Abstract
Phoma stem canker of oilseed rape (Brassica napus), caused by Leptosphaeria maculans/L. biglobosa is a globally important disease. Severe phoma stem canker symptoms have been observed on winter oilseed rape in China but the seed yield loss caused by this disease remains unknown. In May 2012 and May 2013, 17 and 13 crops were surveyed, respectively, in seven counties of Hubei Province, central China. Stems with phoma stem canker disease symptoms were sampled for pathogen isolation and identification. Only L. biglobosa was identified by culture morphology and species-specific PCR; no L. maculans was found. To evaluate the yield losses, yield components (number of branches per plant, number of pods per plant, 1000-seed weight, number of seeds per pod) were assessed on healthy and diseased plants sampled from crops in four counties and on plants from inoculated pot experiments (plants of three cultivars were inoculated at the green bud stage by injecting L. biglobosa conidia into the stem between the first and second leaf scars). Results of the field surveys showed that diseased plants had 14–61% less branches and 32–83% less pods than healthy plants, respectively. The estimated seed yield loss varied from 10% to 21% and from 13% to 37% in 2012 and 2013, respectively. In the pot experiments, there were no differences in numbers of branches or pods but there were differences in number of seeds per pod between inoculated and control plants. For the three cultivars tested, the inoculated plants had yield losses of 29–56% compared with the control. This study indicates that L. biglobosa could cause substantial seed yield loss in China.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phoma stem canker (also known as blackleg) is an important disease of oilseed rape (Fitt et al. 2006a) and cruciferous vegetables (Rimmer and Berg 2007) worldwide. The disease causes serious seed yield loss on oilseed rape in European countries, Australia and Canada. The annual worldwide economic loss is estimated to be more than 1.6 billion USD despite use of fungicides (Zhang et al. 2014). The pathogens that cause phoma stem canker are two closely related Leptosphaeria species, L. maculans and L. biglobosa (Shoemaker and Brun 2001; Fitt et al. 2006a). L. biglobosa is often associated with upper stem lesions, whereas L. maculans is often associated with stem base cankers (West et al. 2002). Generally, L. maculans is considered to be more damaging than L. biglobosa. Air-borne ascospores produced in pseudothecia on the diseased stem debris are the main sources of inoculum for both pathogens (Huang et al. 2005). The ascospores germinate on leaves of oilseed rape and germ tubes/hyphae penetrate through stomata, thereby causing phoma leaf spots (Huang et al. 2003). From the phoma leaf spots, the pathogens usually grow along the leaf petioles towards the stems, where they cause upper stem lesions or stem base cankers (Toscano-Underwood 2003; Huang et al. 2014). Most studies on oilseed rape seed yield loss caused by phoma stem canker did not distinguish whether the yield losses were caused by either L. maculans or L. biglobosa. For example, in 1966, a severe phoma stem canker epidemic in central France caused an average seed yield loss of 40%, compared with the yield of oilseed rape in 1964 (Lacoste et al. 1969). In 1979, the seed yield loss due to phoma stem canker was up to 50% in UK (Gladders and Musa 1979). Since the 1980s, seed yield losses varying from 5.2% to 30% have been reported in many other countries, including Canada (Gugel and Petrie 1992) and the Netherlands (van der Spek 1981). In most regions where oilseed rape is grown (Australia, Canada and western Europe), L. maculans and L. biglobosa coexist, with L. maculans being reported to be isolated most frequently, although there is some spatial and temporal variation in their prevalence (West et al. 2001). In Poland before 2000, phoma stem canker was predominantly caused by L. biglobosa, with 97% of isolations from upper stem lesions and 67% from stem base cankers caused by L. biglobosa (Karolewski et al. 2002). There is good evidence that L. maculans is spreading eastwards and L. maculans has now become predominant in western Poland, whereas L. biglobosa is still predominant in eastern Poland (Karolewski et al. 2002) and only L. biglobosa has been reported in Russia (Jedryczka et al. 2002). However, there is no information about yield losses caused by L. maculans or L. biglobosa in these countries.
China is an important oilseed rape-producing country and the Chinese authorities are very concerned about the potential damage to oilseed rape production from phoma stem canker. This disease was first reported in China 50 years ago (Dai 1979) and the causal pathogen was first identified as L. biglobosa in 2000 (West et al. 2000). Recent disease surveys and pathogen identification have shown that phoma stem canker on both winter and spring oilseed rape in China is caused by L. biglobosa; there was no L. maculans detected from diseased stem samples (Liu et al. 2014; Zhang et al. 2014). Recently, L. maculans was detected in imported oilseed rape seeds at ports in central China (Zhang et al. 2014). Although methods for effective, rapid detection of L. maculans in infected seed lots of oilseed rape have been developed (Song et al. 2016), there is a potential risk that L. maculans may spread into China through importing of seeds of oilseed rape from countries where L. maculans is present. Because there is evidence that L. maculans has been spreading into areas where only L. biglobosa had been present (Fitt et al. 2008). Therefore, there is a need to continue phoma stem canker disease surveys and pathogen identification in China.
