Abstract
Brown planthopper (BPH) is one of the most destructive insect pests of rice. Wild species of rice are a valuable source of resistance genes for developing resistant cultivars. A molecular marker-based genetic analysis of BPH resistance was conducted using an F2 population derived from a cross between an introgression line, ‘IR71033-121-15’, from Oryza minuta (Accession number 101141) and a susceptible Korean japonica variety, ‘Junambyeo’. Resistance to BPH (biotype 1) was evaluated using 190 F3 families. Two major quantitative trait loci (QTLs) and two significant digenic epistatic interactions between marker intervals were identified for BPH resistance. One QTL was mapped to 193.4-kb region located on the short arm of chromosome 4, and the other QTL was mapped to a 194.0-kb region on the long arm of chromosome 12. The two QTLs additively increased the resistance to BPH. Markers co-segregating with the two resistance QTLs were developed at each locus. Comparing the physical map positions of the two QTLs with previously reported BPH resistance genes, we conclude that these major QTLs are new BPH resistance loci and have designated them as Bph20(t) on chromosome 4 and Bph21(t) on chromosome 12. This is the first report of BPH resistance genes from the wild species O. minuta. These two new genes and markers reported here will be useful to rice breeding programs interested in new sources of BPH resistance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brown planthopper (BPH), Nilaparvata lugens Stål (Homoptera: Delphacidae), is one of the most destructive insect pests of rice (Oryza sativa L.), especially of temperate japonica rice cultivars where few resistance genes against BPH have been incorporated (Jena et al. 2006). BPH extracts phloem saps of rice plants using its stylet-type mouthparts, resulting in a severe damage symptom known as ‘hopper-burn’. BPH also transmits rice grassy stunt virus and ragged stunt virus as vectors (Rivera et al. 1966; Heinrichs 1979). On the basis of differential reactions, BPH populations in different countries have been categorized into four biotypes (Khush et al. 1985). The original populations in East and Southeast Asia belonged to biotype 1. Biotype 2 originated in Phillippines, Indonesia and Vietnam (Khush 1979) and is the dominant biotype in these countries. Biotype 3 was produced in the laboratory at the International Rice Research Institute (IRRI) (Pathak and Khush 1979) and in Japan (Ikeda and Vaughan 1991). Biotype 4 is found only in South Asia (Khush 1984). In Southeast Asia, the BPH populations shifted from biotype 1 to biotype 2 in the 1970s (Feuer 1976; Mochida et al. 1977; Stapley et al. 1979), and at present comprise mostly a complex of biotypes 2 and 3 (Medrano and Heinrichs 1985; Sogawa et al. 1987; Huynh and Nhung 1988). On the other hand, Chelliah and Bharathi (1993) categorized BPH populations into five biotypes on the basis of their differential reactions to a set of reference cultivars. Of the biotypes (1, 2, and 3) known to be present in Korea, biotype 1 is the most prevalent (Park and Song 1988). Conventional methods of controlling BPH are highly dependent on spraying poisonous chemicals, which is expensive in terms of labor, money, and the environment. In addition, the occurrence of resurgence, a phenomenon of pest population increase after application of insecticides (Heinrichs et al. 1982), is problematic. Thus introduction of resistant cultivars is beneficial economically and environmentally (Huang et al. 2001).
Incorporating resistance gene(s) from wild species into cultivated species can be an alternative approach to develop BPH resistance in susceptible commercial cultivars. In general, wild species are poor in important agronomic traits such as yield, plant type, grain type, eating quality, seed shattering habit, etc. However, the wild species of Oryza are regarded as a treasure trove of novel genes for resistance to disease or insect pests and tolerance against environmental stresses. Since few useful genes from wild germplasm accessions have been explored, there is still great potential for exploring novel genes. Several wild species including O. minuta, O. latifolia, O. nivara, O. officinalis and O. punctata were reported to possess resistance genes to different BPH biotypes (Wu et al. 1986). Nine resistance loci among a total of 19 BPH resistance loci reported so far in rice have been identified from wild species. They are Bph10 on the long arm of chromosome 12 from O. australiensis (Ishii et al. 1994), Bph12(t) on the short arm of chromosome 4 from O. latifolia (Yang et al. 2002), Bph13(t) on the long arm of chromosome 2 from O. eichingeri (Liu et al. 2001), another Bph13(t) against BPH biotype 4 on the short arm of chromosome 3 from O. officinalis (Renganayaki et al. 2002), Qbp1 and Qbp2 (later named as Bph14 and Bph15, respectively) on the long arm of chromosome 3 and the short arm of chromosome 4, respectively, from O. officinalis (Huang et al. 2001), Bph18(t) on the long arm of chromosome 12 from O. australiensis (Jena et al. 2006), and bph11(t) and bph12(t) on the long arm of chromosome 3 and chromosome 4 respectively, from O. officinalis (Hirabayashi et al. 1999).
