Abstract
Concentrations of quercetin in fish tissues were measured for the first time using HPLC-electrochemical detection method. Its identity was also ascertained with UV-photodiode array detection. Quercetin, in aglycone form, was at measurable concentrations in tilapia plasma, liver, and whole body homogenate when fed with diets containing 1% quercetin (aglycone) for 1 or 15 weeks. Hydrolysis with glucuronidase/sulfatase treatment for the purpose of cleaving conjugates did not increase quercetin levels, suggesting that glucoronide or sulfate conjugates are not the major metabolic forms in Nile tilapia (Oreochromis niloticus). No quercetin was detected in plasma of rainbow trout (Salmo gairdneri) or white sturgeon (Acipenser transmontanus) fed commercial diets. The results suggest that quercetin is absorbed in tilapia and that this flavonoid is deposited mainly in aglycone form in the body after absorption.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Due to its antioxidant activities, quercetin has drawn attention for decades for its efficacy against various pathological conditions such as cancer, cardiovascular disease, and other oxidative stresses (Formica and Regelson 1995). In the field of aquaculture, quercetin has been suggested for its beneficial effects as an antioxidant agent in fish oil storage (Nieto et al. 1993). Despite the ubiquitous occurrence of quercetin in feed stuffs of plant origins, such as cottonseed meal (Shahidi and Naczk 1995), and possible ingestion in fishes (Rinchard et al. 2003), little is known about its nutritional role in fishes ingesting quercetin through formulated fish diets. A study indicated that quercetin is not overly toxic to rainbow trout (Salmo gairdneri) when fed for several months at high levels (Plakas et al. 1985). More recent work by Weber et al. (2002) demonstrated that female medaka (Oryzias latipes) externally treated with quercetin showed an increased number of atretic ovarian follicles. This latter study in particular would imply that quercetin could be absorbed through the skin in fish and exerts its specific toxic effect on the reproductive system. However, the absorption of quercetin has not yet been directly demonstrated in any studies.
Most quercetin consumed through natural sources is not present in aglycone, but occurs in glycosides (Tshushida and Suzuki 1995). The glycosides again generally occur in several different forms depending on their sources. For example, while 4′-glucoside is the major component in onions, they occur as 3-glucoside in apples. Other glucosides such as 3,4′-diglucosdie, 7-O-glucoside, and 7.4′-O-glucoside have also been identified.
Absorbed quercetin, both aglycone and glycosides, have been suggested to be converted rapidly to glucoronide and sulfate conjugates (Piskula and Terao 1998). Apart from conjugation metabolism, the flavonol nucleus of quercetin also undergoes efficient metabolic conversion to isorhamnetin (Boulton et al. 1999).
A variety of detection methods using high-performance liquid chromatography (HPLC) have been employed to quantify the levels of quercetin and its metabolites in biological samples. With HPLC, electrochemical detection and fluorescence detection methods were generally superior to UV detection in sensitivity (Hollman et al. 1996; Jones et al. 1998; Piskula and Terao 1998). As these detection methods depend solely on retention time of standard chemicals, the chemical identification of quercetin in biological tissues would be better validated with additional tools. HPLC coupled with photodiode array detection to obtain the UV spectral signature of quercetin has been used for this purpose (Nielsen and Dragsted 1998; Day and Williamson 2001).
A project is being undertaken by our group to examine whether quercetin can exert an antioxidant role in the diet of Nile tilapia (Oreochromis niloticus) in a long-term feeding with purified, casein–gelatin-based diets containing this flavonoid. Despite several descriptions of HPLC detection of quercetin in different plant and animal samples, there is no report on the quantification of quercetin in fish tissues. In view of the usefulness for analysis of cultured fish tissues, where corn, soybean, rapeseed, or cottonseed meals are used in their diets, it was aimed to establish an HPLC detection method of quercetin in fish tissues.
Materials and methods
Feeding of quercetin in experimental fishes
Quercetin was fed only in tilapia, and other fish species were examined for basal levels when maintained on standard, commercial diets. For long-term quercetin feeding (triplicate tanks, 150 fish in each tank), juvenile Nile tilapia, Oreochromis niloticus, were fed for 15 weeks at 10–20% body weight/day rate with a casein–gelatin-based diet containing 1% quercetin (aglycone form; Sigma Chemicals, St. Louis, MO). Control diet was similar, casein–gelatin-based and devoid of quercetin. Body weights were 0.0096 ± 0.001 and 7.7 ± 2.3 g at the start and termination of feeding (mean ± SD), respectively. Whole body and liver tissues were used for quercetin analysis from this 15-week feeding. A 1-week quercetin feeding experiment was also performed in tilapia (body weight 80.8 ± 13.1 g at start), and these samples were used plasma level analysis. An identical diet (1% quercetin in casein–gelatin-based diet) was fed at 1% body weight/day rate in this 1-week feeding (one tank each containing five fish). Tilapia were maintained at a water temperature of 23.4 ± 0.2°C in a recirculating system during the feeding period; however, water from tanks where fish were fed with a quercetin-containing diet was drained directly to the outside.