Severe epidemics of phoma stem canker on Chinese oilseed rape have not generally been observed, and only L. biglobosa has been found in China (Li et al. 2013; Zhang et al. 2014). Since L. biglobosa is considered less damaging than L. maculans, there is no information about oilseed rape seed yield loss caused by phoma stem canker in China. Assessment of seed yield loss caused by phoma stem canker is very important for developing disease management strategies to control this disease in China. Hubei province is the most important oilseed rape-growing area in central China. The work reported in this paper aimed: (i) to identify the causal pathogen(s) causing phoma stem canker on winter oilseed rape in central China, and (ii) to assess the seed yield loss caused by phoma stem canker on winter oilseed rape in central China.
Materials and methods
Phoma stem canker crop survey and pathogen identification
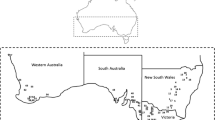
To investigate the incidence of phoma stem canker, seventeen and thirteen crops of winter oilseed rape grown in seven counties in Hubei Province of central China were surveyed in May 2012 and May 2013 before harvest, respectively (Fig. 1, Table 1). In each crop, 500 plants were sampled, from five sites in a zig-zag pattern (‘W’ pattern) with 100 plants per site. The incidence (% of plants affected) of phoma stem canker was calculated for each crop.
Sampling sites for the phoma stem canker survey on winter oilseed rape crops in counties in Hubei province, central China. ‘○’: sites for disease survey only in May 2012; ‘■’ sites for disease survey only in May 2013; ‘△’: sites for disease survey in both seasons; ‘●’: sites for disease survey and yield loss assessment in both seasons
Stems with phoma stem canker symptoms were sampled for pathogen identification. These stems were first classified as affected by phoma stem canker by observation of the visible tissue discoloration and the presence of Leptosphaeria pycnidia. They were cut into small pieces (about 0.5 × 0.5 cm). Each stem piece was divided into two. One half was used for pathogen isolation and identification by morphology/pigment observation and PCR confirmation. The other half was used for direct DNA extraction and identification by nested PCR without isolation.
To isolate the causal pathogen(s), the stem pieces were surface-sterilised in 75% (v/v) ethanol for 1 min, and then dipped in 5% (v/v) NaOCl for 1 min, followed by rinsing in water three times. The surface-sterilized stem pieces were placed on Petri dishes containing potato dextrose agar medium (PDA) amended with streptomycin at 100 μg mL−1. The cultures were incubated at 20 °C for 7 days in darkness. Then, the hyphal tips from these colonies were transferred to new PDA plates containing streptomycin and the cultures were incubated at 20 °C for 7 days. This procedure was repeated three times until a pure culture of each isolate was obtained.
Preliminary identification of the isolates was based on morphological characteristics of the colonies, growth rate and pigment production on PDA (Fitt et al. 2006b). Then, the isolates were cultured and the mycelia were collected for DNA extraction. Six PDA agar plugs (2.5 mm in diameter) were inoculated into 10 ml PDB (potato dextrose broth) liquid medium and maintained on an orbital shaker (25 °C, 150 rpm) for 7 days. The mycelia were harvested by filtration through filter papers in a Buchner-funnel and freeze-dried for 24 h. Genomic DNA was extracted from the freeze-dried mycelia using a CTAB method (Möller et al. 1992).
To confirm whether the isolates were L. maculans or L. biglobosa, Leptosphaeria species-specific primers (Liu et al. 2006) were used in PCR with the DNA from each isolate. The nested PCR amplification with the DNA from diseased stem pieces (DNA was extracted from the freeze-dried stem samples using a CTAB method) consisted of two steps. In the first step of PCR amplification, universal primers ITS1 and ITS4 were used. In the second step of PCR amplification, Leptosphaeria species-specific primers were used. All the PCR products were assessed by electrophoresis in 2% (w/v) agarose gel.
Evaluation of the yield losses caused by phoma stem canker in winter oilseed rape crops
To estimate the yield loss caused by phoma stem canker in winter oilseed rape crops, healthy plants and diseased plants were sampled from four crops in May 2012 and May 2013. The four crops were located in four counties: Jingzhou (N30°11.3291′, E111°58.2264′, cultivar: Deyou No.9); Xiangyang (N32°08.42′, E112°09.51′, cultivar: Zhongyou No.112); Suizhou (N31°43.229′, E112°9.432′, cultivar: Zhongshuang No. 11) and Huanggang (N31°11.382′, E114°58.886′, cultivar: Zhongshuang No. 10) (Fig. 1).