O. minuta (2n = 48, BBCC genome, Acc No.101141), an allotetraploid wild species, belonging to the O. officinalis complex, is endemic to Philippines and Papua New Guinea. It is known to have useful genes for resistance to bacterial blight (BLB), rice blast, and BPH (Brar and Khush 1997). However, few studies have been conducted to transfer the resistance genes from O. minuta to commercial rice cultivars. Amante et al. (1998) evaluated the resistance of advanced progenies introgressed from O. minuta for resistance against BLB and rice blast disease. The introgression line IR71033-121-15 derived from the cross of O. sativa/O. minuta showed resistance to BPH biotypes of Korea (Jena KK, unpublished). However, molecular mapping of these resistance gene(s) has not been reported.
The present study was conducted to identify the BPH resistance genes in IR71033-121-15 and to develop the markers for use in breeding BPH resistance in rice.
Materials and methods
Plant materials
The introgression line, IR71033-121-15, derived from an interspecific cross between IR31917-45-3-2 and a wild species O. minuta (Acc. No. 101141) showed strong resistance to the Korean BPH biotype 1. IR71033-121-15 and Junambyeo (a Korean japonica cultivar susceptible to BPH) were used as parents to develop an F2 population of 190 plants for genetic analysis. The 190 F3 lines harvested from each of the F2 plants were bioassayed for BPH resistance. IR31917-45-3-2 (progenitor of IR71033-121-15) and two Tongil-type rice cultivars, Taebakbyeo and Andabyeo, were used as check cultivars in the bioassays. IR31917-45-3-2, Taebakbyeo, and Junambyeo are susceptible to Korean BPH whereas IR71033-121-15 and Andabyeo are resistant. Seeds of IR71033-121-15, IR31917-45-3-2, and O. minuta were obtained from the Plant Breeding and Genetics and Biotechnology Division of IRRI, Los Baños, Philippines. Seeds of Junambyeo, Taebakbyeo and Andabyeo were obtained from Rice Research Division of National Institute of Crop Science (NICS), Rural Development Administration (RDA), Korea.
Bioassay for BPH resistance
A pure BPH population developed from Korean BPH biotype 1 was used for bioassay of parents and F2/F3 population. Bioassays were conducted at the greenhouse facility of the Rice Research Division of NICS, RDA. The bioassay was performed with a modified bulk seedling test following the method of Pathak et al. (1969). Fifteen seedlings from each of 190 F3 lines were planted in a row per line with three replications. Junambyeo and Taebakbyeo were used as susceptible (S) checks, and IR71033-121-15-2 and Andabyeo were used as resistant (R) checks. Seedlings at the two and a half leaf stage were infested with second or third-instar nymphs at a density of 10–12 nymphs per seedling. The reaction against the BPH was scored following the guidelines of Standard Evaluation Systems for Rice (IRRI, 1988): 0, no damage; 1, very slight damage; 3, first and second leaves of most plants partially yellowing; 5, pronounced yellowing and stunting or about 10 to 25% of the plants wilting; 7, more than half of the plants wilting or dead and remaining plants severely stunted or dying; 9, all plants dead.
PCR amplification and marker detection
Plant DNA was extracted from the frozen leaves of rice plants using the CTAB method (Murray and Thompson 1980). For PCR amplification of markers, each 20 μL reaction mixture contained 50 ng DNA, 5 pmol of each primer, 10× PCR buffer [100 mM Tris (pH 8.3), 500 mM KCl, 15 mM MgCl2, 2 μg gelatin], 250 μM of each dNTP and 0.5 U of Taq polymerase. PCR was performed in a PTC-100 Programmable Thermal Controller thermocycler (MJ Research Inc, Waltham, MA). The thermocycler profile was: 5 min at 94°C, 35 cycles of 1 min at 94°C, 1 min at 48°C (for STS) or 55°C (for SSR), and 2 min at 72°C, with a final extension of 5 min at 72°C. Amplified PCR products were resolved by electrophoresis in 3% agarose gels with ethidium bromide staining or 8% polyacrylamide denaturing gels with silver-staining for SSR markers (Panaud et al. 1996).
