Abstract
The brown planthopper (BPH) is a potent pest of rice in Asia and Southeast Asia. Host resistance has been found to be the most suitable alternative to manage the insect. But varietal resistance has been found to be short-lived. There has been a constant search for alternate resistance genes. We developed an F8 recombinant inbred population for the BPH resistance gene in Salkathi, an indica landrace from Odisha, India. Phenotyping of RILs against the BPH population at Cuttack, Odisha showed continuous skewed variation with four peaks at 2.1–3.0, 4.1–5.0, 6.1–7.0 and 8.1–9.0 SES score, suggesting the involvement of quantitative loci for resistance to BPH in Salkathi. Mapping showed the presence of two QTLs on the short arm of chromosome 4. One QTL, with phenotype variance of 37.02% is located between the markers RM551 and RM335. The other QTL, with phenotype variance of 7.1% is located between markers RM335 and RM5633. The two QTLs have been designated as qBph4.3 and qBph4.4. QBph4.3 seems to be a novel QTL associated with BPH resistance. We have successfully transferred qBph4.3 and qBph4.4 into two elite rice cultivars, Pusa 44 and Samba Mahsuri. Fine mapping of the identified QTLs may lead to a successful transfer of QTLs into other elite germplasm backgrounds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brown planthopper (Nilaparvata lugens Stal) is one of the most destructive insect pests in rice-growing areas of Asia and Southeast Asia. Both adults and nymphs of the insect feed on rice sheaths by sucking sap from phloem. All the growth stages of rice plant in the field are vulnerable to BPH. Mild infestations by the insects lead to yellowing of leaves, reduction in plant height, growth, vigor, number of productive tillers and grain filling. Heavy infestations cause complete drying and death of plants, a condition known as ‘‘hopperburn’’ (Sogawa 1982; Watanabe and Kitagawa 2000; Ali et al. 2012). BPH also transmits rice tungro, grassy stunt and rugged stunt virus (Ling et al. 1978; Khush 1979; Hibino 1989, 1996; Khush and Brar 1991; Rivera et al. 1996; Normile 2008). The habitat of the insect, rapid multiplication, high mobility and survival against selection forces has made this insect a threat to rice cultivars. BPH control by using of chemical pesticides is not efficient. It is environmentally hazardous coupled with the resurgence of the insect. Cultivation of resistant varieties is the most feasible alternative to manage the insect (Sogawa 1982; Hirabayashi and Ogawa 1995; Wu et al. 2005). Since 1960s, several devastating outbreaks have been reported in China, Vietnam, Philippines, Indonesia, Thailand, Japan, Korea, India, Bangladesh and Malaysia (Heong 2010). In 2005 and 2006, China reported a yield loss of 2.7 million tons of rice due to direct damage by BPH (Pathak 1972; Dyck and Thomas 1979; Sogawa 1982; Holt et al. 1996; Gunathilagaraj and Ganesh Kumar 1997; Heong 2010). Hopperburn has been reported regularly from several states of India, namely Kerala, Bihar, Chhattisgarh, Manipur, Andhra Pradesh, Karnataka, Tamil Nadu, West Bengal, Punjab, Odisha and Uttar Pradesh (Das et al. 1973; Chatterjee 1978; Gangrade et al. 1978; Dhaliwal and Singh 1983; Rizvi and Singh 1983; Sidde Gowda and Gubbaiah 2011; Basanth et al. 2013). Cultivation of resistant varieties is the most economic, effective and environmentally safe for management of BPH (Sogawa 1982; Hirabayashi and Ogawa 1995; Wu et al. 2005).
Systematic breeding programs initiated during the late 1960s have led to the identification of several donors in both landraces and wild species of rice. Currently, more than thirty major genes have been identified for BPH resistance from diverse cultivated and wild species of rice using classical genetics and molecular approaches (Jena and Kim 2010; Fujita and Kohli 2013; Wu et al. 2014; Wang et al. 2015). Fourteen resistance genes have been identified from six wild rice species, O. officinalis, O. australiensis, O. rufipogon, O. eichingeri, O. latifolia and O. minuta, while the rest 16 genes have been identified from cultivated rice (Jena and Kim 2010; Cheng et al. 2013; Fujita and Kohli 2013; Huang et al. 2013; Wu et al. 2014). Five genes, Bph14, Bph26, Bph3, bph29 and Bph18 have been cloned and found to encode coiled-coil nucleotide-binding site and leucine-rich repeat (CC-NBS-LRR) protein, CC-NBS-LRR protein, plasma membrane-localized lectin receptor kinases, B3 DNA-binding domain protein and CC-NBS-NBS-LRR protein, respectively (Du et al. 2009; Tamura et al. 2014; Liu et al. 2015; Wang et al. 2015; Ji et al. 2016).
Breeding resistant rice varieties with some of the major resistance genes were successful (Khush 1989). But, the resistance was short-lived because of adaptation of the BPH population to resistant varieties harboring any one of major genes (Gallagher et al. 1994). Four biotypes have been recorded in BPH of which biotype4 is prevalent in the Indian subcontinent (Heinrichs 1986). The short-lived resistance offered by major genes in different elite cultivars suggested that quantitative resistance to BPH could be the most appropriate solution for the resolution of the issue (Bosque-Perez and Buddenhagen 1992). The complex genetic basis of quantitative resistance, greater variability in virulence pattern of BPH insect population, and non-reproducibility of molecular markers across diverse genetic backgrounds are the limiting factors for the application of molecular markers in breeding programs. Thus, there is a need to identify effective resistance genes/QTLs from diverse sources, characterize them genetically and develop reliable tightly linked molecular markers for their introgression through marker-assisted backcross breeding (MABB) into popular rice varieties. Many quantitative trait loci (QTLs) associated with BPH resistance have been discovered in landraces and wild rice, and mapped to different chromosomes 1, 2, 3, 4, 6, 7, 8, 10 and 12 (Alam and Cohen 1998; Huang et al. 2001; Su et al. 2002; Ramalingam et al. 2003; Ren et al. 2004; Liu et al. 2009; Jena and Kim 2010; Fujita and Kohli 2013). With the advent of new genomics tools, availability of ideal mapping populations and reliable phenotyping techniques, it has become possible to look for genes involved in complex traits.
BPH resistance had always been of great challenge for breeders and biotechnologies as the resistance is not durable due to adaptability and the broader range of biotypes infestation. The short-lived resistance offered by the major genes in different elite cultivars and the fact that the quantitative resistance can be the most appropriate solution to resolve the issue. New sources of genetic resistance are necessary to increase bases of resistance. In continuation to the above theory, the objectives were carried out to search the quantitative resistance offered by the indica cultivar Salkathi. Salkathi is a local indica rice cultivar of Odisha, India and found to be highly resistant to BPH in different parts of India (Anonymous 2007). The resistance in Salkathi is due to antixenesis and antibiosis. It also shows moderate levels of field tolerance to yellow stem borer at reproductive stage (Visalakshmi et al. 2014).
