Abstract
Phoma stem canker of oilseed rape (Brassica napus) is a globally important disease that is caused by the sibling ascomycete species Leptosphaeria maculans and L. biglobosa. Sixty fungal isolates obtained from oilseed rape stems with phoma stem canker disease symptoms collected from four provinces in China in 1999, 2005 and 2006 were all identified as Leptosphaeria biglobosa, not L. maculans, by PCR diagnostics based on species-specific primers. There were no differences in cultural characteristics (e.g. pigmentation and in vitro growth) between these L. biglobosa isolates from China and those of 37 proven L. biglobosa isolates from Europe or Canada. In studies using amplified fragment length polymorphism (AFLP) markers, Chinese L. biglobosa populations were genetically more similar to European L. biglobosa populations than to the more diverse Canadian L. biglobosa populations. Sequencing of gene fragments of β-tubulin, actin and the internal transcribed spacer (ITS) region of rDNA from L. biglobosa isolates from China, Europe, Australia and Canada showed a closer taxonomic similarity of Chinese L. biglobosa to the European L. biglobosa ‘brassicae’ than to Canadian L. biglobosa ‘canadensis’ or to the Australian L. biglobosa ‘occiaustralensis’ or ‘australensis’ subclades. These results suggest that the Chinese L. biglobosa population in this study is in the same subclade as European L. biglobosa ‘brassicae’ populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phoma stem canker (blackleg) is a disease of worldwide importance on oilseed rape (Brassica napus L. var. oleifera), causing annual losses of more than £1000 M globally at a price of £370 t−1 (Howlett 2004; Fitt et al. 2008). The disease is caused by two related fungal species, Leptosphaeria maculans (Desm.) Ces. & de Not. (anamorph = Plenodomus lingam) and L. biglobosa Shoemaker & Brun (anamorph = P. biglobosus) (Williams and Fitt 1999; Rouxel and Balesdent 2005). L. maculans is the more aggressive pathogen and usually causes severe epidemics and substantial yield losses (Fitt et al. 2006a, b), associated with damaging stem base cankers (Zhou et al. 1999). However, even though the less damaging lesions caused by L. biglobosa usually occur higher up the stem (West et al. 2001), co-localization within the same niche sometimes occurs (West et al. 2002). Nevertheless, the two species occupy slightly different ecological niches, which enables them to coexist on oilseed rape crops in Europe (Fitt et al. 2006c), North America (Dilmaghani et al. 2009) and Australia (Van de Wouw et al. 2008; Vincenot et al. 2008). Both pathogens are spread by air-borne ascospores (Dawidziuk et al. 2012; Kaczmarek et al. 2012), from which they infect the leaves (Biddulph et al. 1999; Toscano‐Underwood et al. 2001) and then spread along the leaf petioles to the stems (West et al. 2002). The global importance of phoma stem canker has increased over the last 20 years, with long-distance (e.g. inter-continental) spread of L. maculans on infected seed or debris and short-distance spread by air-borne ascospores (Fitt et al. 2008; Zhang et al. 2014).
China is a major oilseed rape producing country, providing ca. 30 % of the total world yield. Since the late 1990s, the annual area of oilseed rape harvested in China has been greater than 8 million ha (http://www.fao.org/), with winter oilseed rape grown in the Yangtze River basin in central China (ca. 7 M ha) and spring oilseed rape in northern China (ca. 1 M ha). However, phoma stem canker has not caused serious economic losses in China (West et al. 2000; Fitt et al. 2006a; Li et al. 2013). In 1999, samples of plants with stem canker symptoms were collected in China from a few winter oilseed rape crops; only the less aggressive L. biglobosa (then known as B-group L. maculans) was isolated (West et al. 2000). When L. maculans has spread into areas where previously only L. biglobosa was present, such as Canada (1975–1998) and Poland (1994–2007) (Fitt et al. 2008; Zhang et al. 2014), the severity of phoma stem canker epidemics has increased greatly (Fitt et al. 2006a). There is thus good evidence that L. maculans should be considered as a global invasive species. Chinese oilseed rape cultivars are extremely susceptible to L. maculans (Li et al. 2008); if it becomes established in China, severe epidemics are likely to occur, with hardship for the subsistence farmers who grow the crop (Fitt et al. 2008). Therefore, it is important to determine the components of Leptosphaeria populations from a wider range of oilseed rape crops in China.