Rainbow trout for basal level analysis were 75–84 g individuals randomly harvested from culture tanks kept for general experimental purposes at 15.5–18.9°C. White sturgeon (Acipenser transmontanus) samples analyzed were from those weighing about 60 g cultured at 18–20°C for similar purposes. Commercial diets (BioVita; Bio-Oregon, Warrenton, OR) were fed to the rainbow trout and sturgeon.
Production of quercetin conjugates in rats
To validate an enzyme hydrolysis step for cleavage of conjugated quercetin metabolites (Piskula and Terao 1998), quercetin metabolite-containing blood plasma was obtained after stomach intubation of quercetin (50 mg/kg in 1.0 ml propylene glycol) to male Sprague-Dawley rats (~250 g; Damul Science, Taejon, Korea). Rats were fasted 12 h prior to quercetin administration (1000 hours), but drinking water was supplied ad libitum. Blood samples were taken from the tail vein 6 h after quercetin administration, and plasma was separated by centrifugation (3,000g, 20 min, 3°C). Rats were kept at 22 ± 2°C room temperature and 55 ± 10% relative humidity with a standard rat chow (Cheil Jedang, Seoul, Korea).
Quercetin extraction from plasma and tissues
Blood was taken from the caudal vein of the fish under 2-phenoxyethanol 6 h after final quercetin feeding, and was centrifuged to separate plasma (3,000g, 20 min, 3°C). Liver was dissected immediately and tissues were homogenized in two volumes of 0.2 M Na-acetate buffer (pH 5.0). Acidified methanol (MeOH:acetic acid, 100:5) was added to the plasma or to an aliquot of tissue homogenate 10–20 times (weight:volume) to extract quercetin and its metabolite isorhamnetin. The extracts were centrifuged for 10 min at 3,000g at 3°C, and filtered through 0.45-μm syringe filters. With this extraction procedure, the recovery was 95.9 ± 4.3% for quercetin and 92.0 ± 6.3% for isorhamnetin (n = 3).
Enzyme hydrolysis of samples
It is well established that quercetin is rapidly converted after absorption to conjugates; most animal and human tissues contain almost exclusively conjugated forms. When enzyme hydrolysis was conducted, tissue homogenate or plasma was treated with hydrolyzing enzymes before acidic methanol extraction described above. For hydrolysis, samples were incubated for 1 h (37°C) with an equal volume of mixture containing 1,000 U β-glucuronidase (E. coli; Sigma) and 50 U sulfatase (H. pomatia; Sigma) dissolved in 100 μl 0.2 M Na-acetate buffer (pH 5.0). The effectiveness of this enzyme hydrolysis procedure was checked with rat plasma administered with quercetin at 50 mg/kg.
HPLC-electrochemical detection condition
Conventional quercetin and isorhamnetin analysis was carried out by HPLC with UV and electrochemical detectors arranged in tandem. As the sensitivity was higher with the electrochemical detector, UV chromatograms were primarily used as an auxiliary measure. Mobile phase was composed of 27% acetonitrile and 73% water–H3PO4 (99.5:0.5) supplied at 1 ml/min with a Beckman HPLC pump (Model 11B). Samples were injected into a Beckman Ultrasphere ODS column (5 μm, 15 cm × 4.6 mm, 30°C) with a manual injector in 20-μl aliquots. Eluted quercetin and isorhamnetin were detected with a Beckman UV detector (Model 116) at 370 nm (AUFS 0.01) and a Bioanalytical System (West Lafayette, IN) electrochemical detector (LC-4C, applied potential 0.8 V, sensitivity 1 nA, full scale). Concentrations were calculated by comparing peak areas of samples with those of known concentrations of standards. Standard quercetin and isorhamnetin were purchased from Sigma and Apin Chemicals (Abingdon, UK), respectively. Detection limits under these conditions, were 5 and 10 ng/ml for quercetin and isorhamnetin, respectively.