Construction of a framework map, detection of QTLs for BPH resistance and high-resolution mapping
Linkage analysis was conducted using MAPMAKER version 3.0 software (Lander et al. 1987). Map distances were estimated by the Kosambi function (Kosambi 1944). The linkage map in this study was basically the same as previously reported (Rahman et al. 2007). In this study, 46 more F2 plants were added to construct a framework map. The M-QTL and E-QTLs between random marker intervals were determined using QTLMapper version 2.0 with the mixed linear model approach (Wang et al. 1999). After detecting QTL positions for BPH resistance based on LOD and R2 values, fine mapping of the resistance loci was performed with SSR and STS markers. Graphical data of the recombinants between markers flanking the target regions were analyzed to clarify QTL positions, and introgressed segments from O. minuta were checked at the QTL regions for confirmation of the QTLs. SSR markers located within the two flanking markers of the resistance QTL regions were adopted from the public database released by the International Rice Microsatellite Initiative (IRMI, http://www.gramene.org/microsat), and PCR-based STS markers were developed according to the sequence information which is available at http://www.ncbi.nlm.nih.gov/ (for indica) and at http://www.rgp.dna.affrc.go.jp/ (for japonica) as described in Chin et al. (2007). For developing new SSR markers, the putative simple sequence repeat motifs were searched and identified using the Simple Sequence Repeat Identification Tool (SSRIT, http://www.gramene.org/db/searches/ssrtool) and for new STS markers, the putative sequences which were highly divergent between the two reference genomes were targeted. All PCR primers were designed using Primer3 version 4.0 (http://frodo.wi.mit.edu/). The markers used for fine mapping of the target regions are listed in Table 1.
Physical map construction
The physical map of the target QTLs was constructed by bioinformatics analysis using bacterial artificial chromosome (BAC) and P1-derived artificial chromosome (PAC) clones of cv. Nipponbare released by the International Rice Genome Sequencing project (IRGSP, http://rgp.dna.affrc.go.jp/IRGSP/index.html). The clones were anchored with the target gene-linked markers and then alignment of sequences was carried out using pairwise BLAST (http://www.ncbi.nlm.nih.gov/blast/bl2seq/b12.html).
Results
Inheritance of BPH resistance in “IR71033-121-15”
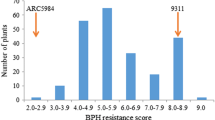
The introgression line IR71033-121-15 expressed strong resistance to the Korean BPH biotype 1, while the recurrent parent, Junambyeo, was completely susceptible to BPH (Fig. 1). The F1 plants from the cross between Junambyeo and IR71033-121-15 showed partial resistance to BPH, indicating that BPH resistance of IR71033-121-15 was controlled by more than one dominant gene and the dominance was not complete. The resistance scores of the 190 F3 lines infested with BPH ranged from 0.0 to 9.0 and showed a continuous variation with two peaks around 1.5 and 3.5 in the segregation curve (Fig. 1), demonstrating the involvement of major genes or QTLs controlling the segregation of resistance.
Linkage map construction and identification of QTLs for BPH resistance
A linkage map was constructed by genotyping 190 F2 individuals using 143 markers. Using the QTLMapper version 2.0, two chromosomal loci for the resistance were identified on the short arm of chromosome 4 delimited by MS10 and RM5953 (3.5 cM distance) and the long arm of chromosome 12, delimited by RM3726 and RM5479 (4.1 cM distance) (Table 2; Figs. 2, 3). At both loci, alleles from the resistant parent IR71033-121-15 significantly increased BPH resistance. These two QTLs were tentatively designated as QTL-4 on chromosome 4 and QTL-12 on chromosome 12. The R 2 values of QTL-4 and QTL-12 were fairly high representing 26.6% and 14.5% of phenotypic variation, respectively, and the summed R 2 of the two QTLs was 41.1% (Table 2). In addition, a total of two significant epistatic interactions between random marker intervals were identified which included a significant interaction between the two QTLs (Table 2). Total phenotypic variation explained by these QTLs and significant interactions was 47.2%. However, there was no dominance effect in the two major QTLs and other marker loci, explaining the intermediate resistance phenotype of F1 plants shown in Fig. 1. The allele effect of each major QTL on BPH resistance was greater in QTL-4 than in QTL-12.