Materials and methods
Plants and insects
The brown planthopper (BPH) population prevalent at Cuttack, Odisha, India was used in the experiment. The insects were reared and maintained on the susceptible cultivar TN1 in the Pest Genomics green house of the Division of Crop Protection, Central Rice Research Institute, Cuttack, Odisha, India. The BPH insects in India are categorized as biotype 4 (Khush 1984). The plant material consisted of the rice cultivar Salkathi (highly resistant) and TN1 (highly susceptible) to BPH population at Cuttack and F8 recombinant inbred lines of the cross TN1/Salkathi, developed by the single-seed-descent method.
Phenotyping of RIL population
Mass rearing and maintenance of pure culture of BPH
BPH insects were collected from the rice fields of Central Rice Research Institute, Cuttack, Odisha, India. Collected insects were mass reared on the susceptible rice cultivar TN1 following the method of Heinrichs et al. (1985).
Standard seed box screening test
Three hundred RILs were evaluated for their reaction to BPH along with the parents TN1 and Salkathi following Standard Seed Box Screening Test method of Jena et al. (2006a, b) with suitable modification. Twenty plants of each RIL line were raised in plastic trays flanked on either side by a line of Salkathi and TN1. Ten days after sowing, seedlings were infested with 2nd instar nymphs at a density of 10–12 nymphs per seedling in insect-proof cages (45 cm × 45 cm × 60 cm). The seedlings were observed daily for damage by BPH. The reaction of each RIL was authentic when all seedlings of TN1 in the tray died. Damage scoring was done according to SES (IRRI) (Heinrichs et al. 1985; SES, IRRI 1988). Mean values of resistance scores/damage severity scores were used for analysis. The complete randomized design was followed with three replications.
Genotyping of RIL
Genomic DNA was isolated from 3 to 4 g young leaf tissues following cetyl trimethyl ammonium bromide (CTAB) method (Murray and Thompson 1980). The extracted genomic DNA samples were then dissolved in TE buffer (10 mM Tris base, 1 mM EDTA) and stored at −20 °C for further analysis. Quality and quantity of DNA of each sample were checked by agarose gel electrophoresis and spectrophotometer. Six hundred ninety-eight SSR markers (http://www.gramene.org) distributed over 12 rice chromosomes were used to identify polymorphic markers between parents. Polymerase chain reaction (PCR) was performed in 20 µl reaction volume containing 40 ng of genomic DNA of parents, 5 pico moles each of forward and reverse primers, 1X PCR buffer [75 mM Tris–HCl (pH9.0), 50 mM KCl, 20 mM (NH4)2SO4], 200 µM dNTP mix (MBI Fermentas, Lithuania, USA), 2 mM of MgCl2 and 1U of Taq polymerase (Biotools, Spain). Amplification was carried out in a thermal cycler (Lark Cycler, England). The thermal cycler profile was initial denaturation at 94 °C for 4 min followed by 35 cycles of denaturation at 94 °C for 1 min, annealing at 55–60 °C (depending upon the Tm value of primer) for 1 min and extension at 72 °C for 2 min with a final extension of 5 min at 72 °C. The amplified PCR products were separated by electrophoresis in 2.5–3.0% agarose gel containing ethidium bromide (EtBr) and visualized under UV using gel imaging system (FluorchemTm 5500 Innotech, USA). The size of amplified bands was determined using Alphaease software (Alpha Innotech, USA). Ninety-two SSR markers, polymorphic between parents were used to genotype the 300 RILs for the construction of linkage map.
Construction of linkage map and detection of QTLs for BPH resistance
The linkage groups and the marker order were determined using integrated QTL software, IcMapping, Version4.0 (http://www.isbreeding.net). Kosambi mapping function was used to convert the recombination frequency to genetic distance in centi-Morgan (cM). The inclusive composite interval additive mapping (ICIM-ADD) program in integrated QTL software, IcMapping, Version4.0 was used to identify QTLs associated with BPH resistance (Li et al. 2010, 2012; Meng et al. 2015). In the first step of ICIM-ADD, a probability value for entering variables (PIN) of 0.001 was used to select the significant markers. One-dimensional scanning of the whole genome was carried out with mapping parameters of 1 cM and a threshold LOD score of 2.5 in order to identify significant QTLs. Additive effects and the percentage of phenotypic variance (PVE) explained by individual QTL were estimated. Two-dimensional interval mapping was conducted to detect epistatic interactions between QTLs using inclusive composite interval mapping of digenic epistatic QTL program (ICIM-EPI).
Validation of QTLs, qBph4.3 and qBph4.4 in breeding lines
The promising resistant breeding lines CR 3006-8-2 (Pusa 44/Salkathi) and CR 3005-230-5 (Samba Mahsuri/Salkathi) were developed in the background of elite susceptible rice cultivars Pusa 44 and Samba Mahsuri, respectively through conventional breeding and validated for resistance QTLs, qBph4.3 and qBph4.4. The genomes of Salkathi, TN1, Pusa 44, Samba Mahsuri, CR 3006-8-2 and CR 3005-230-5 were PCR amplified with the primers specific for flanking linked microsatellite locus RM335, RM551 and RM5633. The amplified PCR products were separated by electrophoresis in 2.5% agarose gel containing ethidium bromide (EtBr) and visualized under UV using gel imaging system (FluorchemTm 5500 Innotech, USA).
Results
Phenotyping of parents and RIL mapping population for BPH resistance
The susceptible parent TN1 showed the mean seedling death of 100% (SES 9) on 6th–7th days of exposure to BPH nymphs while resistant parent Salkathi expressed strong resistance with a mean seedling death of 5.1% (SES 1). Mean resistance scores of 300 RILs ranged from 0.3 to 9.0 with continuous variation skewed towards both the parents having four peaks at 2.1–3.0, 4.1–5.0, 6.1–7.0 and 8.1–9.0 SES (Fig. 1). Twenty RILs showed mean resistance score of 0–1.0 like Salkathi while 104 RILs showed mean resistance score of 8.1–9.0 like TN1. An RIL line was classified as resistant if mean resistance score was ≤5.0 and as susceptible if mean resistance score was >5.0. A total of 122 RILs were found to be resistant whereas 178 RILs were susceptible. The χ2 test showed that the segregation of susceptible plants to resistant plants in RILs fitted into 9:7 ratio (χ2 value: 1.56, P < 0.1), indicating the involvement of two genes/QTLs in controlling resistance to BPH.
