu.12805ro
Hayes J, Volkoff H (2014) Characterization of the endocrine, digestive and morphological adjustments of the intestine in response to food deprivation and torpor in cunner, Tautogolabrus adspersus. Comp Biochem Physiol 170:46–59. https://doi.org/10.1016/j.cbpa.2014.01.014
Heidarieh M, Mirvaghefi AR, Sepahi A, Sheikhzadeh N, Shahbazfar AA, Akbari M (2013) Effects of dietary Aloe vera on growth performance, skin and gastrointestine morphology in rainbow trout (Oncorhynchus mykiss), Turk. J Fish Aquat Sci 13:367–373. https://doi.org/10.4194/1303-2712-v13_2_20
Heredia-Vieira SC, Simonet AM, Vilegas W, Macías FA (2015) Unusual C,O-fused glycosylapigenins from Serjania marginata leaves. J Nat Prod 78:77–84. https://doi.org/10.1021/np500715x
Hirji KN, Courtney WAM (1982) Leucine aminopeptidase activity in the digestive tract of perch, Perca fluviatilis L. J Fish Biol 21:615–622. https://doi.org/10.1111/j.1095-8649.1982.tb02865.x
Howell AB (2007) Bioactive compounds in cranberries and their role in prevention of urinary tract infections. Mol Nutr Food Res 51:732–737. https://doi.org/10.1002/mnfr.200700038
Howell AB, Reed JD, Krueger CG, Winterbottom R, Cunningham DG, Leahy M (2005) A-type cranberry proanthocyanidins and uropathogenic bacterial anti-adhesion activity. Phytochemistry 66:2281–2291. https://doi.org/10.1016/j.phytochem.2005.05.022
Jang S-A, Park DW, Kwon JE, Song HS, Park B, Jeon H, Sohn E-H, Koo HJ, Kang SC (2017) Quinic acid inhibits vascular inflammation in TNF-α-stimulated vascular smooth muscle cells. Biomed Pharmacother 96:563–571. https://doi.org/10.1016/j.biopha.2017.10.021
Karapanagiotidis IT, Psofakis P, Mente E, Malandrakis E, Golomazou E (2018) Effect of fishmeal replacement by poultry by-product meal on growth performance, proximate composition, digestive enzyme activity, haematological parameters and gene expression of gilthead seabream (Sparus aurata). Aquac Nutr 25:1–12. https://doi.org/10.1111/anu.12824
Köhler A (1991) Lysosomal perturbations in fish liver as indicator for toxic effects of environmental pollution. Comp Biochem Physiol Part C. Comp Pharmacol 100:123–127. https://doi.org/10.1016/0742-8413(91)90137-I
Kresty LA, Howell AB, Baird M (2008) Cranberry proanthocyanidins induce apoptosis and inhibit acid-induced proliferation of human esophageal adenocarcinoma cells. J Agric Food Chem 56:676–680. https://doi.org/10.1021/jf071997t
Kuz’mina VV, Gelman AG (1997) Membrane-linked digestion in fish. Rev Fish Sci 5:99–129. https://doi.org/10.1080/10641269709388595
Lima MRF, Luna JS, Santos AF, Andrade MCC, Sant’Ana AEG, Genet JP, Marquez B, Neuville L, Moreau NJ (2006) Anti-bacterial activity of some Brazilian medicinal plants. Ethnopharmacol 105:137–147. https://doi.org/10.1016/j.jep.2005.10.026
Liquori GE, Mastrodonato M, Zizza S, Ferri D (2007) Glycoconjugate histochemistry of the digestive tract of Triturus carnifex (Amphibia, Caudata). J Mol Histol 38:191–199. https://doi.org/10.1007/s10735-007-9087-4
Liu WQ, Zhang J, Liu YS (2009) Research progress in immunity of animal alimentary canal mucosa. Acta Agriculturae Jiangxi 21:155–157 Retrieved from http://caod.oriprobe.com/articles/17373111/Research_Progress_in_Immunity_of_Animal_Alimentary_Canal_Mucosa.htm
Lucon Júnior JF (2016) Estudo da atividade inibitória de extratos vegetais, flavonoides e derivados do ácido cafeico sobre a enzima arginase de Leishmania (Leishmania) amazonensis. Tese (Doutorado), Programa de Pós-Graduação em Biociência Animal, Faculdade de Zootecnia e Engenharia de Alimentos da Universidade de São Paulo