In each crop, ten healthy plants without any phoma stem canker symptoms and ten diseased plants with typical phoma stem canker symptoms were sampled randomly. Stems of diseased plants were cut to examine the internal necrosis. The disease severity was scored using a value scale of 0 to 4 (Zhou et al. 1999): 0, healthy, no disease; 1, less than 50% of the stem cross-section area affected by the disease; 2, more than 50% and less than 90% of the stem cross-section area affected by the disease; 3, the whole stem cross-section area affected by the disease; 4, the whole plant dead. Disease index (DI) for the plants in each crop was calculated by the formula: DI = {[∑(Ni × i)]/Nt ×4} × 100, where Ni is number of plants with disease score i and Nt is the total number of plants assessed. For all the plants sampled, the height (above ground), number of branches, and number of pods (P) of each plant were assessed. Then, the pods from the ten healthy or ten diseased plants were each combined and 50 pods were randomly selected to assess the number of seeds in each pod (S). The seeds from the ten healthy plants or ten diseased plants were each combined and the 1000-seed weight (W) was estimated from 3000 seeds. Oil content of seeds from healthy or diseased plants was measured by Foss NIR Systems 5000 (Foss NIR Systems Inc. Denmark) using the standard procedure in the operation manual (http://www.foss-nirsystems.com). The yield/plant (Y) and percentage crop yield loss (L) were estimated using the formulas: Y = S × P × W/1000 and L = (YH - YD) / YH × Din (YH: yield/plant for healthy plant; YD: yield/plant for diseased plant; Din: disease incidence, % plants affected).
Yield losses caused by L. biglobosa in pot experiments
To assess the yield loss caused by L. biglobosa, plants of three winter oilseed rape cultivars were grown in pots and inoculated with conidia of L. biglobosa isolate W10. The isolate W10 (isolated from oilseed rape in Wuxue County, Hubei in 2010) was cultured on 20% V8 juice agar (Campbell’s, Camden, New Jersey, USA) and incubated at 20 °C with a 12-h photoperiod for two weeks. Sterilized water (10 ml) was added to each culture plate and scrubbed with a sterilized glass spatula to dislodge the conidia. Conidial suspensions were filtered through four layers of gauze to remove mycelial fragments. The concentration of the conidial suspensions was measured using a haemocytometer and adjusted to 1.5 × 107 conidia ml−1 using sterilized water.
Three winter oilseed rape cultivars (Zhongyou 112, Zhongshuang 9 and Ningyou 7) were initially grown in a growth cabinet at 20/16 °C (day/night temperatures) with a 16-h photoperiod for one month. In early October of each season, the seedlings were then transplanted into pots (25 × 35 cm) containing yellow-brown clay soil (pH 6.0) amended with a compound fertilizer (N: P: K = 15:15:15, Hubei Dong Sheng Chemicals Group Co., Ltd., Yuan An County, Hubei, China) at 0.5% (w/w). There were 40 pots with two seedlings per pot for each cultivar. One week later, one seedling was removed from each pot. The pots were kept outdoors under a shade and surrounded by nylon netting. They were arranged in a randomized complete block design and were watered when required.
The oilseed rape plants at the green bud stage were inoculated with L. biglobosa on 12 March 2012 for the first experiment and on 10 March 2013 for the second experiment. To inoculate the plants, 1-mm-diameter holes were made on the stems between the first and the second lowest leaves with a sterilized needle, with one hole per plant. Conidial suspension of L. biglobosa (10 μl) was injected into the hole using a microliter syringe (Gaoge Inc., Shanghai, China). The control treatment was injected with 10 μl of sterilized water. For each cultivar, 25 plants were inoculated with L. biglobosa and 15 plants were inoculated with water. To assess the success of the inoculation, five plants were sampled from each treatment at one month after inoculation to assess the internal symptoms.
On 25 May 2012 or 20 May 2013, plants were harvested to assess the phoma stem canker severity and yield. Phoma stem canker severity was assessed on each plant using a 0–4 scale (Zhou et al. 1999) and then the disease index was calculated as described above. For each plant, the yield components were assessed as in the field survey. The percentage of the plant seed yield loss (P) was calculated using the formula: P = (YC - Yi)/Yc × 100 (Yc: seed yield/plant for the control plant; Yi: seed yield/plant for the inoculated plant).
Data analysis
Analysis of variance (ANOVA) by SAS software (version 9.0, SAS Institute) was used for statistical analysis of data from field and pot experiments. Mean values for different treatments in the pot experiments and crop disease surveys were compared at P < 0.05 according to the Student’s t test. The data for oil content in each crop were arcsine-transformed prior to analysis. To normalize the data, appropriate transformations were determined empirically using normal probability plots, and the transformations were applied before bivariate analysis was performed. To determine if significant correlations existed between the severity of stem canker and yield components, a Pearson’s correlation coefficient was calculated by bivariate analysis using Correlation Procedure (CORR Proc). Effects of season, cultivar (or location) and treatment on yield components were determined using the general linear model (GLM) procedure in ANOVA.