a Genetic and physical map of the Bph20(t) locus on the short arm of chromosome 4. Positions of QTLs (blue vertical bars) previously reported near the Bph20(t) locus were estimated based on the sequence analysis of markers flanking the QTLs using Gramene DB (http://www.gramene.org/): Bph12 from O. latifolia (Yang et al. 2002); Bph15 from O. officinalis (Huang et al. 2001; Yang et al. 2004); Bph17 from Rathu Heenati (Sun et al. 2005). b Phenotype (BPH resistance) and graphical genotype of selected F 3 lines showing crossovers near by the Bph20(t) locus. I IR71033 allele, J Junambyeo allele, H heterozygous
a Genetic and physical map of the Bph21(t) locus on the long arm of chromosome 12. Positions of QTLs (blue vertical bars) previously reported near the Bph21(t) locus were estimated based on the sequence analysis of markers flanking the QTLs using Gramene DB: Bph10 from O. australiensis (Ishii et al. 1994), Bph18(t) from O. australiensis (Jena et al. 2006). b Phenotype (BPH resistance) and graphical genotype of selected F3 lines showing crossovers near by the Bph21(t) locus. I IR71033 allele, J Junambyeo allele, H heterozygous
Position of QTLs on the physical map
All the anchor markers used for fine mapping of the two resistance loci were landed on the reference sequences of cv. Nipponbare by bioinformatics analysis using a software tool BLASTN. The 1.31 Mb virtual contig map of chromosome 4 composed of 14 overlapping BAC/PAC clones was constructed to locate the physical regions for markers MS10 and RM5953 and thus delimit the QTL-4 locus. Similarly, the 1.13 Mb virtual contig map of chromosome 12 composed of 13 overlapping BAC/PAC clones was constructed to locate the physical regions for markers RM3726 and RM5479 and delimit the QTL-12 locus. Additional markers were developed using publicly available sequence databases aided by bioinformatics analysis to narrow down the physical distance between the two flanking markers (Table 1). Finally, two saturated genetic maps and subsequent contig maps were constructed for QTL-4 on the short arm of chromosome 4 (Fig. 2) and QTL-12 on the long arm of chromosome 12 (Fig. 3).
For QTL-4, the markers B43, S4019A, and S4019B derived from OSJBa0028M15, flanked by B42 and B44, co-segregated with the BPH resistance phenotypes. Association of marker genotypes with phenotypes (BPH resistance) of F3 lines that contained the crossover event on the QTL-4 region was analyzed (Fig. 2b). Based on comparisons of the genotypes at both QTL-4 and QTL-12 together with the phenotypes, the lines 73 and 91 delimited the left margin of the QTL-4 position, and similarly, the lines 76 and 115 delimited the right margin of the QTL-4 position. From these results, it was concluded that QTL-4 resides between B42 and B44, which spans 1.2 cM and corresponds to a 193.4-kb region in the reference physical map (http://www.rgp.dna.affrc.go.jp/) (Fig. 2a).
For QTL-12, the markers B120, S12094B and B121 derived from OJ1310_CO3, flanked by S12094A and B122, co-segregated with BPH resistance. Using the same procedure as for QTL-4, the association of marker genotypes with BPH resistance of F3 lines containing the crossover event in the QTL-12 region was analyzed (Fig. 3b). The lines 13 and 28 delimited the left margin of the QTL-12 position, and the lines 43 and 99 delimited the right margin of the QTL-12 position. Therefore, it was concluded that QTL-12 resides between 12094A and B122, which spans 1.0 cM and corresponds to a 194.0 kb region in the reference physical map (Fig. 3a).
Evidence of O. minuta chromosome segment integration in two putative QTLs
To confirm that the two chromosomal regions containing resistance QTLs were introgressed from the wild species O. minuta, the alleles of markers B43, S4019A, S4019B, and B44 on chromosome 4, and B120, S12094B, B121, and B122 on chromosome 12 were compared between IR71033-121-15 and O. minuta. In the QTL-4 region, the same PCR amplicon (≥200 bp) was produced for markers S4019A and S4019B in both O. minuta and IR71033, which was different from the amplicon generated in the recurrent parent IR31917-45-3-2 and Junambyeo (Fig. 4a). However, B43 at the upper position and B44 at the lower position were negative for introgression. Similarly, analysis of markers in the QTL-12 region revealed that the same PCR amplicons were produced for markers S12094B and B121 in IR71033-121-15 and O. minuta, which were different from the amplicons generated in the recurrent parent IR31917-45-3-2 and Junambyeo (Fig. 4b). However, B120 at the upper position and B122 at the lower position were not positive for introgression. These results indicate that the two resistance QTLs correspond to the introgression of O. minuta DNA into chromosome 4 (S4019A to S4019B) and 12 (S12094B to B121) of IR31917-45-3-2.