Lundstedt LM (2003) Aspectos adaptativos dos processos digestivo e metabólico de juvenis de pintado (Pseudoplatystoma corruscans) arraçoados com diferentes níveis de proteína e energia. Tese (Doutorado em Genética e Evolução), Universidade Federal de São Carlos
Mahmood A, Yamagishi F, Eliakim R, De Schryver-Kecskemeti K, Gramlich TL, Alpers DH (1994) A possible role for rat intestinal surfactant-like particles in transepithelial triacylglycerol transport. J Clin Invest 93:70–80. https://doi.org/10.1172/JCI116986
Malagelada JR, Linscheer WG, Fishman WH (1977) The effect of fatty acid perfusion on intestinal alkaline phosphatase: II. Studies on the rat. Am J Digest Dis 22:516–523. https://doi.org/10.1007/BF01072504
Manaf SR, Hassan MD, Noordin MM, Razak AA, Hayati, RH, Faten, AMN, Hamid NH, Geetha MK, Rashidah AR (2017) The effects of dietary supplementation of methanolic extracts of herbal medicine on haematological variable of red hybrid tilapia (Oreochromis sp.). Proceedings of the International Seminar on Livestock Production and Veterinary Technology August 31 Indonesia 540–548. https://doi.org/10.14334/Proc.Intsem.LPVT-2016-p.540-548
Mandal S, Ghosh K (2010a) Accumulation of tannin in different tissues of Indian major carps and exotic carps. Aquac Res 41:945–948. https://doi.org/10.1111/j.1365-2109.2009.02371.x
Mandal S, Ghosh K (2010b) Inhibitory effect of Pistia tannin on digestive enzymes of Indian major carps: an in vitro study. Fish Physiol Biochem 36:1171–1180. https://doi.org/10.1007/s10695-010-9395-6
McManus JFA (1948) Histological and histochemical uses of periodic acid. StainTechnology 23:99. https://doi.org/10.3109/10520294809106232
Mirmazloomi S, Shahsavani D, Baghshani H (2015) Studies on the protective effects of ascorbic acid and thiamine on lead-induced lipid and protein oxidation as well as enzymatic alterations in some tissues of Cyprinus carpio. Comp Clin Pathol 24:1231–1236. https://doi.org/10.1007/s00580-015-2065-4
Moreira RPM, Batista CS, Guarim Neto G (2013) “Check list” de angiospermas da vegetação marginal da estrada Santo Antônio de Leverger—Mimoso, Pantanal de Mato Grosso. Flovet 5:1–21 Retrieved from http://periodicoscientificos.ufmt.br/ojs/index.php/flovet/article/view/1524 Accessed 21 Oct 2018
Moreira SS, Tamashiro LK, Jorge BC, Balin OS, Heredia-Vieira SC, Almeida GL, Cardoso CAL, Kassuya CAL, Arena AC (2019) Toxicological safety evaluation in acute and 28-day studies of aqueous extract from Serjania marginata Casar. (Sapindaceae) leaves in rats. J Ethnopharmacol 231:197–204. https://doi.org/10.1016/j.jep.2018.11.024
Moretti DB, Nordi WM, Cruz TM, Cyrino JE, Machado-Neto R (2014) Histochemical distribution of intestinal enzymes of juvenile pacu (Piaractus mesopotamicus) fed lyophilized bovine colostrum. Fish Physiol Biochem 40(5):1487–1493. https://doi.org/10.1007/s10695-014-9942-7
Munglue P, Dasri K (2015) Effects of Bauhinia strychnifolia Craib leaf extract on growth parameters and intestinal morphology of catfish (Clarias gariepinus). Proceeding B. Sakon Nakhon Rajabhat Univ. Int. Conf, 2015. science.snruic.snru.ac.th. Accessed 20 Oct 2018
Murashita K, Matsunari H, Furuita H, Rønnestad I, Oku H, Yamamoto T (2018) Effects of dietary soybean meal on the digestive physiology of red seabream Pagrus major. Aquaculture 493:219–228. https://doi.org/10.1016/j.aquaculture.2018.05.005
OECD/FAO (2017) OECD-FAO Agricultural Outlook 2017–2026. OECD Publishing, Paris. https://doi.org/10.1787/agr_outlook-2017-en