Results
Phoma stem canker crop survey and pathogen identification
In May 2012, 17 oilseed rape crops in seven counties and in May 2013, 13 oilseed rape crops in six counties were surveyed (Table 1). These fields were representative of a large area of oilseed rape grown in Hubei Province of China (Fig. 1). In May 2012, 11 out of 17 crops had a low incidence (< 1%) of phoma stem canker; disease incidence in the other 6 fields ranged from 12.8 to 75.2%. There were differences in disease incidence between the counties surveyed, with the greatest disease incidence in Xiangyang (49%) and the smallest disease incidence in Yichang (1%). Disease incidence was also significantly different between the crops surveyed within the same county. The results of the disease survey in May 2013 showed a similar pattern to those in May 2012. The disease incidence in seven out of 13 crops was less than 1%, and ranged from 10.7% to 46.2% in the other six crops (Table 1). In May 2013, among the six counties, the crops in Xiangyang had the greatest disease incidence (30.7%) (Fig. 2).
Symptoms of phoma stem canker on stems of winter oilseed rape in Xiangyang County in Hubei province in May of 2013. Symptoms (red arrows) of stem base cankers and upper stem lesions were observed. The incidence of phoma stem canker was 46.2% plants affected. Ten diseased and 10 healthy plants were sampled to estimate crop yield loss
A total of 311 oilseed rape stems with phoma stem canker (208 stems in 2012, 103 stems in 2013) were used for pathogen identification. For isolation and morphological identification, a total of 141 Leptosphaeria isolates (115 isolates in 2012, 26 isolates in 2013) were obtained. After 10 days of incubation on PDA, all the isolates produced black-brown globose pycnidia with pink conidial ooze, suggesting that they were L. biglobosa. The isolates were further identified to be L. biglobosa by species-specific PCR, in which a DNA fragment of 444 bp was amplified. In nested PCR identification of the pathogens in these 311 diseased stems, 115 (in 2012) and 92 (in 2013) diseased stem samples were confirmed to be colonised by L. biglobosa and no L. maculans was found. The frequency of L. biglobosa detected by nested PCR in the diseased stem samples collected in May 2013 (89.3%) was greater than that in the diseased stem samples collected in May 2012 (55.2%).
Seed yield losses caused by phoma stem canker in winter oilseed rape crops
There were significant differences between the 2011–2012 season and the 2012–2013 season for most of the seed yield components assessed. Therefore, the results are presented separately for each season. In the 2011–2012 season, values for plant height, number of pods and yield/plant of healthy plants were significantly greater (P < 0.01) than the corresponding parameters for diseased plants of all the four crops in the four counties (Table 2). However, the differences in the 1000-seed weight and oil content between the diseased and healthy plants were not significant (P > 0.05). For the crops surveyed in Xiangyang, Suizhou and Huanggang counties, numbers of branches per plant on healthy plants were significantly greater (P < 0.01) than that on diseased plants. For example in Huanggang, diseased plants had 61% less branches than healthy plants (Table 2). For the crops surveyed in Jingzhou and Xiangyang counties, number of seeds per pod of healthy plants was also significantly greater (P < 0.01) than that on diseased plants. When the four surveyed crops were analyzed together, there were significant differences between healthy and diseased plants in plant height (P < 0.05), number of branches per plant (P < 0.01), 1000-seed weight (P < 0.0001) and seed yield per plant (P < 0.0001). There were differences between the four crops surveyed in stem canker severity, with the disease index being greater in Suizhou (25.1) than in Jingzhou (9.5), Xiangyang (18.7) or Huanggang (6.9). The severe stem canker in Suizhou was associated with greater crop seed yield loss (21.2%) than that for the diseased plants in Jingzhou (10.1%), Xiangyang (16.2%) or Huanggang (12.5%) (Table 2).
For the crop disease survey in the 2012–2013 season, there were significant differences (P < 0.01) between the diseased and healthy plants in number of branches, number of pods and yield/plant for all four crops in the four counties (Table 2). However, the differences in 1000-seed weight and oil content between the diseased and healthy plants were not significant (P > 0.05). For the crops surveyed in Xiangyang, Suizhou and Huanggang counties, plant height differences between diseased and healthy plants were significant (P < 0.01). For the crop in Jingzhou, there were significant differences in number of seeds per pod between diseased and healthy plants (Table 2). When the four crops were analysed together, the differences between diseased and healthy plants in number of branches, number of pods, 1000-seed weight and yield/plant reached the significant level (P < 0.05 or P < 0.01). For the four crops sampled, the average disease index in 2012–2013 (16.9) was greater than that in 2011–2012 (15.1). The disease index differed between the four crops, with the disease index being greater in Xiangyang (28.6) than those in Jingzhou (14.2), Suizhou (15.7) and Huanggang (9.0). The severe stem canker in Xiangyang led to greater seed yield loss (37.5%) than those losses for the diseased crops in Jingzhou (13.4%), Suizhou (27.3%) and Huanggang (15.2%). The average seed yield loss in the 2012–2013 season (37.5%) was greater than that in the 2011–2012 season (21.2%).