Discussion
Nineteen major BPH resistance loci have been reported in indica cultivars along with four wild species, O. australiensis, O. eichingeri, O. latifolia, and O. officinalis. Of these, 17 resistance loci (Bph1, bph2, Bph3, bph4, Bph6, Bph9, Bph10, bph11, bph12(t), Bph12, Bph13(t), Bph13, Bph14, Bph15, Bph17, Bph18, and bph19) have been assigned to rice chromosomes (Ikeda 1985; Ishii et al. 1994; Hirabayashi and Ogawa 1995; Hirabayashi et al. 1999; Murata et al. 1998, 2001; Kawaguchi et al. 2001; Liu et al. 2001; Jena et al. 2002, 2006; Renganayaki et al. 2002; Sharma et al. 2003, 2004; Yang et al. 2004; Kim and Sohn 2005; Sun et al. 2005; Chen et al. 2006; Jairin et al. 2007). QTL studies involving the BPH-resistant cultivars IR64, Kasalath, DV85, Teqing, Col.5, and B5 introgression line created through the introgression of wild rice O. officinalis have also been carried out (Alam and Cohen 1998; Huang et al. 2001; Su et al. 2002, 2005; Xu et al. 2002; Ren et al. 2004; Soundararajan et al. 2004). Candidate genes for BPH resistance have been reported for the indica cv. Samgangbyeo (Park et al. 2007).
The main goal of this study was to identify new BPH resistance loci originating from the wild species, O. minuta, which has the BBCC genomes. Our analysis resulted in the identification of two major BPH resistance QTLs on chromosomes 4 and 12. These QTLs together explained over 40% of the observed phenotypic variance. Studies involving BPH resistance introgressed from other wild species have also resulted in the identification of loci mapping to chromosome 4. Previously, Huang et al. (2001) reported that two QTLs for BPH resistance, introgressed from O. officinalis (CC), were located on the short arm of chromosome 4 and long arm of chromosome 3. Later, Yang et al. (2004) identified one BPH resistance locus on chromosome 4 (Bph15) using the same introgressed line from O. officinalis. Yang et al. (2002) also reported that a BPH-resistant gene, Bph12(t) from O. latifolia (CCDD), was located on the short arm of chromosome 4. Of the Bph genes that have been mapped to chromosome 4, Bph17 from the Sri Lankan cultivar Rathu Heenati is the closest to QTL-4 described in our study (Fig. 2a). Bph17 is reportedly located between two SSR markers RM8213 and RM5953 with map distances of 3.6 cM and 3.2 cM, respectively (Sun et al. 2005). Part of this region covers the QTL-4 locus; however, the fact that QTL-4 originates from the wild species O. minuta suggests Bph17 is a different gene. Further studies such as the cloning of these genes will clarify their relationship.
For the long arm of chromosome 12, six resistance loci have been reported: Bph1 from ‘Mudgo’, ‘MTU15’, ‘Co22’, ‘MGL2’, ‘Samgangbyeo’ (Kim and Sohn 2005) and ‘Gayabyeo’ (Jeon et al. 1999); bph2 from ‘Karsamba Red, ASD7’ (Murata et al. 1998); Bph9 from ‘Pokkali’ (Murata et al. 2001) and Kaharamana (Su et al. 2006); Bph10 (Ishii et al. 1994),and Bph18(t) (Jena et al. 2006) from O. australiensis. Of these loci, Bph10 and Bph18(t) are located nearby QTL-12. However, as with QTL-4, comparative analysis of marker loci revealed that these resistance genes actually map apart from QTL-12 (Fig. 3a). Given that the two major QTLs in this study differ in chromosomal location and/or origin (O. minuta) from BPH resistance loci reported previously, we have designated these loci as Bph20(t) and Bph21(t) on chromosome 4 and 12, respectively. This is the first report on BPH resistance loci introduced from a BBCC genome wild species.
It is notable that BPH resistance genes are clustered in the same regions of chromosome 4 and 12 despite their different origins. Similar findings were also observed in the case of BPH resistance loci Bph13(t) (Renganayaki et al. 2002) and the recessive bph19(t) clustered on the short arm of chromosome 3 (Chen et al. 2006), and Bph10 (Ishii et al. 1994) with Bph18(t) (Jena et al. 2006) on the long arm of chromosome 12.
Besides the two major QTLs, significant epistatic interactions between two random markers were found to play a certain role in the expression of resistance to BPH (Table 2). This suggests that epistatic interactions as well as major QTLs should be taken into consideration for breeding BPH-resistant cultivars through MAS, although this might complicate the MAS process (Qiao et al. 2008). The existence of an additive effect for the two major QTLs reported here indicates that lines with stronger BPH resistance can be developed by incorporating multiple QTLs. We are now developing resistant japonica lines by backcrossing the resistant progenies with the recurrent parent Junambyeo using MAS for Bph20(t) and Bph21(t).