Confirmation of quercetin with UV-photodiode array
Selected samples were analyzed for confirmation of quercetin and isorhamnetin by HPLC, using a Waters (Milford, MA) 2690 separation module and 996 photodiode array detector (Bonello and Blodgett 2003). This analytical system was utilized because of its identification capability for a wide range of flavonoids including quercetin and isorhamnetin. The system was managed by a workstation running the Waters Millennium HPLC software. A Beckman Ultrasphere ODS, 5 μm, 15 cm × 4.6 mm column was used to separate samples. The autosampler temperature was 4°C and column temperature was 30°C. The photodiode array detector was set to scan between 237 and 376 nm. The acidified water–methanol mobile phase developed by Rosemann et al. (1991) was used. Composition of solvent A was 980 ml H2O, and 20 ml of 5% ammonium formate in formic acid. Composition of solvent B was 882 ml methanol, 96 ml H2O, and 20 ml of 5% ammonium formate in formic acid. The following linear gradient [cumulative run time (min), flow rate (ml/min), % solvent A] was used: 2.0, 1.0, 100.0; 4.0, 1.0, 90.0; 20.0, 1.0, 52.0; 38.0, 1.75, 0.0; 39.0, 1.75, 0.0; 41.0, 1.75, 100.0; 43.8, 1.75, 100.0; and 44.0, 0.5, 100.0 (total run time 44 min). Acidic methanol extracts of fish tissue containing quercetin were concentrated 20-fold by freeze-drying and redissolving in methanol before HPLC analysis. Under these HPLC conditions, quercetin and isorhamnetin were eluted at 23.2 and 25.9 min, respectively.
Results and discussion
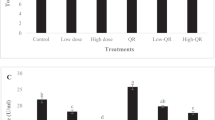
In this study, quercetin and its most abundant metabolite isorhamnetin (3′-O-methylated form) concentrations were analyzed using HPLC-electrochemical detection in both control and quercetin-fed tilapia. Some other fish tissues were also analyzed for baseline survey. The retention time for quercetin and isorhamnetin were 5.4 and 10.5 min, respectively (Fig. 1a). Table 1 shows quercetin concentrations in tissues from tilapia fed a special diet to administer quercetin, and also from other fish species fed with commercial diets and sampled for reference purposes. Following feeding tilapia with quercetin at 1% feed level for 15 weeks; both quercetin and isorhamnetin were detected in the liver and the whole body. The presence of quercetin was ascertained in selected samples with UV-photodiode array detection (Figs. 1b, c). The level of quercetin or isorhamnetin did not change when the quercetin-fed tilapia tissue was treated with the hydrolyzing enzymes β-glucuronidase and sulfatase (18.5 ± 6.7, non-hydrolyzed vs 18.0 ± 5.0 μg/g, hydrolyzed, n = 4). In contrast, in the plasma of rats administered with quercetin 50 mg/kg by stomach intubation, the same hydrolysis procedure elevated quercetin levels from 57.0 ± 57.0 to 823.8 ± 106.4 ng/ml (n = 3). This indicates that the metabolic conjugation, at least glucuronidation/sulfation, is not important in tilapia. This does not, however, exclude the possibility of metabolism to other conjugate(s) resistant to glucuronidase or sulfatase enzymes.
Chromatogram and UV-photodiode array spectrum of quercetin from the Nile tilapia (Oreochromis niloticus) whole body homogenate after 15-weeks feeding. a Chromatogram of quercetin and isorhamnetin with electrochemical detection (full scale = 1 nA). Quercetin, 5.4 min; Isorhamnetin, 10.5 min. b Standard quercetin (500 ng/ml) spectrum obtained with diode array detection. c Spectrum of the peak corresponding to quercetin retention time. A whole body tissue (containing 47 ng/g quercetin) extract was concentrated 20-fold before injection onto the column
It is not clear at this time why quercetin was detected in tilapia but not in rainbow trout fed cottonseed-based diets which contained 0.8% natural quercetin, mostly in glycosides (Dabrowski and Lee 2001). One possible explanation for this difference can be the absorption discrepancy between the aglycone form in this study and the glycosidic ones mainly found in natural sources. Some studies indicate that quercetin glycosides are not well absorbed in animals and humans (Manach et al. 1997; Walle et al. 2000). Indeed, quercetin concentrations in feces were enhanced, but absorption was hardly measurable in rainbow trout (Dabrowski and Lee 2001).