The global spread of L. maculans into areas where only L. biglobosa was previously present suggests that L. maculans may be ‘younger’ than L. biglobosa, in evolutionary terms. Further support for this conclusion is provided by genetic evidence, since L. biglobosa is genetically more diverse than L. maculans (Gall et al. 1995; Purwantara et al. 2000; Mendes-Pereira et al. 2003; Voigt et al. 2005). Mendes-Pereira et al. (2003) studied the phylogeny of the L. maculans–L. biglobosa species complex and other related Leptosphaeria species, using parsimony analysis of the sequences of the entire ribosomal internal transcribed spacer (ITS) region, including the 5.8S rDNA. L. biglobosa isolates were clustered into five subclades, i.e. L. biglobosa ‘brassicae’ (from various Brassica species, mostly in Europe), L. biglobosa ‘canadensis’ (mostly found in central Canada), L. biglobosa ‘thlaspii’ (from Thlaspi arvense), L. biglobosa ‘erysimii’ (from Erysimum sp.) and L. biglobosa ‘australensis’ (from Australia). In Australia, a further subclade, L. biglobosa ‘occiaustralensis,’ has now been identified (Vincenot et al. 2008) and L. biglobosa ‘canadensis’ has now been found (Van de Wouw et al. 2008). Whereas there were mixed populations of different L. biglobosa subclades in some countries (e.g. Australia), populations in Europe were exclusively L. biglobosa ‘brassicae’ and those in Canada were exclusively L. biglobosa ‘canadensis’. From an evolutionary point of view, L. biglobosa may have evolved earlier than L. maculans from a common ancestor (Gudelj et al. 2004; Rouxel and Balesdent 2005).
Whilst two Chinese isolates from Guizhou province were classified as L. biglobosa ‘brassicae’ (Mendes-Pereira et al. 2003), it is not clear how representative they are of populations of L. biglobosa in China. Furthermore, it is unclear how genetically diverse Chinese populations are in comparison to L. biglobosa populations from Europe or Canada. Although Hao et al. (2014) and Zhang et al. (2014) did surveys of Leptosphaeria species infecting Chinese B. napus and confirmed the presence of only L. biglobosa, neither the genetic structure nor subclade of this species was determined. This paper reports work to confirm whether phoma stem canker in China is caused solely by L. biglobosa and to use banding patterns of Amplified Fragment Length Polymorphism (AFLP) markers to study the genetic diversity of Chinese L. biglobosa isolates and their relatedness to L. biglobosa isolates from different parts of the world. While this method has been employed for evaluation of geographical differentiation of Leptosphaeria spp. within Australia (Barrins et al. 2004) and between Australian populations and those from Europe and North America (Pongam et al. 1999; Purwantara et al. 2000), our study presents the first use of AFLP for investigation of genetic diversity in L. biglobosa populations from China, Europe and Canada. Furthermore, this paper reports studies to identify the subclade of a sub-set of these Chinese phoma stem canker L. biglobosa isolates.
Materials and methods
Fungal sampling and isolation
Sixty isolates of Leptosphaeria species were obtained from four provinces of China. Oilseed rape stems with symptoms similar to those of phoma stem canker disease, generally on upper stems, were collected from three provinces in China in different years shortly before (or after) oilseed rape crops were harvested (samples were collected in May 2005 from winter oilseed rape crops in Wuhan, Hubei province, in September 2005 from spring oilseed rape crops in Hailar, Inner Mongolia and in May 2006 from winter oilseed rape crops in Hefei, Anhui province). Stem bases were always inspected for symptoms of canker but these were absent. Details of sampling procedures used in Chinese surveys to identify the pathogen causing phoma stem canker disease in oilseed rape crops are provided by Zhang et al. (2014). Such stems had previously been collected from Guiyang, Guizhou province and Hefei in 1999 (West et al. 2000) (Fig. 1). Diseased stems were first classified as affected by phoma stem canker disease by observation of the visible tissue discolouration and the presence of pycnidia. Pieces of the necrotic stem lesions (ca. 0.2 × 0.2 cm) were excised from these stems.
Sites in China from which oilseed rape stems with phoma stem canker were sampled to obtain isolates of Leptosphaeria species. Spring oilseed rape samples were from crops near Hailar, Inner Mongolia Province in 2005 (1). Winter oilseed rape samples were from crops near Hefei, Anhui in 2006 (2), near Wuhan, Hubei Province in 2005 (3) and near Guiyang, Guizhou Province in 1999 (4). Areas of winter oilseed rape (ca. 7 M ha) production are indicated by solid diagonal lines; areas of spring oilseed rape (ca. 1 M ha) production are indicated by dashed diagonal lines
To isolate the causal pathogen(s), these pieces of stem were surface sterilised in 70 % (v/v in water) ethyl alcohol for 2–3 s, and then immersed in 10 % (v/v) sodium hypochlorite solution containing 8 % available chlorine (Fisher Scientific, UK #S/5040/21) for 2 min, followed by a thorough rinse with sterile distilled water. Surface-sterilized pieces were placed on water agar (WA) plates (9 cm diameter Petri dish, five samples per plate) and incubated at 15 °C in darkness for 5–7 days to allow fungal colonies to grow from them. Then the hyphal tips were excised from these colonies and transferred to PDA+ medium [potato dextrose agar (Oxoid Ltd., Basingstoke, Hampshire, UK) containing the antibiotics streptomycin (100 mg l−1) and penicillin (50 mg l−1)] for 7 days at 15 °C (five colonies per dish). The transfer was done using a sterilised Pasteur pipette (Fisherbrand, Fisher Scientific, UK) by cutting a plug (ca. 1 mm in diameter) containing only a few hyphal tips from the edge of each actively growing fungal colony. The colonies were then sub-cultured onto PDA medium and incubated for 3–6 days at 20 °C (five colonies per dish). Finally, a pure culture of each isolate was sub-cultured onto PDA medium (one colony per dish) and incubated for 3–4 weeks at 15 °C.