Seed yield losses caused by L. biglobosa in pot experiments
One month after inoculation in each year (in April), the stem cross-section of the plants inoculated with L. biglobosa showed blackened stem piths and vascular tissues (Fig. 3). By the end of the experiment (at harvest in late May), all inoculated plants showed typical stem canker symptoms (Fig. 4a), while the plants in the control treatment (inoculated with water) showed no disease symptoms (Fig. 4b). When the plants were cut horizontally and vertically at the inoculation sites, all the inoculated plants had blackened or rotted stem piths (Fig. 4c) and the symptoms had spread along the stem (Fig. 4e), while the control plants had stem piths that were healthy or had little blackening (Fig. 4d) that was only around the inoculation site and did not spread along the stem (Fig. 4f).
Symptoms of phoma stem canker on stems of oilseed rape at one month after inoculation in the pot experiments. Symptoms on cross-section of two oilseed rape stems inoculated with Leptosphaeria biglobosa (right) or water (left) (a); Internal symptoms in the stem pith of oilseed rape inoculated with L. biglobosa on cultivar Ningyou 7 (b), cultivar Zhongshuang 9 (c) or with water on Ningyou 7 (d)
There were differences between the experiments in the 2011–2012 season and the 2012/2013 season for all the seed yield components assessed. Therefore, the results are presented separately for each experiment. For the experiment, in the 2011–2012 season, the number of seed/pods and yield/plant for all three cultivars were significantly different (P < 0.01) between the inoculated and control plants. However, there were no differences (P > 0.05) between the inoculated plants and control plants in number of branches, number of pods, 1000-seed weight or oil content (Table 3). When the three cultivars were analyzed together, the differences between inoculated plants and control plants in plant height, number of seed/pods and yield/plant were significant (P < 0.0001). For inoculated plants, the disease index was greater on cultivar Zhongshuang 9 (50.0) than on cultivars Zhongyou 112 (38.1) and Ningyou 7 (38.3). The seed yield loss caused by L. biglobosa was greater for cultivar Zhongyou No.112 (42.1%) than that for cultivars Zhongshuang No. 9 (38.5%) and Ningyou No. 7 (37.2%).
For the pot experiment in the 2012–2013 season there were significant differences between the inoculated and control plants in number of seeds/pod and yield/plant but no differences in plant height, number of branches, number of pods, 1000-seed weight or oil content for all the three cultivars (Table 3). When the three cultivars were analyzed together, there were significant differences between inoculated plants and control plants in number of seeds/pod (P < 0.0001) and yield/plant (P < 0.0001) but no differences in plant height, number of branches, number of pods, 1000-seed weight or oil content. For inoculated plants, the average disease index was greater in the 2012/2013 season (67.9%) than that in the 2011/2012 season (42.1%), with the disease index greater on Zhongyou 112 (70.0) than on Zhongshuang 9 (66.3) and Ningyou 7 (66.5). The mean plant yield loss in the 2012/2013 growing season (44.1%) was greater than that in the 2011/2012 season (39.3%), with the plant yield loss caused by L. biglobosa greater on Ningyou 7 (56.4%) than on Zhongyou 112 (47.1%) or Zhongshuang 9 (28.8%) (Table 3).
The combined data from the field surveys (four crops in two seasons) and the combined data from the two pot experiments were used for analysis of the correlations between disease levels and yield. Coefficient of correlation between crop yield and severity of stem canker showed that crop yield was inversely correlated to the severity of stem canker both in the field surveys (r = −0.65, P < 0.0001) and in the pot experiment (r = −0.30, P < 0.01). In the field surveys, the severity of stem canker was negatively correlated with plant height (r = −0.374, P < 0.0001), number of branches (r = −0.49, P < 0.0001), number of pods (r = −0.65, P < 0.0001), and number of seeds per pod (r = −0.37, P < 0.005). In contrast, the severity of stem canker was not significantly (P > 0.05) correlated with any of these four yield components in the pot experiments.
Discussion
Results of the disease surveys in May 2012 and May 2013 showed that phoma stem canker occurred widely in the winter oilseed rape growing area in Hubei Province of China. This is consistent with the previous surveys showing that phoma stem canker was commonly observed in China, both on spring and on winter oilseed rape crops, with disease incidence varying between crops, provinces and seasons (Li et al. 2013). In this study, a large variation in phoma stem canker incidence (from <1 to 75%) was observed between crops/counties and between seasons. One reason for the variation may due to the differences in cultivar resistance because different cultivars were used in different counties. This is supported by the results from pot experiments showing that there were differences between cultivars in severity of stem canker (Table 3). The large variation in phoma stem canker incidence between crops and seasons suggests that there is a need to regularly monitor phoma stem canker in China in order to assess the risk of phoma stem canker epidemics. Furthermore, phoma stem canker symptoms have recently been found on many cruciferous vegetables in central China (Cai et al. 2014a, 2014b). Considering the pattern of phoma stem canker spread in Europe and Canada (Fitt et al. 2008; Zhang et al. 2014), there is a need to control this disease on oilseed rape and to prevent it spreading to other cruciferous vegetables in China.