Genotyping of RIL mapping population
A total of 698 microsatellite primers were used to find polymorphism between resistant parent, Salkathi and susceptible parent, TN1. Out of which ninety-two (13.18%) primers generated polymorphic markers between TN1 and Salkathi. The genotypic analysis of RILs with 92 markers indicated that 50.62% were Salkathi alleles while 47.62% were TN1 alleles. The overall heterozygosity and missing data were found to be 1.63 and 0.13%, respectively.
Linkage map construction, identification of QTLs and epistatic interactions for BPH resistance
The linkage map was constructed using 92 markers. Sixteen linkage groups comprising of 75 markers were formed. The linkage map covered 1251.78 cM involving ten of the twelve chromosomes with an average marker interval of 17.30 cM (Table 1). The QTL analysis using Inclusive Composite Interval Mapping (ICIM) showed the presence of two QTLs on the short arm of chromosome 4 with LOD scores of 34.2 and 4.61, respectively. These QTLs were designated as qBph4.3 and qBph4.4. The QTL qBph4.3 was mapped to the telomeric region of the short arm of chromosome 4 between microsatellite markers RM551 (0.177 Mb) and RM335 (0.688 Mb) with 1.42 and 0.56 cM distance from RM551 to RM335, respectively. It explained 37.02% of the phenotypic variance. qBph4.4 was mapped between RM335 (0.688 Mb) and RM5633 (13.07 Mb) with 12.46 and 8.23 cM distance from RM335 to RM5633, respectively. It explained PVE of 7.1%. Both the QTLs have negative additive effects of −1.75 and −0.77, respectively in contributing to BPH resistance (Table 2, Figs. 2, 3). The haplotype analysis for RM551, RM335 and RM5633 markers revealed the statistical significance of these markers to be linked with resistance QTLs qBph4.3 and qBph4.4. The Chi square values for phenotypes (resistant and susceptible) and haplotypes (A, B and H) of all the three markers were highly significant (P < 0.01). Hence, in accordance with the null hypothesis, the markers are linked with the resistance QTLs qBph4.3 and qBph4.4 (Table 3). The Chi square values for resistant and susceptible haplotypes for all the three linked markers were non-significant ((P > 0.05) indicating that haplotypes are segregating at 1:1 ratio in RIL mapping population. In addition to the main effect, the significant additive digenic epistatic interaction was identified between two genomic positions on chromosome 4 (LG8) at marker intervals RM551-RM518 (qBph4.3, position 10 cM) and RM335-RM5633 (qBph4.4, position 35 cM). 69.01% phenotypic variance was contributed for BPH resistance by an epistatic interaction between two QTLs qBph4.3 and qBph4.4 with LOD score of 6.93 (Table 4, Fig. 4).
One-dimensional scanning of whole genome for identification of QTLs associated with BPH resistance using integrated QTL IcMapping software. Upper half shows position of QTLs on linkage group8 (chromosome 4) with LOD score while lower half shows additive effects of QTLs. Two peaks corresponding to two QTLs were identified on LG8 (chromosome 4)
Location of two BPH resistance QTLs qBph4.3 and qBph4.4 on linkage group8 (chromosome 4). Left side of graph shows SSR markers and their positions in cM. LG8 represents short arm of chromosome 4(LG8). Right side of graph shows two peaks corresponding QTLs qBph4.3 and qBph4.4 with LOD score of 34.2 and 4.61, respectively
Cyclic graph showing location of epistatic QTLs qBph4.3 and qBph4.4. The two-dimensional interval mapping using ICIM- EPI program of QTL IciMapping software identified significant additive epistatic interaction between two marker intervals RM551-RM518 (qBph4.3, position 10 cM) and RM335-RM5633 (qBph4.4, position 35 cM) on chromosome 4 (LG8) with LOD score of 6.9 contributing 69.01% phenotypic variance towards resistance to BPH. The genetic linkage map is shown as a colorized circle. The parts with different colors on circle are represented as linkage groups and are arranged from LG1 to LG16. Two circles on LG8 (chromosome 4) with different color indicate the position and additive effect of two epistatic QTLs qBph4.3 and qBph4.4 involved in BPH resistance. The connecting line (red color) indicates significant epistatic interaction between QTLs with LOD values of 6.9
Validation of QTLs qBph4.3 and qBph4.4 in elite breeding lines
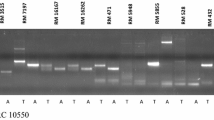
The resistance specific allele of 115 bp was amplified by RM335 in resistant parent Salkathi and promising resistant breeding lines CR 3006-8-2 and CR 3005-230-5, while susceptible specific allele of 150 bp was amplified in susceptible genotypes TN1and recurrent parents Pusa 44 and Samba Mahsuri. RM551 amplified resistance specific allele of 215 bp in resistant genotypes Salkathi, CR 3006-8-2 and CR 3005-230-5, while susceptible specific allele of 165 bp was amplified in susceptible genotypes TN1, Pusa 44 and Samba Mahsuri. RM5633 amplified resistance specific allele of 250 bp in resistant genotypes Salkathi, CR 3006-8-2 and CR 3005-230-5, while susceptible specific allele of 190 bp was amplified in susceptible genotypes TN1, Pusa 44 and Samba Mahsuri. This indicated that the QTLs qBph4.3 and qBph4.4 of Salkathi were contributing resistance to BPH in the promising resistant breeding lines CR 3006-8-2 and CR 3005-230-5 developed in the background of Pusa 44 and Samba Mahsuri, respectively (Fig. 5).
PCR amplification with linked microsatellite markers, a RM551, b RM335 and c RM5633 to validate presence of QTLs qBph4.3 and qBph4.4 in the promising resistant breeding lines. Lanes M- Molecular weight marker (50 bp DNA ladder plus), 1-TN1 (susceptible parent), 2-Salkathi (resistant parent), 3-Pusa 44 (elite susceptible rice variety), 4-CR3006-8-2(promising resistant breeding line in the background of Pusa 44), 5-Samba Mahsuri (elite susceptible rice variety), 6-CR3005-230-5 (promising resistant breeding line in the background of Samba Mahsuri). White arrow indicates the presence of resistant specific band/allele while black arrow indicates the susceptible specific bands/alleles. a Amplification of resistant (215 bp) and susceptible (165 bp) alleles by RM551 confirming the presence of QTL qBph4.3 in resistant breeding lines, b Amplification of resistant (115 bp) and susceptible (150 bp) alleles by RM335 confirming the presence of QTLs qBph4.3 and qBph4.4 in resistant breeding lines, c Amplification of resistant (250 bp) and susceptible (190 bp) alleles by RM5633 confirming the presence of QTL qBph4.4 in resistant breeding lines
Discussion
Resistance in rice is governed by many traits of BPH like host preference, egg mortality, nymphal survival, oviposition, feeding rate, developmental period, adult longevity, population builds up and functional impairment of the host (Soundararajan et al. 2004). Previous studies have enhanced the understanding of BPH resistance/tolerance. The complex nature of resistance has indicated the involvement of several loci or multiple gene families controlling resistance against BPH.