Preliminary identification of isolates was based on morphological characters of each colony on PDA medium (Williams and Fitt 1999). Colonies of L. biglobosa can be distinguished from those of L. maculans by size and pigment production (larger colonies, yellow to brown pigment, L. biglobosa; relatively smaller size, no brown/yellowish pigment production, L. maculans). No Leptosphaeria cultures were discarded.
A collection of 39 L. biglobosa ‘brassicae’ isolates from the UK (Rothamsted Research fungal culture collection), 35 isolates from Poland (provided by Malgorzata Jedryczka, Institute of Plant Genetics, Poznan, Poland), 34 isolates from France (provided by Hortense Brun, Institut National de la Recherche Agronimique, Le Rheu, France, and Marie-Hélène Balesdent, INRA, Thiverval-Grignon, France), nine isolates from Austria (Rothamsted Research fungal culture collection) and 10 L. biglobosa ‘canadensis’ isolates from Canada (provided by Randy Kutcher, Crop Development Centre, Saskatoon, Canada) was also assembled for this work (Supplementary Table 1). All these isolates were confirmed as L. biglobosa by PCR using L. biglobosa-specific primers (Mahuku et al. 1996, specific to all L. biglobosa sensu lato; Liu et al. 2006, specific to L. biglobosa ‘brassicae’ sensu stricto) and maintained on PDA medium at 4 °C for short-term storage. All isolates, together with Chinese L. biglobosa isolates (confirmed as L. biglobosa by PCR with either or both pairs of diagnostic primers), were grown on PDA medium and the cultural characteristics of a subset were assessed.
DNA extraction
All the 60 isolates obtained from China and the 64 reference isolates (54 from Europe and 10 from Canada) were transferred to PDB (potato dextrose broth, Sigma-Aldrich® Inc., USA) liquid medium (six pieces of 2.5 × 2.5 mm PDA agar plugs with mycelia into 10 ml PDB medium) and maintained on an orbital shaker (23 °C, 180 rpm) for 10 days. Mycelia were harvested by centrifugation at 14,000 × g for 5 min at 20 °C, frozen and freeze-dried for at least 24 h. Genomic DNA from each isolate was extracted using a modified version of the method of Graham et al. (1994). Freeze-dried mycelial samples (150 μl fungal powder and 50 μl sterilised sand) were placed in 1.5 ml microfuge tubes. To each was added 600 μl CTAB (hexadecyltrimethyl ammonium bromide = cetyltriammonium bromide; Sigma, UK) lysis buffer with 2 % β-mercapto-ethanol and the content was homogenised with a plastic pestle. The samples were vortexed and incubated at 70 °C for 30 min, followed by centrifugation (10 min at 15,000 × g) in a microcentrifuge. The supernatant was collected into a fresh tube and extracted against an equal volume of a chloroform : isoamyl alcohol (24 : 1) mixture by vortexing for 30 s before centrifugation at 14,000 × g for 10 min. The upper, aqueous phase was collected and a 0.1 volume of 3 M sodium acetate (pH 5.0) and two volumes of ice-cold absolute ethanol were added. Samples were mixed by gentle inversion and placed at −20 °C for 1 h to precipitate the genomic DNA. Pellets were collected by centrifuging the samples at 14,000 × g for 10 min and discarding the supernatant. The precipitate was washed twice with ice-cold 70 % ethanol. Pellets were dried at 37 °C and then dissolved in 200 μl 1 mM TE (tris-ethylenediamine tetra acetic acid, pH 7.5; Sigma, UK) buffer. DNA extract was stored at −20 °C.
PCR screening
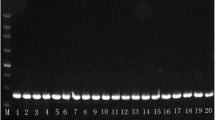
Species identification was done with PCR using L. maculans- and L. biglobosa- specific primers (Mahuku et al. 1996; Liu et al. 2006; Table 1). The PCR reaction was done in a GeneAmp® 2700 PCR thermal cycler (Applied Biosystems Inc., Foster City, USA). Each 15 μl PCR reaction solution was made up of 7.5 μl RedTaq™ ReadyMix™ (2 × concentrate) PCR reaction mix with MgCl2 (Sigma®), 0.3 μl of each primer (10 pmol μl−1), 5.9 μl sterile distilled water and 1 μl fungal genomic DNA. The uniplex PCR was programmed for initial denaturation at 95 °C for 2 min, followed by 40 cycles of denaturation at 95 °C for 30 s, annealing at 55–68 °C, depending on the primer pair used, for 30 s and elongation at 72 °C for 1 min, followed by elongation at 72 °C for 10 min and kept at 4 °C. PCR products were electrophoresed on 1 % agarose gel in 1 × TAE (1 M tris-ethylenediamine tetra-acetic acid plus ethylenediamine tetra-acetic, pH 7.2; Sigma, UK) buffer at 90 V for 1 h and visualized under UV light. Only DNA from isolates confirmed by PCR as L. biglobosa was used in the population genetic diversity analysis.