Based on colony morphology and/or species-specific PCR, the pathogen causing phoma stem canker on winter oilseed rape crops in seven counties in Hubei province was identified as L. biglobosa. Results from this study support the previous evidence that only L. biglobosa (or B-type L. maculans) is currently associated with phoma stem canker on oilseed rape crops in China (West et al. 2000; Fitt et al. 2008; Li et al. 2013; Liu et al. 2014). It is not clear why L maculans has not been found on oilseed rape or other cruciferous crops in China. One of the reasons may be China had adopted effective quarantine measures. There is evidence that L. maculans has been spreading into areas where only L. biglobosa was previously present (Fitt et al. 2008). Previous studies showed that Chinese oilseed rape cultivars are very susceptible to L. maculans (Li et al. 2008; Fitt et al. 2008; Zhang et al. 2014). Furthermore, the climatic and agronomic conditions required for development of L. maculans stem canker epidemics appear similar to those for L. biglobosa (West et al. 2002; Huang et al. 2003; Fitt et al. 2006b). L. biglobosa is already present in China; if L. maculans spreads into China, phoma stem canker may destroy the Chinese oilseed rape industry. Considering the potential risk of L. maculans spreading into China through imported infected seeds together with infected crop debris (Fitt et al. 2008; Zhang et al. 2014), quarantine restrictions on imported seeds of oilseed rape and regular monitoring of the pathogen populations will help to reduce or avoid the risk of severe phoma stem canker epidemics in China.
Results of the field disease surveys and the pot experiments suggest that L. biglobosa can cause considerable seed yield loss on oilseed rape in China. Traditionally, L. biglobosa has been considered to be ‘weakly virulent’ and its impact on seed yield loss in most oilseed rape growing areas was ignored. Before 2000, phoma stem canker was rarely found in central China and only L. biglobosa was isolated. The oilseed rape-rice rotation in central China may suppress the production of the inoculum of L. biglobosa. However after 2000, the oilseed rape-rice rotation measure has gradually decreasing in central China. Meanwhile, Chinese breeders have not made efforts to breed oilseed cultivars resistant to Leptosphaeria spp. This may be one of the reasons why phoma stem canker was commonly observed recently on oilseed rape in China (Li et al. 2013; Zhang et al. 2014). Our field survey data showed that L. biglobosa could cause seed yield losses ranging from 10 to 37% in crops with the phoma stem canker incidence ranging from 13 to 46%. Previous crop disease surveys showed that L. biglobosa could cause seed yield losses up to 50% (Rong et al. 2015). In some crops, both stem base cankers and upper stem lesions were observed. This result suggests that phoma stem canker is currently a potential threat to oilseed rape production in China. Absence of L. maculans might allow severe colonization of oilseed rape by L. biglobosa. However, it is difficult to accurately estimate the seed yield loss caused by this pathogen under the field conditions, because no fungicides are currently used to control phoma stem canker in China. Furthermore, another important disease, namely sclerotinia stem rot caused by Sclerotinia sclerotiorum, often co-exists with phoma stem canker caused by L. biglobosa, making it difficult to estimate the yield loss caused by phoma stem canker alone and the severity of phoma stem canker may be under estimated due to the co-infection by S. sclerotiorum. In this study, the crop seed yield loss caused by phoma stem canker was estimated from yields of diseased/healthy plants sampled from the same crop and the disease incidence (% plants affected) in the crop. This may lead to overestimation of the seed yield loss. There is a need for further investigation of the seed yield loss caused by L. biglobosa in field conditions in China by doing field experiments with/without fungicides targeting L. biglobosa and S. sclerotiorum and by inoculating the plants with naturally affected stem debris.
The severity of phoma stem canker differed between different cultivars both in the field surveys and in the pot experiments. These results suggest that it is possible to use cultivar resistance to control phoma stem canker in China. Use of host resistance to control phoma stem canker caused by L. maculans has been well studied (Delourme et al. 2006; Larkan et al. 2015). However, there is no information on use of host resistance to control L. biglobosa. Differences in disease index between the three cultivars were observed in the pot experiments. It is necessary to examine a large number of cultivars using different inoculation methods to screen for resistance against L. biglobosa. To guarantee the infection of plants, in this study, conidial suspensions of L. biglobosa were injected into the stems of oilseed rape at the green bud stage. Other inoculation methods used to screen for resistance against L. maculans, such as cotyledon inoculation and petiole inoculation (Huang et al. 2014), should be used in future work. It has been reported that inoculation at different times and on different tissues can result in different seed yield losses on plants of oilseed rape. In south-eastern Australia, B. napus plants inoculated with L. maculans conidia at the third- to fifth-true-leaf stage did not develop phoma stem canker (Marcroft et al. 2005). Furthermore, the study by Li and colleagues (2006) reported that the resistance response of B. napus plants to L. maculans was affected both by the growth stage of the plant at inoculation and the temperature at which the inoculated plants were grown. Late inoculation with L. biglobosa on the oilseed plants at the green bud stage, rather than at the seedling stage, might be the reason for no significant differences in plant height, number of branches, number of pods, 1000-seed weight or oil content for all the three cultivars in these pot experiments. Ascospores of L. biglobosa and L. maculans are the main inoculum in natural conditions (West et al. 2001; Huang et al. 2005). Therefore, besides use of conidia as inoculum, ascospores should also be used as inoculum in future work to screen cultivars of oilseed rape for resistant against L. biglobosa in China.