Of the five biotypes reported (Chelliah and Bharathi 1993), resistance genes have been identified against four of them (Panda and Khush 1995). Bph1 confers resistance against biotypes 1 and 3, bph2 is closely linked with Bph1 and provides resistance against biotypes 1 and 2, Bph3 and bph4, which are closely linked, confer resistance against four biotypes, and Bph5, Bph6, and Bph7 provide resistance against only biotype 4. The relationship between other resistance genes and corresponding BPH biotypes has not been comprehensively documented. Considering the potential changes of BPH biotypes, due to the extensive cultivation of high yielding rice cultivars and different environmental factors, new resistance genes need to be identified to ensure the durability of host resistance. Wild species of rice are important genetic resources for breeding insect resistant cultivars. The parental line, IR71033-121-15, used in this study carries two new resistance genes, Bph20(t) and Bph21(t), introgressed from O. minuta, and is expected to be a valuable source of BPH resistance.
References
Alam SN, Cohen MB (1998) Detection and analysis of QTLs for resistance to the brown planthopper, Nilaparvata lugens, in a double-haploid rice population. Theor Appl Genet 97:1370–1379
Amante AD, Sitch LA, Nelson R, Dalmacio RD, Oliva NP, Aswidi H, Leung H (1998) Transfer of bacterial blight and blast resistance from the tetraploid wild rice Oryza minuta to cultivated rice O. sativa. Theor Appl Genet 84:345–354
Brar DS, Khush GS (1997) Alien introgression in rice. Plant Mol Biol 35:35–47
Chelliah S, Bharathi M (1993) Biotypes of the brown planthopper, Nilaparvata lugens (Homoptera: Delphacidae)—host influenced biology and behavior. In: Ananthakrishnan TN, Raman A (eds) Chemical ecology of phytopathogous insects. International Science Publishers, New York, pp 133–148
Chen JW, Wang L, Pang XF, Pan QH (2006) Genetic analysis and fine mapping of a rice brown planthopper (Nilaparbata lugens stal) resistance gene bph19(t). Mol Gen Genomics 275:321–329
Chin JH, Kim JH, Jiang W, Chu SH, Woo MO, Han L, Brar D, Koh HJ (2007) Identification of subspecies-specific STS markers and their association with segregation distortion in rice (Oryza sativa L.). J Crop Sci Biotech 10(3):175–184
Feuer R (1976) Biotype 2 brown planthopper in the Philippines. Int Rice Res New 1(1):15
Heinrichs EA (1979) Control of leafhopper and planthopper vectors of rice viruses. In: Moramorosch K, Arris KF (eds) Leafhopper vectors and planthopper disease agents. Academic Press, New York, pp 529–558
Heinrichs EA, Aquino GB, Chelliah S (1982) Resurgence of Nilaparvata lugens (Stål) populations as influenced by method and timing of insecticide applications in lowland rice. Environ Entomol 11:78–84
Hirabayashi H, Ogawa T (1995) RFLP Mapping of Bph-1 (Brown planthopper resistance gene) in rice. Breed Sci 45:369–371
Hirabayashi H, Kaji R, Angeles ER, Ogawa T, Brar DS, Khush GS (1999) RFLP analysis of a new gene for resistance to brown planthopper derived from O. officinalis on rice chromosome 4. (abstract in Japanese). Breed Sci 48(Suppl 1):48
Huang Z, He G, Shu L, Li X, Zhang Q (2001) Identification and mapping of two brown planthopper resistance genes in rice. Theor Appl Genet 102:929–934
Huynh NV, Nhung HT (1988) High virulence of new brown planthopper (BPH) populations in the Mekong Delta, Vietnam. Int Rice Res New 13(5):16
Ikeda R (1985) Studies of the inheritance of resistance to the rice brown planthopper (Nilaparvata lugens Stål) and the breeding of resistance rice cultivar. Bull Natl Agric Res Cent 3:1–54 (In Japanese)
Ikeda R, Vaughan DA (1991) The distribution of resistance genes to the brown planthopper in rice germplasm. RGN 8:1–3
IRRI (1988) Standard evaluation system for rice. IRRI, Manila
Ishii T, Brar DS, Multani DS, Khush GS (1994) Molecular tagging of genes for brown planthopper resistance and earliness introgressed from Oryza australiensis into cultivated rice, O. sativa. Genome 37:217–221
Jairin J, Phengrat K, Teangdeerith S, Vanavichit A, Toojinda T (2007) Mapping of a broad-spectrum brown planthopper resistance gene, Bph3, on rice chromosome 6. Mol Breed 19:35–44