Mass screening at seedling stage using bulk seed box screening test is fast, easy and well adopted for screening of a large number of rice germplasm accessions, breeding lines, and mapping populations. Such large-scale evaluation, where insects are offered a free choice of plant materials has been found to be authentic in screening of mapping population for resistance/susceptibility. Using the screening methodology, molecular genotyping and mapping, we identified two QTLs for resistance in Salkathi. Sun et al. (2007) used 147 F3 families for resistance in Col.5 and found continuous distribution ranging from SES scores of 1.0 to 9.0 with three peaks at 1, 5 and 8. They identified two major QTLs associated with BPH resistance on chromosomes 2 and 6 contributing 29.4 and 46.2% phenotypic variance, respectively. Hou et al. (2011) used 303 F2:3 families of the cross 93-11/WB01 (introgression line from Oryza rufipogon) and identified two QTLs bph22(t) and bph23(t). This F2:3 families showed a continuous distribution, ranging from resistance score of 2.6 to 9.0 with two peaks at 7.0 and 9.0. Hu et al. (2015a) observed seedling resistance scores after 10 days of infestation for the 188 F2:3 families derived from the cross between susceptible variety Zhenshan97 and resistant introgression line IR65482-17-511-5-7(derived from O. australiensis), and found continuous variation of resistance with two peaks at mean SES scores of 3.5 and 5.5. One major and one minor QTL were identified by ICIM using IcMapping QTL software. These QTLs explained 41.38 and 4.11% of the phenotypic variation in BPH resistance, respectively. Hu et al. (2015b) found mean resistance scores in seedlings of 166 BC1F3 which was developed from susceptible cultivar Zhenshan97 and resistance introgression line IR02W101. The score ranged from 1.0 to 9.0, with continuous variation having two peaks around 3.5 and 5 at 10 days after infestation, and 5 and 9 at 12 at 12 days after infestation. Two QTLs QBph3 and Qbph4 were identified at 12 DAI on chromosomes 3 and 4, respectively by composite interval mapping using WinCarto 2.0 QTL software. These QTLs explained 27.59 and 35.43%, respectively. Three less effective QTLs were detected in addition to QBph3 and Qbph4 at 10DAI on chromosomes 6, 7 and 9, and they collectively explained 23.57% of phenotypic variation.
Ninety-two (13.18%) out of 698 microsatellite loci showed polymorphism between Salkathi and TN1. Santhanalakshmi et al. (2010) detected 10% polymorphism between indica parents PTB33 and TN1 using 6% polyacrylamide gel while mapping QTLs associated with BPH resistance in PTB33. Hu et al. (2015b) observed 14% polymorphism between Zhensan97 and IR6582-17-511-5-7 using 4% polyacrylamide gel. However, higher polymorphism was detected when indica and japonica parents were used for mapping of genes, bph2 (28% between ASD7 and C418; Sun et al. 2006), Bph9 (34% between Kaharamana and 02428; Su et al. 2006), Bph12 (37.1% between B14 and TN1; Yang et al. 2002), Bph17 (32.5% between Rathu Heenati and 02428; Sun et al. 2005), and QTLs, QBph2, QBph6 and QBph4.2 (33.6% between Col 5T and 02428; Sun et al. 2007). The 13.18% polymorphism observed by us was probably sufficient to map as both parents are indica.
Previous studies indicated that the BPH resistance genes are clustered in specific regions of rice chromosomes and closely linked to each other similar disease resistance genes (Jena and Kim 2010; Cheng et al. 2013). Interestingly, about half of the BPH resistance genes were identified and mapped to chromosomes 4 and 12 despite their different origins. Eight genes, Bph3 (Zhiyong et al. 2004; Liu et al. 2014), Bph6 (Qiu et al. 2010), Bph12 (Yang et al. 2002), Bph15 (Yang et al. 2004), bph16 (Hirabayashi et al. 1998), Bph17 (Sun et al. 2005), Bph20 (Rahman et al. 2009) and Bph27 (Huang et al. 2013) were mapped to chromosome 4 while other eight genes, Bph1 (Kim and Sohn 2005), bph2 (Sun et al. 2006), Bph9 (Su et al. 2006), Bph10 (Nguyen and Bui 2003), Bph18 (Jena et al. 2006a, b), bph19 (Chen et al. 2006), Bph21 (Rahman et al. 2009) and Bph26 (Yara et al. 2010) were mapped to chromosome 12, suggesting that these two chromosomes harbor hot-spots for BPH resistance genes. The Bph6 gene was identified in the cultivar Swarnalata between SSR markers RM5742 and RM6997 (21.1–21.38 Mb) (Qiu et al. 2010). Two genes, Bph3 and Bph17(Qbph4) were identified in the landrace Rathu Heenati of Sri Lanka, and mapped on chromosome 4 between markers HJ16 and J417(6.94, 6.97 Mb), and RM8213-RM5953 (4.44–9.38 Mb), respectively (Sun et al. 2005; Liu et al. 2014). Five genes Bph12, Bph15, bph16, Bph20, and Bph27 were mapped to region on chromosome 4 flanked by markers RM1305-RM16459 (5.21–5.66 Mb), RG1-RG2(6.57–6.7 Mb), G271-R93 (20.17–21.15 Mb), MS10-RM5953(8.5–9.38 Mb), and RM16846-RM16853(18.94–19.03 Mb), respectively (Hirabayashi et al. 1998; Yang et al. 2004; Rahman et al. 2009; Huang et al. 2013). Hu et al. (2015a) identified a major QTL, qBph4.2 on chromosome 4 between RM261 and Xc4-27 (6.57–6.9 Mb). Hu et al. (2015b) identified and fine mapped a major QTL Qbph4 on chromosome 4 in the introgression line, IR02W101 derived from O. officinalis. It was delimited to a 360 kb region between markers P16 and Xc4-27 (6.7–6.9 Mb), and it explained 35.42% phenotypic variation for the trait. Hence, five genes, Bph12, Bph15, Bph17, Bph3, Bph20 and two QTLs, qBph4.2, Qbph4, are closely linked and distributed in a narrow region between RM8213 and RM5953.