References
Cai, X., Yang, L., Zhang, J., & Li, G. Q. (2014a). First report of Leptosphaeria biglobosa causing blackleg on Brassica campestris ssp. chinensis var. purpurea in central China. Plant Disease, 98, 1156.
Cai, X., Yang, L., Zhang, J., & Li, G. Q. (2014b). First report of Leptosphaeria biglobosa causing blackleg on Raphanus sativus in central China. Plant Disease, 98, 993.
Dai, F. L. (1979). Flora Fungoru Sinicorum (pp. 1027–1028). Beijing, China: Science Press.
Delourme, R., Chèvre, A. M., Brun, H., Rouxel, T., Balesdent, M. H., Dias, J. S., et al. (2006). Major gene and polygenic resistance to Leptosphaeria maculans in oilseed rape (Brassica napus). European Journal of Plant Pathology, 114, 41–52.
Fitt, B. D. L., Brun, H., Barbetti, M. J., & Rimmer, S. R. (2006a). World-wide importance of phoma stem canker (Leptosphaeria maculans and L. biglobosa) on oilseed rape (Brassica napus). European Journal of Plant Pathology, 114, 3–15.
Fitt, B. D. L., Huang, Y. J., van den Bosch, F., & West, J. S. (2006b). Coexistence of related pathogen species on arable crops in space and time. Annual Review of Phytopathology, 44, 163–182.
Fitt, B. D. L., Hu, B. C., Li, Z. Q., Liu, S. Y., Lange, R. M., Kharbanda, P. D., et al. (2008). Strategies to prevent spread of Leptosphaeria maculans (phoma stem canker) onto oilseed rape crops in China; costs and benefits. Plant Pathology, 57, 652–664.
Gladders, P., & Musa, T. (1979). The development of Leptosphaeria maculans in winter oilseed rape and its implications for disease control. Proceedings of the British Crop Protection Conference-Pests and Diseases, 129–136.
Gugel, R., & Petrie, G. (1992). History, occurrence, impact, and control of blackleg of rapeseed. Canadian Jorunal of Plant Pathology, 14, 36–45.
Huang, Y. J., Toscano-Underwood, C., Fitt, B. D. L., Hu, X. J., & Hall, A. M. (2003). Effects of temperature on ascospore germination and penetration of oilseed rape (Brassica napus) leaves by A- or B-group Leptosphaeria maculans (phoma stem canker). Plant Pathology, 52, 245–255.
Huang, Y. J., Fitt, B. D. L., Jedryczka, M., Dakowska, S., West, J. S., Gladders, P., et al. (2005). Patterns of ascospore release in relation to phoma stem canker epidemiology in England (Leptosphaeria maculans) and Poland (Leptosphaeria biglobosa). European Journal of Plant Pathology, 111, 263–277.
Huang, Y. J., Qi, A. M., King, G. J., & Fitt, B. D. L. (2014). Assessing quantitative resistance against Leptosphaeria maculans (phoma stem canker) in Brassica napus (oilseed rape) in young plants. PloS One, 9, e84924.
Jedryczka, M., Nikonorenkov, V. A., Levitin, M., Gasich, E., Lewartowska, E., & Portenko, L. (2002). Spectrum and severity of fungal diseases on spring oilseed rape in Russia. IOBC WPRS Bulletin, 25, 13–20.
Karolewski, Z., Kosiada, T., Hylak-Nowosad, B., & Nowacka, K. (2002). Changes in population structure of Leptosphaeria maculans in Poland. Phytopathologia Polonia, 25, 27–34.
Lacoste, L., Louvet, J., Anselme, C., Alabouvette, C., Brunin, B., & Pierre, J. (1969). Rôle de Phoma lingam (Tode) Desm. et de sa forme parfaite, Leptosphaeria maculans (Desm.) Ces. et de Not. dans les épidémies de nécrose du collet de colza (Brassica napus L. var. oleifera Metzer). Comptes Rendus de l'Academie d'Agriculture de France, 981–987.
Larkan, N. J., Ma, L., & Borhan, M. H. (2015). The Brassica napus receptor-like protein RLM2 is encoded by a second allele of the LepR3/Rlm2 blackleg resistance locus. Plant Biotechnology Journal, 13, 983–992.
Li, C. X., Wratten, N., Salisbury, P. A., Burton, W. A., Potter, T. D., Walton, G., et al. (2008). Response of Brassica napus and B. juncea germplasm from Australia, China and India to Australian populations of Leptosphaeria maculans. Australasian Plant Pathology, 37, 162–170.