Growth in culture of L. biglobosa from China, Europe and Canada
Sixty L. biglobosa isolates from China and, from the global collection, 32 isolates from the UK, 33 isolates from Poland, 33 isolates from France and eight isolates from Canada were compared for cultural characteristics and growth. Each L. biglobosa isolate was sub-cultured on PDA medium by transferring a mycelial ‘plug’ from the colony edge with a Pasteur pipette and incubating at 15 °C in darkness for 10 days. Colony size (diameter of the colony) and pigmentation were assessed.
Pathogenicity assay
Three Chinese isolates that had been confirmed as L. biglobosa by PCR (CN60, CN22 and CN13) were tested for their pathogenicity to Chinese winter oilseed rape to satisfy Koch’s postulates in relation to phoma leaf spot lesions. In the first experiment in which isolate CN60 was used, seeds of Chinese Brassica napus cvs Deyou 829, Huiyou 50, Shifeng 1, Xingxuan 2 and Zhongshuang 10 were sown in a glasshouse at 20 °C/16 °C (16 h day/8 h night). There were five plants of each cultivar. To test for development of phoma leaf spot symptoms, seedlings with two to three true leaves were chosen for inoculation. A L. biglobosa conidial suspension was prepared by the method of Ansan-Melayah et al. (1995) and adjusted to 107 spores ml−1. When plants were 4 weeks old, one leaf from each plant was wounded with a needle in six places and then a 10 μl drop of conidial suspension was placed on each wound. The inoculated plants were each covered with a polyethylene bag to maintain leaf wetness (ca. 100 % relative humidity) for 48 h before removing it. Phoma leaf spot lesions were assessed 14 days post inoculation (dpi). In the second experiment in which all three isolates were used, Chinese winter oilseed rape cultivars Deyou 829 and Shifeng 1 were sown in a growth cabinet (20 °C/16 °C; 16 h day/8 h night). There were 10 plants of each cultivar. When plants were 4 weeks old, they were inoculated with L. biglobosa conidia at six wounded sites on one leaf from each plant (two inoculation points per isolate) with a 10 μl droplet of L. biglobosa conidial suspension (107 spores ml−1). Leaf spot lesions were assessed 15 dpi. In both experiments, phoma leaf spot symptoms were photographed. In the second experiment, after assessment, lesions were excised from the leaves and the pathogen was re-isolated from them, and identified by cultural characteristics and PCR.
AFLP analysis
Genetic diversity of L. biglobosa populations was analysed by DNA fingerprinting, using amplified fragment length polymorphism (AFLP) markers, of a selection of 97 isolates from L. biglobosa populations in China, the UK, Poland, France, Austria and Canada (Supplementary Table 1). These 97 isolates included Chinese isolates Gui2b2 and Gui2b2 (CN59 and CN60 in this study) and UK isolate BW70-11 (UK28) that had all been previously identified as L. biglobosa ‘brassicae’ (Mendes-Pereira et al. 2003; Liu et al. 2006). Samples of isolate DNA were used for the AFLP analysis after confirmation as L. biglobosa by PCR. DNA concentration was determined using a NanoDrop® spectrophotometer ND-1000 (Labtech International Ltd., East Sussex, UK). AFLP was done using an AFLP® Microorganism Primer Kit from Invitrogen™, following the instructions in the manual with slight modifications. Fungal genomic DNA (125 ng in 9 μl aqueous solution) was digested at 37 °C with restriction enzymes MseI and EcoRI for 2 h and then complementary double stranded adaptors were ligated at 20–22 °C to the digested fragment ends with T4 DNA ligase. This mixture was pre-amplified with MseI and EcoRI specific primers [0.5 μl each of primer E-0 and primer M-0 provided by the Kit (Table 1)]. Pre-amplification was done over 30 cycles of denaturation at 94 °C for 30 s, annealing at 46 °C for 30 s and elongation at 72 °C for 1 min. PCR products of each reaction were diluted 10 times in TE buffer.
The selective AFLP amplification was done with 5 μl of the resulting diluted PCR samples in 20 μl (final volume) using primer E-AC and primer M-G in a touch-down PCR procedure. Cycling conditions were as follows. Firstly, one cycle was run at 94 °C for 30 s, 58 °C for 30 s and 72 °C for 1 min. Secondly, 13 cycles were run, with the annealing temperature decreased by 0.7 °C in each successive cycle. Thirdly, 28 additional cycles of amplification with an annealing temperature of 49 °C were done. Finally, products were elongated at 72 °C for 2 min and kept at 4 °C. AFLP amplicons were denatured at 95 °C for 5 min, and then placed on ice immediately.
The amplicons were electrophoresed on polyacrylamide gel. Sequi-Gen® GT Sequencing Cell plates (Bio-Rad Laboratories Ltd, Herts, UK) were assembled and the gel was poured and allowed to set horizontally for 1 h. After warming at 80 W for 1 h, samples (AFLP amplicons) were loaded and separated on the denaturing polyacrylamide gel in 1 × TBE buffer for 1.75 h at 50 W.