The present study revealed that BPH resistance in the landrace Salkathi is controlled by two QTLs qBph4.3, and qBph4.4. The qBph4.3 explained 37.02% of the phenotypic variance and thus it is a major QTL. The other locus qBph4.4 explaining 7.1% of the phenotypic variance is a minor QTL. The qBph4.3 was mapped to the telomeric region on the short arm of chromosome 4 between the SSR markers RM551 (0.177 Mb) and RM335 (0.688 Mb) with 1.42 and 0.56 cM distance from RM551 to RM335, respectively. No gene or QTL for BPH resistance has been mapped to this region. Hence, qBph4.3 appears to be novel BPH resistance locus which needs further confirmation by fine mapping and cloning. The qBph4.4 was mapped to a region between RM335 (0.688 Mb) and RM5633 (13.07 Mb) with 12.44 and 8.23 cM distance from RM335 to RM5633, respectively. This QTL region has five major genes namely, Bph12, Bph15, Bph17, Bph3 and Bph20, and 2 QTLs qBph4.2 and Qbph4. These genes and QTLs except Bph3 originated from wild species while Bph3 originated from Sri Lankan indica landrace, Rathu Heenati. Further, studies are necessary to know whether qBph4.4 is different or same as above mentioned genes/QTLs.
Epistasis is a commonly observed genetic phenomenon. It is an important source of variation of complex traits, which could maintain additive variance and therefore assure the long-term genetic gain in breeding (Lark et al. 1995; Holland et al. 1997; Luo et al. 2009). Qiao et al. (2008) indicated that epistatic interactions, as well as major QTLs, should be taken into consideration for breeding BPH-resistant cultivars through MAS. The significant additive epistatic interaction was identified between QTLs qBph4.3 and qBph4.4 as high as 69.01% that could play a certain role in the expression of resistance to BPH. Similar to our observations, Rahman et al. (2009) observed significant epistatic interactions between two random markers bracketing major QTLs, which were designated as Bph20(t) and Bph21(t). Xu et al. (2002) identified seven main QTLs and many epistatic QTLs associated with quantitative resistance to BPH using a recombinant inbred line population derived from Teqing and Lemont.
The use of resistant cultivars has proven to be the most efficient way to reduce economic damage caused by BPH. The insect appears to be effectively overcoming single major resistance gene under natural conditions. Few major resistance genes have been introduced into elite cultivars through conventional breeding approaches (Fujita and Kohli 2013; Hu et al. 2016). A number of BPH-resistant varieties with single resistance gene (i.e., Bph1) were developed and released, which saved rice production from massive BPH damage in the field (Khush and Virk 2005; Jena and Kim 2010). However, the resistance broke down in 1976 with the development of a new BPH population (biotype 2). Varieties with bph2 showing effective resistance to biotype 2 were then released and widely grown. In 1981, another BPH population (biotype 3) capable of overcoming the resistance of bph2 was detected. Consequently, pyramiding of two or more major resistance genes and QTLs was proposed to provide stronger and more durable resistance in susceptible rice cultivars. Sharma et al. (2004) reported that the pyramided lines carrying Bph1 and bph2 genes showed higher resistance than lines with the only bph2. Hu et al. (2011) introgressed Bph14 and Bph15 resistance genes into several rice hybrids. The planting of resistant pyramided hybrids around conventional susceptible hybrids significantly reduced the overall BPH population in the field. Myint et al. (2012) found that the level of BPH resistance in Bph25/Bph26-NILs was significantly higher than Bph25-NILs or Bph26-NILs. Qui et al. (2012) found that the pyramided lines of 9311 and Nipponbare with Bph6 and Bph12 resistance genes showed less damage as compared to monogenic lines. Hu et al. (2012) introgressed Bph14 and Bph15 resistance genes into Minghui 63 and its derived hybrids. The results showed that the improved hybrids containing resistance genes showed enhanced resistance. Hu et al. (2013) pyramided three dominant BPH resistance genes Bph14, Bph15 and Bph18 into elite indica rice 9311 and its hybrids. The results showed an additive effect of pyramided genes in the order of Bph14/Bph15/Bph 18 ≥ Bph 14/Bph 15 > Bph 15/Bph 18 ≥ Bph 15 > Bph 14/Bph 18 ≥ Bph 14 ≥ Bph 18 > none. Hu et al. (2015b) developed eight near-isogenic lines (NILs) and four pyramided lines (PLs) containing both Qbph3 and Qbph4 or with Bph14 and Bph15 in the background of Shenzhen97 and 9311. The BPH bioassay showed that the PL-QBph3/Qbph4 was the most resistant of the lines. Wang et al. (2016) pyramided Bph14 and Bph15 resistance genes and improved the BPH resistance in Huahui938 and its derived hybrids.
The landrace, Salkathi used in this study carries two QTLs qBph4.3 and qBph4.4 and is a valuable source of BPH resistance. These two QTLs may be belonging to different resistance gene families with different mechanisms for death response. Cloning of five resistance genes, Bph14, Bph26, Bph3, bph29 and Bph18 indicated the existence of different mechanisms for controlling BPH infestation (Du et al. 2009; Tamura et al. 2014; Liu et al. 2015; Wang et al. 2015; Ji et al. 2016). The existence of the strong additive epistatic interaction between two QTLs qBph4.3 and qBph4.4 is very much impressive enough to use Salkathi as a donor for developing elite cultivars with stronger BPH resistance. We have successfully transferred BPH resistance from Salkathi into two elite cultivars, Pusa 44 and Samba Mahsuri through conventional back-cross breeding method. The resistant breeding lines, CR 3006-8-2 (Pusa 44/Salkathi) and CR 3005-230-5 (Samba Mahsuri/Salkathi) were found to be promising in the AICRIP trials (Annual Report, DRR, Annual Report 2012–2013) (Table 5). These lines were found to carry QTLs qBph4.3 and qBph4.4, and would be useful for MAS breeding programs. Further, these breeding lines were also found to be resistant to white backed planthopper (WBPH), green leafhopper and gall midge (Annual Report, DRR, 2011). Visalakshmi et al. (2014) screened breeding lines CR 3006-8-2 and CR3005-230-5 along with other entries against yellow stem borer under natural field conditions of Agricultural Research Station, Ragolu, Srikakulam district of Andhra Pradesh, India during Kharif 2011 and 2012. These breeding lines were found to be moderately tolerant to yellow stem borer at reproductive stage. The presence of BPH resistance QTLs in these breeding lines was detected using flanking linked markers RM335 and RM551, and RM335 and RM5633. Fine mapping of these resistance loci would identify closely linked markers for future use in the molecular breeding programs. The QTLs qBph4.3 and qBph4.4 can be integrated into multiple resistance breeding programs using MAS.