Quercetin is rapidly metabolized to isorhamnetin, which is a weaker antioxidant compared to its parent compound (Manach et al. 1999). In consideration of the rapid conversion of quercetin in mammalian cells (Manach et al. 1998), the prolonged presence in the parent form in tilapia may be an advantageous asset vis-a-vis its pharmacological effects.
As mentioned above, quercetin does not seem to exist in glucuronide or sulfate conjugates in fish tissues. This is in contrast to the fact that both quercetin and isorhamnetin exist almost exclusively as glucuronides/sulfates in human (Erlund et al. 2000; Wittig et al. 2001) and other mammalian animal samples (Piskula and Terao 1998; Manach et al. 1999; Ader et al. 2000; Noroozi et al. 2000; Hou et al. 2003).
Trace levels of quercetin and isorhamnetin were occasionally detected in liver and body tissues of tilapia that were reared as control for 15 weeks without supplementation of quercetin (data not shown). The source of quercetin in this case might have been algae growing on tank walls, but this was not ascertained. Morrice et al. (2000) reported similar quercetin-like peaks from normal rat plasma, but they did not confirm the identity of these mysterious peaks. Sample tissues from other fish species, rainbow trout and sturgeon, fed commercial feeds that may contain plant ingredients were also examined (Table 1). Most of these samples did not exhibit significant quercetin residues except the sturgeon liver. The occurrence in some unexpected tissues needs further investigation.
It is not yet clear yet whether quercetin absorption is more efficient in tilapia compared to other fishes. It is certain, however, from this study that quercetin does not undergo metabolic conjugation to glucuronides and sulfates in tilapia, dissimilarly to other animal species examined so far.
References
Ader P, Wessmann A, Wolffram S (2000) Bioavailability and metabolism of the flavonol quercetin in the pig. Free Radic Biol Med 28:1056–1067. doi:10.1016/S0891-5849(00)00195-7
Bonello P, Blodgett JT (2003) Pinus nigra-Sphaeropsis sapinea as a model pathosystem to investigate local and systemic effects of fungal infection of pines. Physiol Mol Plant Pathol 63:249–261. doi:10.1016/j.pmpp.2004.02.002
Boulton DW, Walle UK, Walle T (1999) Fate of the flavonoid quercetin in human cell lines: chemical instability and metabolism. J Pharm Pharmacol 51:353–359. doi:10.1211/0022357991772367
Dabrowski K, Lee K-J (2001) Quercetin—a new phytochemical in fish diets formulations. Paper presented at the International Triennial Conference & Exposition of Aquaculture, Disney’s Coronado Springs Resort, Lake Buena Vista, Florida, 21–25 January 2001
Day AJ, Williamson G (2001) Biomarkers for exposure to dietary flavonoids: A review of the current evidence for identification of quercetin glycosides in plasma. Br J Nutr 86(Suppl 1):S105–S110
Erlund I, Kosonen T, Alfthan G, Mäenpää J, Perttunen K, Kenraali J et al (2000) Pharmacokinetics of quercetin from quercetin aglycone and rutin in healthy volunteers. Eur J Clin Pharmacol 56:543–553. doi:10.1007/s002280000197
Formica JV, Regelson W (1995) Review of the biology of quercetin and related bioflavonoids. Food Chem Toxicol 33:1061–1080. doi:10.1016/0278-6915(95)00077-1
Hollman PCH, van Trijp JMP, Buysman MNCP (1996) Fluorescence detection of flavonols in HPLC by postcolumn chelation with aluminum. Anal Chem 68:3511–3515. doi:10.1021/ac960461w
Hou YC, Chao PDL, Ho HJ, Wen CC, Hsiu SL (2003) Profound difference in pharmacokinetics between morin and its isomer quercetin in rats. J Pharm Pharmacol 55:199–203. doi:10.1211/002235702487
Jones DJL, Lim CK, Ferry DR, Gescher A (1998) Determination of quercetin in human plasma by HPLC with spectrophotometric or electrochemical detection. Biomed Chromatogr 12:232–235. doi:10.1002/(SICI)1099-0801(199807/08)12:4<232::AID-BMC740>3.0.CO;2-1
Manach C, Morand C, Demigné C, Texier O, Régérat F, Rémésy C (1997) Bioavailability of rutin and quercetin in rats. FEBS Lett 409:12–16