The gel was stained with silver nitrate before reading. At the end of each run, plates were separated and the gel which bound to the larger plate was fixed by shaking in 10 % acetic acid for 20–30 min. The gel was then rinsed in cold ultra-pure water (3 times) and shaken in staining solution (2 g AgNO3 and 3 ml 33 % formaldehyde in 2 l ultra-pure water) for 30 min. At the end of the staining phase, the gel was rinsed (5–10 s) in cold ultra-pure water and developed in a solution of 60 g sodium carbonate in 2 l ultra-pure water plus 400 μl sodium thiosulphate and 3 ml 33 % formaldehyde until the bands became visible. The reaction was stopped by adding the fixing solution. Gels were rinsed three times with ultra-pure water and left to dry at room temperature overnight. Gel images were scanned using an HP ScanJet 5470c scanner (Hewlett-Packard Development Company, L.P.).
Sequencing
Shake cultures of 15 isolates of L. biglobosa, including six from China (CN01, CN21, CN57, CN58; and CN59 and CN60, previously identified as L. biglobosa ‘brassicae’), four from Canada (CA02, CA03, CA07 and CA08), and five from Europe (Austrian AT01 & AT03; French FR08; British UK28 (previously identified as L. biglobosa ‘brassicae’) and Polish PL30) were grown in PDB (potato dextrose broth; 10 days at 180 rpm and 23 °C) medium. A culture of the Canadian L. maculans isolate (LEROY, IBCN80) was included in the study to serve as an outlier. DNA samples were subjected to polymerase chain reaction for the amplification of fragments from genomic regions corresponding to internal transcribed spacer (ITS) of rDNA (using primers PN3 & PN10, Ta = 54 °C, Table 1; Mendes-Pereira et al. 2003), actin (primers Ta = 56 °C, Table 1; Van de Wouw et al. 2008) or β-tubulin (primers Ta = 58 °C, Table 1; Vincenot et al. 2008). Amplicons from each of the isolates were excised from 1.5 % agarose after electrophoresis in 1 x TAE buffer, purified and sent to Eurofin MWG Operon (Ebersberg, Germany) for bi-directional sequencing using these primers.
Data analyses
AFLP gels were scored manually for the presence or absence of bands to create a binary matrix. Data were collected for each isolate, band by band, with ‘presence’ or ‘absence’ recorded as 1 or 0, respectively. The binary data were analysed (Cluster Analysis) with a Multivariate Analysis using GenStat® (edition 9) software to draw a hierarchical tree illustrating the relatedness of the different groups of isolates (Payne et al. 2011). These band-based AFLP marker data were further analysed by principal coordinate analysis using a freely downloaded programme: PCO by Anderson (2003). The analysis was done on the basis of Bray-Curtis dissimilarities calculated on a binary matrix (i.e. 1 for the marker presence and 0 for the marker absence) of 97 isolates by 80 polymorphic bands. The first two principal coordinate axes were used to contrast and compare groups of isolates from different countries/regions. Then the individual isolate estimate of the first principal coordinate axis was used to test for significant differences between isolates from China and those from other countries/regions. In addition, binary data from these AFLP gels were grouped on the basis of geographical origin and assessed with the POPGENE software (Yeh et al. 1997) for the evaluation of population differentiation and Nei’s genetic diversity (Nei 1972, 1987). The mean values for colony diameters measured on PDA plates were compared statistically using Analysis of Variance (ANOVA; GenStat® (edition 9) software; Payne et al. 2011). Nucleotide sequences of these gene fragments from the 16 Leptosphaeria isolates and those for three other isolates that had been published previously and obtained from the NCBI database (PHW 1268 (AJ550870.1, AY749001.1, AY748953.1), L. biglobosa ‘australensis’; UWA A21-8 (AM410082.1, AM410084.1, AM410083.1), L. biglobosa ‘occiaustralensis’; IBCN 82 (AJ550866.1, AY748958.1, AY749006.1), L. biglobosa ‘canadensis’) were aligned either separately or using a concatenated, multi-locus approach. Genetic distances were calculated using the Neighbor-Joining tree option (Saitou and Nei 1987; Tamura et al. 2004) of the MEGA4 software (Tamura et al. 2007) and dendrogram stability was assessed using 100,000 bootstrap replications (Felsenstein 1985). Nucleotide sequences of all gene fragments were lodged with the NCBI database and ascribed the following GenBank (Bankit) Accession numbers: for Actin sequences; Roth_LbCA02 (KJ574225), Roth_LbCA03 (KJ574229), Roth_LbCA07 (KJ574227), Roth_LbCA08 (KJ574228), Roth_LbCN 01 (KJ574238), Roth_LbCN21 (KJ574239), Roth_LbCN57 (KJ574234), Roth_LbCN58 (KJ574232), Roth_LbCN59 (KJ574230), Roth_LbCN60 (KJ574230), Roth_LbPL30 (KJ574224), Roth_LbUK28 (KJ574231), Roth_LbAT01 (KJ574235), Roth_LbAT03 (KL574237), Roth_LbFR08 (KJ574236), L. biglobosa PHW 1268 (KJ574224), L. biglobosa UWA A21-8 (KJ574226), L. maculans LEROY (KJ574242). For β–tubulin sequences; Roth_LbCA02 (KJ574253), Roth_LbCA03 (KJ574256), Roth_LbCA07 (KJ574257), Roth_LbCA08 (KJ574254), Roth_LbCN01 (KJ574243), Roth_LbCN21 (KJ574244), Roth_LbCN57 (KJ574255), Roth_LbCN58 (KJ574249), Roth_LbCN59 (KJ574248), Roth_LbCN60 (KJ574245), Roth_LbPL30 (KJ574251), Roth_LbUK28 (KJ574252), Roth_LbAT01 (KJ574246), Roth_LbAT03 (KJ574247), Roth_LbFR08 (KJ574250), L. biglobosa PHW 1268 (KJ574258), L. biglobosa UWA A21-8 (KJ574259), L. maculans LEROY (KJ574260). For ITS sequences; Roth_LbCA02 (KJ574220), Roth_LbCA03 (KJ574219), Roth_ LbCA07 (KJ574217), Roth_LbCA08 (KJ574218), Roth_LbCN01 (KJ574216), Roth_LbCN21 (KJ574208), Roth_LbCN57 (KJ574215), Roth_LbCN58 (KJ574209), Roth_LbCN59 (KJ574211), Roth_LbCN60 (KJ574213), Roth_LbPL30 (KJ574214), Roth_LbUK28 (KJ574210), Roth_LbAT01 (KJ574212), Roth_LbAT03 (KJ574206), Roth_LbFR08 (KJ574207), L. biglobosa PHW 1268 (KJ574222), L. biglobosa UWA A21-8 (KJ574221) and L. maculans, LEROY (KJ574223).