References
Alam SN, Cohen MB (1998) Detection and analysis of QTLs for resistance to the brown planthopper Nilaparvata lugens, in a doubled haploid rice population. Theor Appl Genet 97:1370–1379
Ali MP, Alghamdi SS, Begum MA, Anwar Uddin ABM, Alam MZ, Huang D (2012) Screening of rice genotypes for resistance to the brown planthopper, Nilaparvata lugens stal. Cereal Res Commun 40(4):502–508
Annual Report (2011) Multiple resistance screening trial. In: Directorate of Rice Research (ed), Hyderabad, pp 2.12–2.13
Annual Report (2012–2013) Host plant resistance. In: Directorate of rice research (ed), Hyderabad, p 14
Anonymous (2007) Proceedings of 42nd Annual All India Rice Group Meeting. In: Directorate of Rice Research (ed), Hyderabad, 9–11th April, p 34
Basanth YS, Sannaveerappanavar VT, Siddegowda DK (2013) Susceptibility of different populations of Nilaparvata lugens from major rice growing areas of Karnataka, India to different groups of insecticides. Rice Sci 20(5):371–378
Bosque-Perez NA, Buddenhagen IW (1992) The development of host-plant resistance to insects pests: outlook for the topics. In: Menken SBJ, Visser JH, Harrewijn P (eds) Proceedings 8th international symposium insect plant relationships. Springer-Science+Business Media, Dordrecht, pp 235–249
Chatterjee PB (1978) Occurrence of brown planthopper on rice in West Bengal, India. Int Rice Res Newsl 3:12
Chen JW, Wang L, Pang XF, Pan QH (2006) Genetic analysis and fine mapping of a rice brown planthopper (Nilaparvata lugens Stal) resistance gene bph19(t). Mol Genet Genomics 275:321–329
Cheng XY, Zhu LL, He CC (2013) Towards understanding of molecular interactions between rice and the brown planthopper. Mol Plant 6:621–634
Das NM, Mammen KV, Christudas SP (1973) Occurrence of Nilaparvata lugens (Stål) (Delphacidae: Homoptera) as a serious pest of paddy in Kerala. Agric Res J Kerala 10:191–192
Dhaliwal GS, Singh J (1983) Outbreaks of white backed planthopper and brown planthopper in Punjab, India. Int Rice Res Newsl 32:26–28
Du B, Zhang WL, Liu BF, Hu J, Wei Z, Shi ZY, He RF, Zhu LL, Chen RZ, Han B (2009) Identification and characterization of Bph14, a gene conferring resistance to brown planthopper in rice. Proc Natl Acad Sci USA 52:22163–22168
Dyck VA, Thomas B (1979) The brown planthopper problem. In: International Rice Research Institute (ed) Brown planthopper-threat to rice production in Asia. International Rice Research Institute, Philippines, pp 3–17
Fujita D, Kohli A, Horgan FG (2013) Rice resistance to planthoppers and leafhoppers. Crit Rev Plant Sci 32(3):162–191
Gallagher KD, Kenmore PE, Sogawa K (1994) Judicial use of insecticides deter planthopper outbreaks and extend the life of resistant varieties in southeast Asian rice. In: Denno RF, Perfect JT (eds) Planthoppers: their ecology and management. Chapman and Hall, Boca Raton, pp 599–614
Gangrade GA, Kaushik UK, Patidar GL, Shukla BC, Shrivastava SK, Deshmukh PD, Pophaly DJ (1978) Insect pest of summer paddy in MP. Int Rice Res Newsl 3:16
Gunathilagaraj K, Ganesh Kumar M (1997) Rice insect outbreaks: an analysis. Madras Agric J 84:298–311
Heinrichs EA (1986) Perspectives and directions for the continued development of insect-resistant rice varieties. Agric Ecosyst Environ 18:9–36
Heinrichs EA, Medrano FG, Rapusas HR (1985) Genetic evaluation for insect resistance in rice. International Rice Research Institute, Manila, p 365
Heong KL (2010) Assessing BPH outbreak risks of commonly used insecticides. Available: http://ricehoppers.net/2010/09/farmers-in-central-thailand-remain-trapped-by-the-bph-problem/
Hibino H (1989) Insect-borne viruses in rice. In: Harris KF (ed) Advances in disease vector research. Springer-Verlag, New York, pp 209–241
Hibino H (1996) Biology and epidemiology of rice viruses. Annu Rev Phytopathol 34:249–274
Hirabayashi H, Ogawa T (1995) RFLP mapping of Bph-1 (Brown planthopper resistance gene) in rice. Breed Sci 45:369–371
Hirabayashi H, Angeles ER, Kaji R, Ogawa T, Brar DS, Khush GS (1998) Identification of brown planthopper resistance gene derived from O. officinalis using molecular markers in rice. Breed Sci 48:82
Holland JB, Moser HS, O’Donoughue LS, Lee M (1997) QTLs and epistasis associated with vernalization responses in oat. Crop Sci 37:1309–1414
Holt J, Chancellor TCB, Reynolds DR, Tiongco ER (1996) Risk assessment for rice planthopper and tungro disease outbreaks. Crop Prot 15(4):359–368
Hou L, Yu P, Xu Q, Yuan X, Yu H, Wang Y, Wang C, Wan G, Tang S, Peng S, Wei X (2011) Genetic analysis and preliminary mapping of two recessive resistance genes to brown planthopper, Nilaparvata lugens Stal in rice. Rice Sci 18:238–242
Hu J, Yang CJ, Zhang QL et al (2011) Resistance of pyramided rice hybrids to brown planthoppers. Chin J Appl Entom 48:1341–1347
Hu J, Li X, Wu C, Yang C, Hua H, Gao G, Xiao J, He Y (2012) Pyramiding and evaluation of the brown planthopper resistance genes Bph14 and Bph15 in hybrid rice. Mol Breed 29:61–69
Hu J, Cheng MX, Gao GJ et al (2013) Pyramiding and evaluation of three dominant brown planthopper resistance genes in the elite indica rice 9311 and its hybrids. Pest Manag Sci 69:802–808
Hu J, Xiao C, Cheng M, Gao G, Zhang Q, He Y (2015a) A new finely mapped Oryza autraliensis- derived QTL in rice confers resistance to brown planthopper. Gene 561:132–137
Hu J, Xiao C, Cheng M, Gao G, Zhang Q, He Y (2015b) Fine mapping and pyramiding of brown planthopper resistance genes QBph3 and QBph4 in an introgression line from wild rice O. officinalis. Mol Breed 35:3. doi:10.1007/s11032-015-0228-2
Hu J, Xiao C, He Y (2016) Recent progress on the genetics and molecular breeding of brown planthopper resistance in rice. Rice 9:30. doi:10.1186/s12284-016-0099-0