Ortiz-Delgado JB, Darias MJ, Cañavate JP, Yúfera M, Sarasquete C (2003) Organogenesis of the digestive tract in the white seabream, Diplodus sargus. Histological and histochemical approaches. Histol Histopathol 18:1141–1154 http://www.hh.um.es. Accessed 04 Nov 2018
Osman AGM, Koutb M, Sayed AE-DH (2010) Use of hematological parameters to assess the efficiency of quince (Cydonia oblonga Miller) leaf extract in alleviation of the effect of ultraviolet—a radiation on African catfish Clarias gariepinus (Burchell, 1822). J Photochem Photobiol B Biol 99:1–8. https://doi.org/10.1016/j.jphotobiol.2010.01.002
Park JT, Johnson MJ (1949) A submicro determination of glucose. J Biol Chem 181:149–151. https://www.ncbi.nlm.nih.gov/pubmed/15390401
Park K, de Oca G, Bonello P, Lee KJ, Dabrowski K (2009) Determination of quercetin concentrations in fish tissues after feeding quercetin-containing diets. Aquacult Int 17:537–544. https://doi.org/10.1007/s10499-008-9222-6
Périco LLA, Heredia-Vieira SC, Beserra FP, Dos Santos RC, Weiss MB, Resende FA, Ramos MAS, Bonifácio BV, Bauab TM, Varanda EA, Gobbi JIF, Rocha LRM, Vilegas W, Hiruma-Lima CA (2015) Does the gastro protective action of a medicinal plant ensure healing effects? An integrative study of the biological effects of Serjania marginata Casar (Sapindaceae) in rats. J Ethnopharmacol 172(22):312–324. https://doi.org/10.1016/j.jep.2015.06.025
Pero RW, Lund H, Leanderson T (2009) Antioxidant metabolism induced by quinic acid. Increased urinary excretion of tryptophan and nicotinamide. Phytother Res 23:335–346. https://doi.org/10.1002/ptr.2628
Petrere M Jr (1989) River fisheries in Brazil: a review. Regul Rivers Res Manag 4:1–16. https://doi.org/10.1002/rrr.3450040102
Piccinelli AL, Veneziano A, Passi S, Simone FD, Rastrelli L (2007) Flavonol glycosides from whole cottonseed by-product. Food Chem 100:344–349. https://doi.org/10.1016/j.foodchem.2005.09.053
Plakas SM, T-C LEL, Wolke RE (1985) Absence of overt toxicity from feeding the flavonol, quercetin, to rainbow trout (Salmo gairdneri). Food Chem Toxicol 23(12):1077–1080. https://doi.org/10.1016/0278-6915(85)90055-9
Porter LJ (1994) Flavans and proanthocyanidins. In: Harborne JB (ed) The flavonoids: advances in research since 1986. Chapman & Hall/CRC Press, London. Chapter 2, p 23
Quesada SP, Paschoal JAR, Reyes FGR (2013) Considerations on the aquaculture development and on the use of veterinary drugs: special issue for fluoroquinolones—a review. J Food Sci 78:1321–1333. https://doi.org/10.1111/1750-3841.12222
Reitman S, Frankel S (1957) A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol 28:56–63. https://doi.org/10.1093/ajcp/28.1.56
Reverter M, Bontemps N, Lecchini D, Banaigs B, Sasal P (2014) Use of plant extracts in fish aquaculture as an alternative to chemotherapy: current status and future perspectives. Aquaculture 433:50–61. https://doi.org/10.1016/j.aquaculture.2014.05.048
Rhaman ANA, Khalil AA, Abdalah HM, ElHady M (2018) The effects of the dietary supplementation of Echinacea purpurea extract and/or vitamin C on the intestinal histomorphology, phagocytic activity, and gene expression of the Nile tilapia. Fish Shellfish Immunol 82:312–318. https://doi.org/10.1016/j.fsi.2018.08.024
Roby MHH, Sarhan MA, Selim KA-H, Khalel KI (2013) Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme (Thymus vulgaris L.), sage (Salvia officinalis L.), and marjoram (Origanum majorana L.) extracts. Ind Crop Prod 43:827–831. https://doi.org/10.1016/j.indcrop.2012.08.029
Roessner U, Wagner C, Kopta J, Trethewey RN, Willmitzer L (2000) Simultaneous analysis of metabolites in potato tuber by gas chromatography-mass spectrometry. Plant J 23(1):131–142. https://doi.org/10.1046/j.1365-313x.2000.00774.x