Li, Q. S., Rong, S. B., Hu, B. C., Jiang, Y. F., Hou, S. M., Fei, W. X., et al. (2013). Distribution of blackleg disease on oilseed rape in China and its pathogen identification. Chinese Journal of Oil Crop Sciences, 35, 415–423.
Liu, S. Y., Liu, Z., Fitt, B. D. L., Evans, N., Foster, S. J., Huang, Y. J., et al. (2006). Resistance to Leptosphaeria maculans (phoma stem canker) in Brassica napus (oilseed rape) induced by L. biglobosa and chemical defence activators in field and controlled environments. Plant Pathology, 55, 401–412.
Liu, Z., Latunde-Dada, A., Hall, A., & Fitt, B. D. L. (2014). Phoma stem canker disease on oilseed rape (Brassica napus) in China is caused by Leptosphaeria biglobosa ‘brassicae’. European Journal of Plant Pathology, 140, 841–857.
Marcroft, S. J., Sosnowski, M. R., Scott, E. S., Ramsey, M. D., Salisbury, P. A., & Howlett, B. J. (2005). Brassica napus Plants infected by Leptosphaeria maculans after the third to fifth leaf growth stage in south-eastern Australia do not develop blackleg stem canker. European Journal of Plant Pathology, 112, 289–292.
Möller, E. M., Bahnweg, G., Sandermann, H., & Geige, H. H. (1992). A simple and efficient protocol for isolation of high molecular weight DNA from filamentous fungi, fruit bodies, and infected plant tissues. Nucleic Acids Research, 20, 6115–6116.
Rimmer, R., & Berg, V. D. (2007). Blackleg (phoma stem canker). In S. R. Rimmer, V. I. Shattuck, & L. Buchwaldt (Eds.), Compendium of brassica diseases (pp. 19–22). St Paul, MN, USA: APS Press.
Rong, S. B., Hu, B. C., Chen, F. X., Wu, X. J., Hou, S. M., Fei, W. X., et al. (2015). Effects of Leptosphaeria biglobosa on grain yield and yield related traits of oilseed rape. Crops, 169, 159–161.
Shoemaker, R. A., & Brun, H. (2001). The teleomorph of the weakly aggressive segregate of Leptosphaeria maculans. Canadian Journal of Botany, 79, 412–419.
Song, P. L., Jedryczka, M., Irzykowski, W., Yan, M. J., Huangfu, H. Y., Hao, L. F., et al. (2016). Efficient detection of Leptosphaeria maculans from infected seed lots of oilseed rape. Journal of Phytopathology, 164, 1097–1104.
Toscano-Underwood, C, Huang, Y.J, Fitt, B.D.L., & Hall, A.M. (2003). Effects of temperature on maturation of pseudothecia of Leptosphaeria maculans and L. biglobosa on oilseed rape stem debris. Plant Pathology, 52, 726–736.
Van Der Spek, J. (1981). Blackleg of oilseed rape in Netherlands. Medelelingen van de Faculteit Landbouwwetenschappen Rijksuniversiteit Gent, 46, 813–822.
West, J. S., Evans, N., Liu, S., Hu, B., & Peng, L. (2000). Leptosphaeria maculans causing stem canker of oilseed rape in China. Plant Pathology, 49, 800.
West, J. S., Kharbanda, P. D., Barbetti, M. J., & Fitt, B. D. L. (2001). Epidemiology and management of Leptosphaeria maculans (phoma stem canker) on oilseed rape in Australia, Canada and Europe. Plant Pathology, 50, 10–27.
West, J. S., Balesdent, M. H., Rouxel, T., Narcy, J. P., Huang, Y. J., Roux, J., et al. (2002). Colonization of winter oilseed rape tissues by a/Tox(+) and B/Tox(0) Leptosphaeria maculans (phoma stem canker) in France and England. Plant Pathology, 51, 311–321.
Zhang, X., White, R. P., Demir, E., Jedryczka, M., Lange, R. M., Islam, M., et al. (2014). Leptosphaeria spp., phoma stem canker and potential spread of L. maculans on oilseed rape crops in China. Plant Pathology, 63, 598–612.
Zhou, Y., Fitt, B. D. L., Welham, S. J., Gladders, P., Sansford, C., & West, J. S. (1999). Effects of severity and timing of stem canker (Leptosphaeria maculans) symptoms on yield of winter oilseed rape (Brassica napus) in the UK. European Journal of Plant Pathology, 105, 715–728.
Acknowledgements
We thank the China Agriculture Research System (Grant CARS-13), China National Science and Technology Supporting Program (Grant No. 2010BAD01B04), the UK Biotechnology and Biological Sciences Research Council (BBSRC), AHDB Cereals and Oilseeds and the Felix Thornley Cobbold Agricultural Trust for supporting the work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cai, X., Huang, Y., Jiang, D. et al. Evaluation of oilseed rape seed yield losses caused by Leptosphaeria biglobosa in central China. Eur J Plant Pathol 150, 179–190 (2018). https://doi.org/10.1007/s10658-017-1266-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-017-1266-x