Results
Occurrence of only L. biglobosa on oilseed rape in China
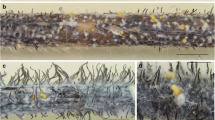
Phoma stem canker symptoms were observed on both winter type (Anhui, Hubei, Guizhou provinces) and spring type (Inner Mongolia) oilseed rape (B. napus) crops in China in the period before or after harvest (May/June in the Yangtze River basin; September in north China). The canker symptoms on Chinese oilseed rape plants were mostly observed on parts of stems above ground level (Fig. 2a) rather than at the stem base where lesions are often observed in Europe (West et al. 2000; Fitt et al. 2006a, b, c). Pycnidia were observed in the pale grey lesions. Discolouration of central stem pith tissues caused by the pathogen was also observed (Fig. 2b).
Phoma stem canker caused by Leptosphaeria biglobosa in China. Diseased winter oilseed rape stems collected from Hefei, Anhui province in May 2005, showing phoma lesions at the site of leaf scars near the base of stems, with black pycnidia (P) observed in the lesions (a), and colonisation of the stem pith tissue (b). Phoma leaf spotting was observed on leaves of Chinese winter oilseed rape (cv. Deyou 829) point-inoculated with conidia of a Chinese L. biglobosa isolate (CN60) after wounding (14 days post inoculation at 20 °C) (c). The pathogen responsible for symptoms was isolated from diseased stems from China (a, b) and diseased leaves (c) from controlled environment experiments and identified as L. biglobosa by pigment production in culture and by PCR. Range of pigmentation observed amongst Leptosphaeria biglobosa isolates grown on PDA (potato dextrose agar) medium, for Chinese isolates (d) (clockwise from top-left: CN53, CN57, CN26, CN52, CN55, CN49), European isolates (e) (clockwise, PL35, UK09, FR02, PL30) and Canadian isolates (f) (clockwise, CA16, CA19, CA01, CA12). All the isolates were incubated at 15 °C on PDA medium in darkness for 2 weeks
The phoma stem canker fungus was isolated from 113 diseased oilseed rape stems collected from three regions in China (namely Hailar in Inner Mongolia, Hefei in Anhui and Wuhan in Hubei). All cultures were morphologically similar to those of L. biglobosa and no cultures similar to those of L. maculans were observed. These comprised 10 L. biglobosa isolates obtained from stem samples from Hefei (2006), 26 from Wuhan (2005) and 20 from Hailar (2005). In addition, two isolates from Hefei and two isolates from Guiyang, that had been collected in 1999 (West et al. 2000), were added. When cultures were tested by PCR using L. maculans- and L. biglobosa-specific primers, all of the 60 isolates were identified as L. biglobosa with both sets of primers. With the pair of primers of Mahuku et al. (1996), the L. biglobosa PCR amplicon was a single band at 230 bp while the L. maculans amplicon was a single 370 bp band. When the diagnostic primers of Liu et al. (2006) were used, however, the PCR amplicons were 444 bp (L. biglobosa) and 330 bp (L. maculans).
Growth in culture of L. biglobosa from China, Europe and Canada
L. biglobosa isolates collected from China demonstrated a wide range of pigmentation when cultured on PDA medium (Fig. 2d). Some Chinese L. biglobosa isolates produced a typical yellow-brown pigment on nutrient medium (e.g. CN53, CN57 and CN26), whilst others produced weak or even no pigmentation on PDA (e.g. CN49, CN55, CN52). A similar variation in pigment production was also observed in European L. biglobosa isolates, when a range of isolates was tested (Fig. 2e). Variation in pigmentation was also observed amongst Canadian L. biglobosa isolates; they were not as variable as Chinese or European L. biglobosa isolates (Fig. 2f), due perhaps, to the smaller sample size.