Romarheim OH, Zhang C, Penn M, Liu Y-J, Tian L-X, Skrede A, Krogdahl A, Storebakken T (2008) Growth and intestinal morphology in cobia (Rachycentron canadum) fed extruded diets with two types of soybean meal partly replacing fish meal. Aquac Nutr 14(2):174–180. https://doi.org/10.1111/j.1365-2095.2007.00517.x
Roubaty C, Portmann P (1988) Relation between intestinal alkaline phosphatase activity and brush border membrane transport of inorganic phosphate, D-glucose, and D-glucose-6-phosphate. Pfluegers Arch 412:482–490. https://doi.org/10.1007/BF00582536
Rust MB (2002) Nutritional physiology. In: Halver, J.E., Hardy, R. (Eds.), Fish nutrition. Third (pp. 367–452). San Diego: Academic Press, An imprint of Elsevier Science. Retrieved from: www.agrifs.ir/sites/default/files/Fish%20Nutrition.pdf. Accessed 14 Sept 2018
Salinas-Sánchez DO, Jiménez-Ferrer E, Sánchez-Sánchez V, Zamilpa A, González-Cortazar M, Tortoriello J, Herrera-Ruiz M (2017) Anti-inflammatory activity of a polymeric proanthocyanidin from Serjania schiedeana. Molecules 22:863. https://doi.org/10.3390/molecules22060863
Santos SAO, Freire CSR, Domingues MRM, Silvestre AJD, Pascoal Neto C (2011) Characterization of phenolic components in polar extracts of Eucalyptus globulus Labill. bark by high-performance liquid chromatography-mass spectrometry. J Agric Food Chem 59:9386–9393. dx.doi.org. https://doi.org/10.1021/jf201801q
Shin HS, Yoo JH, Min TS, Lee K-Y, Choi CY (2010) The effects of quercetin on physiological characteristics and oxidative stress resistance in olive flounder, Paralichthys olivaceus. Asian-Aust J Anim Sci 23(5):588–597. https://doi.org/10.5713/ajas.2010.90624
Shivashri C, Rajarajeshwari T, Rajasekar P (2013) Hepatoprotective action of celery (Apium graveolens) leaves in acetaminophen-fed freshwater fish (Pangasius sutchi). Fish Physiol Biochem 39:1057–1069. https://doi.org/10.1007/s10695-012-9762-6
Silva JLV, Carvalho VS, Silva FL, Barbosa-Filho JM, Rigoni VLS, Nouailhetas VLA (2012) Gastrointestinal property of Serjania caracasana (Jacq.) Willd. (Sapindaceae) on rats. Pharmacologyonline 1:22–26 Retrieved from http://pharmacologyonline.silae.it/front/specialissues_2012_1. Accessed 04 Sept 2018
Silva FL, da Silva JLV, Silva JM, Marcolin LSA, Nouailhetas VLA, Yoshida M, Vendramini PH, Eberlin MN, Barbosa-Filho JM, Moreno PRH (2017) Antispasmodic activity from Serjania caracasana fractions and their safety. Rev Bras Farmacogn 27:346–352. https://doi.org/10.1016/j.bjp.2016.12.002
Simirgiotis MJ, Bórquez J, Schmeda-Hirschmann G (2013) Antioxidant capacity, polyphenolic content and tandem HPLC-DAD-ESI/MS profiling of phenolic compounds from the South American berries Luma apiculata and L. chequén. Food Chem 139(1–4):289–299. https://doi.org/10.1016/j.foodchem.2013.01.089
Soares LAL (1997) Padronização do extrato aquoso e desenvolvimento de produto seco por aspersão de Phyllanthus niruri L. - Euphorbiaceae (quebra-pedra). Dissertação (Mestrado), Universidade Federal do Rio Grande do Sul
Soltan MA, Hanaf MA, Wafa MIA (2008) Effect of replacing fish meal by a mixture of different plant protein sources in Nile tilapia (Oreochromis niloticus L.) diets. Glob Vet 2(4):157–164 https://www.researchgate.net/publication/242486604. Accessed 17 Oct 2018
Souza VC, Lorenzi H (2013) Botânica Sistemática: Guia ilustrado para identificacão das famiĺias de fanerógamas nativas e exóticas no Brasil, baseado em APG III. Instituto Plantarum, Nova Odessa, pp 454–459