Jena KK, Pasalu IC, Rao YK, Varalaxmi Y, Krishnaiah K, Khush GS, Kochert G (2002) Molecular tagging of a gene for resistance to brown planthopper in rice (Oryza sativa L.). Euphytica 129:81–88
Jena KK, Jeung JU, Lee JH, Choi HC, Brar DS (2006) High-resolution mapping of a new brown planthopper (BPH) resistance gene, Bph18(t), and marker-assisted selection for BPH resistance in rice (Oryza sativa L.). Theor Appl Genet 112:288–297
Jeon YH, Ahn SN, Choi HC, Hahn TR, Moon HP (1999) Identification of a RAPD marker linked to a brown planthopper resistance gene. Euphytica 107:23–28
Kawaguchi M, Murata K, Ishii T, Takumi S, Mori N, Nakamura C (2001) Assignment of a brown planthopper (Nilaparvata lugens Stal) resistance gene bph4 to rice chromosome 6. Breed Sci 51:13–18
Khush GS (1979) Genetics and breeding for resistance to brown planthopper. In: IRRI (ed) Brown planthopper: threat to rice production in Asia. IRRI, Los Banõs, Philippines, pp 321–322
Khush GS (1984) Breeding rice for resistance to insects. Prot Ecol 7:147–165
Khush GS, Karim AR, Angeles EG (1985) Genetics of resistance of rice cultivar ARC10550 to Bangladesh brown planthopper biotype. J Genet 64(2):121–125
Kim SM, Sohn JK (2005) Identification of a rice gene (Bph1) conferring resistance to brown planthopper (Nilaparvata lugens Stål) using STS markers. Mol Cells 20(1):30–34
Kosambi DD (1944) The estimation of map distances from recombination values. Ann Eugen 12:172–175
Lander ES, Green P, Abrahamson J, Barlow A, Daly MJ, Lincoln SE, Newburg L (1987) MAPMAKER: an interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics 1:174–181
Liu GQ, Yan H, Fu Q, Qian Q, Zhang ZT, Zhai WX, Zhu LH (2001) Mappping of a new gene for brown planthopper resistance in cultivated rice introgressed from Oryza eichingeri. Chin Sci Bull 46:738–742
Medrano FG, Heinrichs EA (1985) Response of resistant rices to brown planthoppers (BPH) collected in Mindanao, Philippines. Int Rice Res New 10(6):14–15
Mochida O, Oka IN, Dandi S, Harahap Z, Sutjipto P, Beachell HM (1977) IR26 found susceptible to the brown planthopper in North Sumatra, Indonesia. Int Rice Res New 2(5):10–11
Murata K, Fujiwara M, Kaneda C, Takumi S, Mori N, Nakamura C (1998) RFLP mapping of a brown planthopper (Nilaparvata lugens Stål) resistance gene bph2 of indica rice introgressed into a japonica breeding line ‘Norin-PL4. Genes Genet Syst 73:359–364
Murata K, Fujiwara M, Murai H, Takumi S, Mori N, Nakamura C (2001) Mapping of a brown planthopper (Nilaparvata lugens Stål) resistance gene Bph9 on the long arm of rice chromosome 12. Cereal Res Commun 29:245–250
Murray MG, Thompson WF (1980) Rapid isolation of high molecular-weight plant DNA. Nucleic Acids Res 8:4321–4325
Panaud O, Chen X, McCouch SR (1996) Development of microsatellite markers and characterization of simple sequence length polymorphism (SSLP) in rice (Oryza sativa L.). Mol Gen Genet 252:597–607
Panda N and Khush GS (1995) Host plant resistance to insects. CAB International, Wallingford, 431 pp
Park YD, Song YH (1988) Studies on the distribution of the brown planthopper (Nilaparvata lugens Stal) biotypes migrated in the southern regions of Korea. Korean J Appl Entomol 27(2):63–67
Park DS, Lee SK, Lee JH, Song MY, Song SY, Kwak DY, Yeo US, Jeon NS, Park SK, Yi G, Song YC, Nam MH, Ku YC, Jeon JS (2007) The identification of candidate rice genes that confer resistance to the brown planthopper (Nilaparvata lugens) through representational difference analysis. Theor Appl Genet 115:357–547
Pathak MD, Khush GS (1979) Studies of varietal resistance in rice to the brown planthopper at the International Rice Research Institute. In: IRRI (ed) Brown planthopper: threat to rice production in Asia. pp 285–301.IRRI, Los Banõs, Philippines
Pathak MD, Cheng CH, Fortuno ME (1969) Resistance to Nephotettix impictiveps and Nilaparvata lugens in varieties of rice. Nature 223:502–504
Qiao Y, Jiang W, Rahman ML, Chu SH, Piao R, Han L, Koh HJ (2008) Comparison of molecular linkage maps and QTLs for morphological traits in two reciprocal backcross populations of rice. Mol Cells 25(3):417–427
Rahman ML, Chu SH, Choi MS, Qiao YL, Jiang W, Piao R, Khanam S, Cho YI, Jeung JU, Jena KK, Koh HJ (2007) Identification of QTLs for some agronomic traits in rice using an introgression line from Oryaza minuta. Mol Cells 24(1):16–26