After incubating isolates on PDA medium at 15 °C in darkness for 10 days, the colony diameter of L. biglobosa isolates from China was 3.57 ± 0.55 cm (mean ± SD); for the UK isolates it was 3.07 ± 0.42 cm, for the Polish isolates it was 3.36 ± 0.41 cm, for the French isolates it was 3.14 ± 0.49 cm and for Canadian isolates it was 3.25 ± 0.16 cm. Thus, there were no significant differences between Chinese L. biglobosa isolates and European or Canadian L. biglobosa isolates in in vitro colony diameter (P > 0.05).
Pathogenicity of the L. biglobosa isolates to Chinese oilseed rape cultivars
In the pathogenicity experiment, the Chinese L. biglobosa isolate (CN60) caused typical phoma leaf spot lesions on Chinese oilseed rape (cv. Deyou 829) seedling leaves (Fig. 2c). After 14 dpi, small, dark leaf spots without pycnidia surrounded by yellow margins were observed. These leaf symptoms were similar to those observed by Brun et al. (1997), Ansan-Melayah et al. (1995) and West et al. (2001) for European L. biglobosa ‘brassicae’ on European B. napus seedling leaves. In the second experiment, phoma leaf spot lesions were also observed after Chinese winter oilseed rape (cv. Deyou 829 or Shifeng 1) was inoculated with Chinese L. biglobosa isolates (CN60, CN13 or CN22). Both cultural and PCR identification confirmed that the isolates obtained from these leaf spots on inoculated Chinese winter oilseed rape were L. biglobosa.
Genetic relatedness of L. biglobosa isolates from China, Europe and Canada
In total, 97 L. biglobosa isolates from the collection were used for AFLP analysis. These comprised 33 isolates from China (12 from Hefei, 10 from Wuhan, nine from Hailar and the two from Guiyang previously identified as L. biglobosa ‘brassicae’), 15 isolates from each of the UK, France and Poland, nine isolates from Austria and 10 isolates from Canada (Supplementary Table 1). AFLP fingerprints of L. biglobosa isolates revealed that they were genetically diverse. A total of 86 amplified DNA fragments from the primer-combination were recorded. The size of the DNA fragments ranged from 200 bp to 5 kb. Amongst these amplified fragments, 80 showed polymorphism and were scored as discrete character data (as ‘1’ for presence and ‘0’ for absence). The combined character data matrix was analysed, assuming that co-migrating bands in an AFLP gel are homologous.
The 97 L. biglobosa isolates were clustered as two distinct groups, based on genetic relatedness from principal component analysis of the AFLP data (Fig. 3). One group comprised all the isolates from China and Europe and the other group consisted of all the Canadian isolates. The L. biglobosa isolates from China and Europe were separated from the Canadian isolates at a similarity level of ca. 25 %. Except for one Canadian L. biglobosa isolate (CA08) with a similarity of 52 %, the similarity between it and the other nine Canadian L. biglobosa isolates was more than 75 %.
Genetic relatedness (0–1 scale) of Leptosphaeria biglobosa isolates obtained from China, the UK, France, Poland, Austria and Canada, assessed using AFLP markers and analysed using GenStat software. There were 33 L. biglobosa isolates from China (CN  ), 54 L. biglobosa isolates from Europe and 10 L. biglobosa isolates from Canada (CA
), 54 L. biglobosa isolates from Europe and 10 L. biglobosa isolates from Canada (CA  ) used for the AFLP analysis. In total, 80 polymorphic bands were compared between isolates. European isolates were from the UK (UK
) used for the AFLP analysis. In total, 80 polymorphic bands were compared between isolates. European isolates were from the UK (UK  ), Poland (PL
), Poland (PL  ), France (FR
), France (FR  ) and Austria (AT
) and Austria (AT  ). These isolates are in the following order, starting from the top (CN01, 03, 06, 08, 10, 12, 14, 18, 16, 21, 25, 31, 27, 29, 33, 35, 39, 41, 45, 51; AT03, 07, 08, 06, 09; CN57, 58, 59, 60, 47, 48, 50, 54, 55, 49, 52, 53; FR26; CN56; FR14, 04, 12, 32, 24, 34, 35, 06, 08, 33, 10; UK01, 32; PL34, 24, 30, 29, 33; AT01; FR18, 30; UK08, 22, 21, 28, 31; PL19, 28, 35, 22, 23, 27; AT05; PL25, 26; UK03, 13, 36, 16, 15, 18, 10, 25; PL32; FR28; PL31; AT02, 04; CA01, 02, 10, 03, 09, 05, 04, 07, 06, 08). Details of the origins of these isolates are given in Supplementary Table 1
). These isolates are in the following order, starting from the top (CN01, 03, 06, 08, 10, 12, 14, 18, 16, 21, 25, 31, 27, 29, 33, 35, 39, 41, 45, 51; AT03, 07, 08, 06, 09; CN57, 58, 59, 60, 47, 48, 50, 54, 55, 49, 52, 53; FR26; CN56; FR14, 04, 12, 32, 24, 34, 35, 06, 08, 33, 10; UK01, 32; PL34, 24, 30, 29, 33; AT01; FR18, 30; UK08, 22, 21, 28, 31; PL19, 28, 35, 22, 23, 27; AT05; PL25, 26; UK03, 13, 36, 16, 15, 18, 10, 25; PL32; FR28; PL31; AT02, 04; CA01, 02, 10, 03, 09, 05, 04, 07, 06, 08). Details of the origins of these isolates are given in Supplementary Table 1
Chinese L. biglobosa isolates clustered together and similarities between them were over 90 % (Fig. 3). No differences were apparent in AFLP data either within one region or between winter and spring oilseed rape producing regions of China. Some of the isolates showed 100 % similarity to each other (e.g. CN08, CN10, CN12, CN14 and CN18), suggesting genotypic homology. This analysis suggested that Chinese isolates were closer to European L. biglobosa isolates than to Canadian L. biglobosa isolates. The similarities between Chinese isolates and most French, Austrian, UK and Polish isolates, for example, were at least 80 %. Several isolates from Hefei (e.g. CN56 and CN53) clustered very closely with French isolates, with nearly 100 % similarity. With only a few exceptions, L. biglobosa isolates from China and Europe showed considerable similarity (about 75 %). For many European L. biglobosa isolates, isolates that were collected from each country grouped together regardless of when they were collected. Population differentiation of the AFLP data and POPGENE version 1.31 analyses of the 86 polymorphic bands generated a phenogram (Fig. 4) that confirmed the short genetic distance between Chinese L. biglobosa and L. biglobosa isolates from European countries. As shown in Fig. 4, there was a greater genetic distance between Chinese and Canadian isolates.