Stratev D, Zhelyazkov G, Noundou XS, Krause RWM (2018) Beneficial effects of medicinal plants in fish diseases. Aquacult Int 26:289–308. https://doi.org/10.1007/s10499-017-0219-x
Subramanian S, MacKinnon SL, Ross NW (2007) A comparative study on innate immune parameters in the epidermal mucus of various fish species. Comp Biochem Physiol B Biochem Mol Biol 148:256–263. https://doi.org/10.1016/j.cbpb.2007.06.003
Teixeira JRM, Lapa AJ, Souccar C, Valle JR (1984) Timbós: ichthyotoxic plants used by brazilian Indians. J Ethnopharmacol 10:311–318. https://doi.org/10.1016/0378-8741(84)90018-7
Tengjaroenkul B, Smith BJ, Caceci T, Smith SA (2000) Distribution of intestinal enzymes activities along the intestinal tract of cultured Nile tilapia, Oreochromis niloticus L. Aquaculture 182(3):317–327. https://doi.org/10.1016/S0044-8486(99)00270-7
Verma P, Nath A (2015) Modulatory role of Withenia somnifera root extract mixed pelleted feed on pesticide induced hepatic anomalies’ in fresh water catfish Clarias batrachus (Linn.). Int J Pharm Sci Res 6(10):4243–4251. https://doi.org/10.13040/IJPSR.0975-8232.6(10).4243-51
Walter HE (1984) Proteinases: methods with hemoglobin, casein and azocoll as substrates. In: Bergmeyer HU (ed) Methods of enzymatic analysis, vol 5. Verlag Chemie, Weinheim, pp 270–277
Yamakoshi J, Saito M, Kataoka S, Kikuchi M (2002) Safety evaluation of proanthocyanidin-rich extract from grape seeds. Food Chem Toxicol 40:599–607. https://doi.org/10.1016/S0278-6915(02)00006-6
Yokota K, Kimura H, Ogawa S, Akihiro T (2013) Analysis of A-type and B-type highly polymeric proanthocyanidins and their biological activities as nutraceuticals. J Chem:Article ID 352042 7 pages. https://doi.org/10.1155/2013/352042
Yuan X-Y, Jiang G-Z, Wang C-C, Abasubong KP, Zou Q, Zhou, Y-Y, Liu W-B (2018) Effects of partial replacement of fish meal by yeast hydrolysate on antioxidant capability, intestinal morphology, and inflammation-related gene expression of juvenile Jian carp (Cyprinus carpio var. Jian). Fish Physiol Biochem 1–11. https://doi.org/10.1007/s10695-018-0552-7
Zhai S-W, Liu S-L (2013) Effects of dietary quercetin on growth performance, serum lipids level and body composition of tilapia (Oreochromis niloticus). Ital J Anim Sci 12(4):e85. https://doi.org/10.4081/ijas.2013.e85
Zhai S-W, Lu J-J, Chen X-H (2014) Effects of dietary grape seed proanthocyanidins on growth performance, some serum biochemical parameters and body composition of tilapia (Oreochromis niloticus) fingerlings. Ital J Anim Sci 13(3357):336–540. https://doi.org/10.4081/ijas.2014.3357
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001. Also, it was financed by Fundação de Apoio ao Desenvolvimento do Ensino, Ciência e Tecnologia do Estado de Mato Grosso do Sul (FUNDECT) (concession number 59/300.222/2016), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (concession number CALC 310801/2015-0), Universidade Estadual de Mato Grosso do Sul (UEMS), and Programa Institucional de Bolsas aos Alunos de Pós Graduação/UEMS (PIBAP).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The assay has been approved by the Ethics Committee for Animals Use of Centro Universitário da Grande Dourados (protocol number 003/14).
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
do Carmo Ota, E., Honorato, C.A., Heredia-Vieira, S.C. et al. Hepatic and gastroprotective activity of Serjania marginata leaf aqueous extract in Nile tilapia (Oreochromis niloticus). Fish Physiol Biochem 45, 1051–1065 (2019). https://doi.org/10.1007/s10695-019-00622-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-019-00622-9