Ren X, Wang X, Yuan H, Weng Q, Zhu L, He G (2004) Mapping quantitative trait loci and expressed sequence tags related to brown planthopper resistance in rice. Plant Breed 123:342–348
Renganayaki K, Feitz AK, Sadasivam S, Pammi S, Harrington SE, McCouch SR, Kumar SM, Reddy AS (2002) Mapping and progress toward map-based cloning of brown planthopper biotype-4 resistance gene introgressed from Oryza officinalis into cultivated rice, O. sativa. Crop Sci 42:2112–2117
Rivera CT, Ou SH, Lida TT (1966) Grassy stunt disease of rice and its transmission by Nilaparvata lugens (Stal). Plant Dis Rep 50:453–456
Sharma PN, Ketipearachchi Y, Murata K, Torii A, Takumi S, Mori N, Nakamura C (2003) RFLP/AFLP mapping of a brown planthopper (Nilaparvata lugens Stål) resistance gene Bph1 in rice. Euphytica 129(1):109–117
Sharma PN, Torii A, Takumi S, Mori N, Nakamura C (2004) Marker-assisted pyramiding of brown planthopper (Nilaparvata lugens Stål) resistance genes Bph1 and Bph2 on rice chromosome 12. Heriditas 140:61–69
Sogawa K, Soekirno Raksadinata Y (1987) New genetic makeup of brown planthopper (BPH) populations in Central Java, Indonesia. Int Rice Res New 12(6):29–30
Soundararajan RP, Kadirvel P, Gunathilagaraj K, Maheswaran M (2004) Mapping of Quantitative trait loci associated with resistance to brown planthopper in rice by means of a double haploid population. Crop Sci 44:2214–2220
Stapley JH, May-Jackson YY, Golden WG (1979) Varietal resistance to the brown planthopper in the Solomon Islands. In: International Rice Research Institute (ed) Brown planthopper: threat to rice production in Asia. International Rice Research Institute, Los Banõs, pp 233–239
Su CC, Cheng XN, Zhai HQ, Wan JM (2002) Detection and analysis of QTL for resistance to brown planthopper, Nilaparvata lugens (Stål), in rice (Oryza sativa L.), using backcross inbred lines. Acta Genetica Sinica 29:332–338
Su CC, Wan J, Zhai HQ, Wang CM, Sun LH, Yasui H, Yoshimura A (2005) A new locus for resistance to brown planthopper identified in the indica rice variety DV85. Plant Breed 124:93–95
Su CC, Zhai HQ, Wang CM, Sun LH, Wan JM (2006) SSR mapping of brown planthopper resistance gene Bph9 in Kaharamana, an indica rice (Oryza sativa L.). Acta Genetica Sinica 33:262–268
Sun L, Su C, Wang C, Zhai H, Wan J (2005) Mapping of a major resistance gene to the brown plant hopper in the rice cultivar Rathu Heenati. Breed Sci 55:391–396
Wang DL, Zhu J, Li ZK, Paterson AH (1999) Mapping QTLs with epistatic effects and QTL × environment interactions by mixed linear model approaches. Theor Appl Genet 99:1255–1264
Wu JT, Heinrichs EA, Medrano FG (1986) Resistance of wild rice, Oryza spp., to the brown planthopper, Nilaparvata lugens (homoptera: Delphacidae). Environ Entomol 15:648–653
Xu XF, Mei HW, Luo LJ, Cheng XN, Li ZK (2002) RFLP-facilitated investigation of the quantitative resistance of rice to brown planthopper (Nilaparvata lugens). Theor Appl Genet 104:248–253
Yang H, Ren X, Weng Q, Zhu L (2002) Molecular mapping and genetic analysis of a rice brown planthopper (Nilaparvata lugens Stål) resistance gene. Hereditas 136:39–43
Yang H, You A, Yang Z, Zhang F, He R, Zhu L, He G (2004) High-resolution genetic mapping at the Bph15 locus for brown planthopper resistance in rice (Oryza sativa L.). Theor Appl Genet 110:182–191
Acknowledgments
This research was supported by a grant (code# CG3111) from the Crop Functional Genomics Center of the 21st Century Frontier Research Program funded by the Ministry of Science and Technology, Republic of Korea. We are also thankful to Rural Development Administration, Korea for providing necessary greenhouse facilities for BPH bioassay.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T. Tai.
Rights and permissions
About this article
Cite this article
Rahman, M.L., Jiang, W., Chu, S.H. et al. High-resolution mapping of two rice brown planthopper resistance genes, Bph20(t) and Bph21(t), originating from Oryza minuta . Theor Appl Genet 119, 1237–1246 (2009). https://doi.org/10.1007/s00122-009-1125-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-009-1125-z