Manach C, Morand C, Crespy V, Demigné C, Texier O, Régérat F et al (1998) Quercetin is recovered in human plasma as conjugated derivatives which retain antioxidant properties. FEBS Lett 426:331–336. doi:10.1016/S0014-5793(98)00367-6
Manach C, Texier O, Morand C, Crespy V, Régérat F, Dimigné C et al (1999) Comparison of the bioavailability of quercetin and catechin in rats. Free Radic Biol Med 27:1259–1266. doi:10.1016/S0891-5849(99)00159-8
Morrice PC, Wood SG, Duthie GG (2000) High-performance liquid chromatographic determination of quercetin and isorhamnetin in rat tissues using β-glucuronidase and acid hydrolysis. J Chromatogr B Analyt Technol Biomed Sci Appl 738:413–417. doi:10.1016/S0378-4347(99)00520-4
Nielsen SE, Dragsted LO (1998) Column-switching high-performance liquid chromatographic assay for the determination of quercetin in human urine with ultraviolet absorbance detection. J Chromatogr B Biomed Sci Appl 707:81–89. doi:10.1016/S0378-4347(97)00574-4
Nieto S, Garrido A, Sanhueza J, Loyola LA, Morales G, Leighton F et al (1993) Flavonoids as stabilizers of fish-oil—an alternative to synthetic antioxidants. J Am Oil Chem Soc 70:773–778. doi:10.1007/BF02542599
Noroozi M, Burns J, Crozier A, Kelly IE, Lean MEJ (2000) Prediction of dietary flavonol consumption from fasting plasma concentration or urinary excretion. Eur J Clin Nutr 54:43–149. doi:10.1038/sj.ejcn.1600908
Piskula MK, Terao J (1998) Quercetin’s solubility affects its accumulation in rat plasma after oral administration. J Agric Food Chem 46:313–4317. doi:10.1021/jf980117v
Plakas SM, Lee T-C, Wolke RE (1985) Absence of overt toxicity from feeding the flavonol, quercetin, to rainbow trout (Salmo gairdneri). Food Chem Toxicol 23:077–1080
Rinchard J, Lee K-J, Dabrowski K, Ciereszko A, Blom JH (2003) Influence of gossypol from dietary cottonseed meal on haematology, reproductive steroids and tissue gossypol enantiomer concentrations in male rainbow trout (Oncorhynchus mykiss). Aquacult Nutr 9:275–282. doi:10.1046/j.1365-2095.2003.00253.x
Rosemann D, Heller W, Sandermann H Jr (1991) Biochemical plant responses to ozone: II. Induction of stilbene biosynthesis in Scots pine Pinus sylvestris L. seedlings. Plant Physiol 97:1280–1286
Shahidi F, Naczk M (1995) Food phenolics: sources, chemistry, effects, applications. Technomic, Lancaster, PA, USA
Tshushida T, Suzuki M (1995) Isolation of flavonoid-glycosides in onion and identification by chemical synthesis of the glycoside: Flavonoids in fruits and vegetables Part I. Nippon Shokuhin Kagaku Kogaku Kaishi 42:100–108
Walle T, Otake Y, Walle UK, Wilson FA (2000) Quercetin glycosides are completely hydrolyzed in ileostomy patients before absorption. J Nutr 130:2658–2661
Weber LP, Kiparissis Y, Hwang GS, Niimi AJ, Janz DM, Metcalfe CD (2002) Increased cellular apoptosis after chronic aqueous exposure to nonylphenol and quercetin in adult medaka (Oryzias latipes). Comp Biochem Physiol C Toxicol Pharmacol 131:51–59
Wittig J, Herderich M, Graefe EU, Veit M (2001) Identification of quercetin glucoronides in human plasma by high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr B Biomed Sci Appl 753:237–243. doi:10.1016/S0378-4347(00)00549-1
Acknowledgments
Part of this paper was prepared with support from the Pond Dynamics/Aquaculture Collaborative Research Support Program (PD/A CRSP), funded by USAID Grant No. LAG-G-00-96-90015-00 and by contributions from the participating institutions. The Aquaculture CRSP accession number is 1290. The opinions expressed herein are those of the author(s) and do not necessarily reflect the views of the US Agency of International Development. Part of this paper was also funded by the United States Department of Agriculture-Cooperative State Research, Education, and Extension Service National Research Initiative (USDA-CSREES NRI) Grant No. 2003-35206-12858.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, K.H., de Oca, G.A.RM., Bonello, P. et al. Determination of quercetin concentrations in fish tissues after feeding quercetin-containing diets. Aquacult Int 17, 537–544 (2009). https://doi.org/10.1007/s10499-008-9222-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-008-9222-6