Huang Z, He G, Shu L, Li X, Zhang Q (2001) Identification and mapping of two brown planthopper resistance genes in rice. Theor Appl Genet 102:929–934
Huang D, Qiu Y, Zhang Y, Huang F, Meng J, Wei S, Li R, Chen B (2013) Fine mapping and characterization of Bph27, a brown planthopper resistance gene from wild rice (Oryza rufipogon Griff). Theor Appl Genet 126:219–229
Jena KK, Kim SM (2010) Current status of brown planthopper (BPH) resistance and genetics. Rice 3:161–171
Jena M, Sahu RK, Marndi BC (2006a) Screening of rice varieties for resistance against brown planthopper (Nilaparvata lugens Stal.). Oryza 43(4):334–335
Jena KK, Jeung JU, Lee JH, Cho HC, Brar DS (2006b) High-resolution mapping of a new brown planthopper (BPH) resistance gene, Bph18(t) and marker-assisted selection for BPH resistance in rice (Oryza Sativa L.). Theor Appl Genet 112:288–297
Ji H, Kim SR, Kim YH et al (2016) Map-based cloning and characterization of the BPH18 gene from wild rice conferring resistance to brown plant hopper (BPH). Insect Pest Sci Rep 6:34376. doi:10.1038/srep34376
Khush GS (1979) Genetics of and breeding for resistance to the brown planthopper. In: International Rice Research Institute (ed) The brown planthopper threat to rice production. International Rice Research Institute, Los Banos, pp 321–332
Khush GS (1984) Breeding rice for resistance to insects. Prot Ecol 7:147–165
Khush GS (1989) Progress in irrigated rice research. International Rice Research Institute, Los Banos, pp 79–92
Khush GS, Brar DS (1991) Genetics of resistance to insects in crop plants. Adv Agron 45:223–274
Khush GS, Virk PS (2005) IR varieties and their impact. International Rice Research Institute, Los Banos
Kim SM, Sohn JK (2005) Identification of a rice gene Bph1 conferring resistance to brown planthopper (Nilaparvata lugens Stal.) using STS markers. Mol Cells 20(1):30–34
Lark KG, Chase K, Adler FR, Mansur LM, Orf JJ (1995) Interactions between quantitative trait loci in soybean in which trait variation at one locus is conditional upon a specific allele at another. Proc Natl Acad Sci USA 92:4656–4660
Li H, Hearne S, Banziger M, Li Z, Wang J (2010) Statistical properties of QTL linkage mapping in biparental genetic populations. Heredity 105:257–267
Li H, Zhang L, Wang J (2012) Estimation of statistical power and false discovery rate of QTL mapping methods through computer simulation. Chin Sci Bull 57:2701–2710
Ling KC, Tiongco ER, Aguiero VM (1978) Rice ragged stunt, a new virus disease. Plant Dis Rep 62:701–705
Liu YQ, Su CC, Jiang L, He J, Wu H, Peng C, Wan JM (2009) The distribution and identification of brown planthopper resistance genes in rice. Hereditas 146:67–73
Liu Y et al (2014) A gene cluster encoding lectin receptor kinases confers broad-spectrum and durable insect resistance in rice. Nat Biotechnol 33:301–305
Liu Y, Wu H, Chen H, Liu Y, He J, Kang H et al (2015) A gene cluster encoding lectin receptor kinases confers broad-spectrum and durable insect resistance in rice. Nat Biotechnol 33(3):301–305
Luo X, Fu Y, Zhang P, Wu S, Tian F (2009) Additive and over-dominant effects resulting from epistatic loci are the primary genetic basis of heterosis in rice. J Integr Plant Biol 51:393–408
Meng L, Li H, Zhang L, Wang J (2015) QTL IciMapping: integrated software for genetic linkage map construction and quantitative trait locus mapping in biparental populations. Crop J 3:269–283
Murray MG, Thompson WF (1980) Rapid isolation of high molecular- weight plant DNA. Nucleic Acids Res 8:4321–4432
Myint K, Fujita D, Matsumura M et al (2012) Mapping and pyramiding of two major genes for resistance to the brown planthopper (Nilaparvata lugens Stål) in the rice cultivar ADR52. Theor Appl Genet 124:495–504
Nguyen TL, Bui CB (2003) Genetic and physical maps of gene Bphl0 controlling brown planthopper resistance in rice (Oryza sativa L.). Omonrice 11:35–41
Normile D (2008) Reinventing rice to feed the world. Science 321:330–337
Pathak MD (1972) Resistance to insect pests in rice varieties. In: International Rice Research Institute (ed) Rice breeding. IRRI, Los Banos, pp 325–341
Qiao Y, Jiang W, Rahman ML, Chu SH, Piao R, Han L, Koh HJ (2008) Comparison of molecular linkage maps and QTLs for morphological traits in two reciprocal backcross populations of rice. Mol Cells 25(3):417–427
Qiu Y, Guo J, Jing S, Zhu L, He G (2010) High-resolution mapping of the brown planthopper resistance gene Bph6 in rice and characterizing its resistance in the 9311 and Nipponbare near isogenic backgrounds. Theor Appl Genet 121:1601–1611
Qiu Y, Guo J, Jing S et al (2012) Development and characterization of japonica rice lines carrying the brown planthopper-resistance genes BPH12 and BPH6. Theor Appl Genet 124:485–494
Rahman ML, Jiang W, Chu SH, Qiao Y, Ham Th, Mo Woo, Lee J, Khanam MS, Chin JH, Jeung JU (2009) High resolution mapping of two rice brown planthopper resistance genes, Bph20 (t) and Bph21(t), originating from Oryza minuta. Theor Appl Genet 119(7):1237–1246
Ramalingam J, Vera Cruz C, Kukreja K, Chittoorr JM, Wu JL, Lee SW (2003) Candidate defense genes from rice, barley and maize and their association with qualitative and quantitative resistance in rice. Mol Plant-Microbe Interact 16:14–24
Ren X, Wang X, Yuan H, Weng Q, Zhu L, He G (2004) Mapping quantitative trait loci and expressed sequence tags related to brown planthopper resistance in rice. Plant Breed 123:342–348
Rivera CT, Ou SH, Lida TT (1996) Grassy stunt disease of rice and its transmission by Nilaparvata lugens(stal.). Plant Dis Rep 50:453–456
Rizvi SMA, Singh HM (1983) Brown planthopper in Eastern Uttar Pradesh, India. Int Rice Res Newsl 8:16
Santhanalakshmi S, Saikumar S, Rao S, Saiharini A, Khera P, Shashidhar HE, Kadirvel P (2010) Mapping genetic locus linked to brown planthopper resistance in rice Oryza sativa. Int J Plant Breed Genet 4(1):13–22
Sharma PN, Torii A, Takumi S, Mori N, Nakamura C (2004) Marker-assisted pyramiding of brown planthopper (Nilaparvata lugens Stal) resistance genes Bph1 and Bph2 on rice chromosome 12. Hereditas 140:61–69
Sidde Gowda DK, Gubbaiah (2011) Insect pests of rice and their management in Karnataka state of India: a review. Agric Rev 32:55–62
Sogawa K (1982) The rice brown planthopper: feeding physiology and host plant interactions. Annu Rev Entomol 27:49–73
Sogawa K, Liu GJ, Shen JH (2003) A review on the hyper-susceptibility of Chinese hybrid rice to insect pests. Chin J Rice Sci 17:23–30
Soundararajan RP, Kadirvel P, Gunathilagaraj K, Maheswaran M (2004) Mapping of quantitative trait loci associated with resistance to brown planthopper in rice by means of a doubled haploid population. J Agron Crop Sci 44:2214–2220
Su CC, Cheng XN, Zhai HQ, Wan JM (2002) Detection and analysis QTL for resistance to brown planthopper, Nilaparvata lugens (Stål), in rice (Oryza sativa L) using backcross inbred lines. Acta Genet Sin 29:332–338
Su CC, Zhai HQ, Wang CM, Sun LH, Wan JM (2006) SSR mapping of brown planthopper resistance gene Bph9 in Kaharamana, an indica rice (Oryza sativa L.). Acta Genet Sin 33:262–268
Sun L, Su C, Wang C, Zhai H, Wan J (2005) Mapping of a major resistance gene to the brown planthopper in the rice cultivar Rathu Heenati. Breed Sci 55:391–396
Sun LH, Wang CM, Su CC, Liu YQ, Zhai HQ, Wan J (2006) Mapping and marker-assisted selection of a brown planthopper resistance gene bph2 in rice (Oryza sativa L.). Acta Genet Sin 33:171–723
Sun LH, Liu YQ, Jiang L, Su CC, Wang CM, Zhai HQ, Wan JM (2007) Identification of quantitative trait loci associated with resistance to brown planthopper in the indica cultivar Col. 5 Thailand. Hereditas 144:48–52
Tamura Y, Hattori M, Yoshioka H, Yoshioka M, Takahashi A, Wu J, Sentoku N, Yasui H (2014) Map-based cloning and characterization of a brown planthopper resistance gene BPH26 from Oryza sativa L. ssp. indica cultivar ADR52. Sci Rep 4:5872
Visalakshmi V, Satyanarayna H, Jyothula DPB, Raju MRB, Murthy KVR (2014) Screening of rice germplasm for resistance to yellow stem borer Scirpophaga insertulas Walker. Int J Plant Animal Environ Sci 4(1):129–133
Wang Y, Cao L, Zhang Y, Cao C, Liu F, Huang F et al (2015) Map-based cloning and characterization of BPH29, a B3 domain-containing recessive gene conferring brown planthopper resistance in rice. J Exp Bot 66:6035–6045
Wang H, Ye S, Mou T (2016) Molecular breeding of rice restorer lines and hybrids for brown planthopper (BPH) resistance using the Bph14 and Bph15 genes. Rice 9:53. doi:10.1186/s12284-016-0126-1
Watanabe T, Kitagawa H (2000) Photosynthesis and translocation of assimilates in rice plants following phloem feeding by the planthopper Nilaparvata lugens (Homoptera: Delphacidae). J Econ Entomol 93:1192–1198
Wu CJ, Jiang GH, Li X, Liu T, Xu CG, He YQ (2005) Dynamic detection and analysis of QTL for resistance to the brown planthopper using a double haploid rice population. Mol Plant Breed 3(4):456–462
Wu H, Liu Y, He J, Yanling Liu, Jiang L, Liu L, Wang C, Cheng X, Wan J (2014) Fine mapping of brown planthopper (Nilaparvata lugens Stàl) resistance gene Bph28(t) in rice (Oryza sativa L.). Mol Breed 33:909–918
Xu XF, Mei HW, Luo LJ, Cheng XN, Li ZK (2002) RFLP facilitated investigation of the quantitative resistance of rice to brown planthopper (Nilaparvata lugens). Theor Appl Genet 104:248–253
Yang H, Ren X, Weng Q, Zhu L, He G (2002) Molecular mapping and genetics of a rice brown planthopper (Nilaparvata lugens Stal) resistance gene. Hereditas 136:39–43
Yang HY, You AQ, Yang ZF, Zhang FT, He RF, Zhu LL, He GC (2004) High-resolution genetic mapping at the Bph15 locus for brown planthopper resistance in rice (Oryza sativa L.). Theor Appl Genet 110:182–191
Yara A, Phi CN, Matsumura M, Yoshimura A, Yasui H (2010) Development of near isogenic lines for Bph25(t) and Bph26(t), which confers resistance to the brown planthopper, Nilaparvata lugens (Stàl) in indica rice ‘ADR52’. Breed Sci 60:639–647
Zhiyong X, Guangxuan TAN, Aiqing YOU, Guangyuan HE, Chaowen SHE, Lijia LI, Yunchun SONG (2004) Comparative physical mapping of rice BAC clones linked to resistance genes Glh, Bph-3 and xa-5 in Oryza sativa L. and O. granulate Nees et Arn ex Watt. Chin Sci Bull 49:591–596
Acknowledgements
We express our gratitude to the Department of Biotechnology (DBT), Ministry of Science and Technology, Govt. of India for financial support to carry out this research. We also thank the Director, NRRI, Odisha for providing all necessary infrastructure facility to carry out this research.
Author contribution statement
SKM, RSP, SLM, AN conducted phenotyping and genotyping of RIL mapping population experiment, RKS, SCS and LB developed RIL mapping population, SCS and MJ which contributed to phenotyping of RIL mapping population, LB and SKM carried out QTL analysis, LB, SKM and RSP prepared manuscript, TM helped in preparation and critical review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Mohanty, S.K., Panda, R.S., Mohapatra, S.L. et al. Identification of novel quantitative trait loci associated with brown planthopper resistance in the rice landrace Salkathi. Euphytica 213, 38 (2017). https://doi.org/10.1007/s10681-017-1835-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10681-017-1835-2