Differentiation of L. biglobosa isolates into geographical populations based on AFLP markers using the POPGEN32 software. The dendrogram (Neighbour-joining) is based on Nei’s genetic distance to illustrate difference relationships within 86 AFLP bands from 97 isolates of Leptosphaeria biglobosa obtained from different countries
The principal coordinate analysis (PCA) showed that the first and second coordinate axes combined to account for 72.4 % of the variation in the 97 isolates based on the 80 polymorphic AFLP markers (Fig. 5). The first principal coordinate axis explained 50.7 % while the second coordinate axis accounted for 21.7 % of the variation. It was again clear that isolates from Canada were distinctly different from isolates from both China and Europe (i.e. isolates combined from the UK, Poland, France and Austria). Since the first principal coordinate axis had the greatest discriminant power, an individual estimate of this axis was used to test differences between isolates from different countries/regions. As the isolate estimates of the first coordinate axis were not normally distributed across countries/regions, Kruskal-Wallis one-way analysis of variance on ranks was done to test the median variations between groups of isolates from different countries/regions. The results showed that the median (3.8) of first principal coordinate axis for isolates from China was significantly different from the median (−27.3) for isolates from Canada but was not different from the median (4.1) for isolates from Europe. The median values for isolates from European countries were also significantly different from the median for isolates from Canada.
Results of the principal coordinate analyses based on the binary matrix of 97 Leptosphaeria biglobosa isolates tested by 80 polymorphic AFLP markers. Data represent means ± SD within each country/region. Of the 97 isolates, 33 were from China, 15 from the UK, 15 from Poland, 15 from France, 9 from Austria and 10 from Canada. Isolates from Austria, France, Poland and the UK were combined into a single group (Europe)
Table 2 shows the genetic diversity indices from the 86 AFLP bands for isolates from China, Europe and Canada. Chinese isolates had genetic diversity and Shannon’s Information Index values that were comparable to values for European (the UK, Poland, France and Austria) isolates; for these indices, Canadian L. biglobosa isolates had the greatest values (Table 2). Furthermore, Nm, the estimate of gene flow (Table 3) indicated both the infrequency and the unlikelihood of gene exchange between the Chinese L. biglobosa population and European L. biglobosa populations with which it shared the closest genetic similarity (Table 4), as judged by Nei’s (1972) genetic identity and genetic distance.
Multilocus nucleotide sequencing and phylogeny of L. biglobosa to identify the subclade of Chinese L. biglobosa isolates
Phylogenetic analyses of the sequenced actin, β-tubulin and ITS gene fragments showed through clustering that L. biglobosa isolates from all four Chinese provinces were L. biglobosa ‘brassicae’ (Fig. 6). A comparison of the Clustal Omega (EMBL-EBI software) phylogram based on ITS sequences alone against a phylogram that was generated from a concatenation of the β-tubulin and actin sequences of the L. biglobosa isolates that were used in this study, confirmed the superiority of the 3-loci approach that was adopted. Chinese isolates Gui2b2 and Gui2b2 (CN59 and CN60 in this study) and UK isolate BW70-11 (UK28) had all been previously described as L. biglobosa ‘brassicae’ (Mendes-Pereira et al. 2003; Liu et al. 2006) and served as references for this infra-specific classification in this study. In addition, isolate IBCN 82 was also included (Mendes-Pereira et al. 2003) as a reference for the L. biglobosa ‘canadensis’ subclade. Similarity (and difference) matrices for the six isolates from the Hailar, Hefei, Wuhan and Guizhou regions of China that were sequenced showed >99.1 % similarity across the entire 1451 bp gene fragments used in the combined analysis (Table 5).
Evolutionary relationships of 18 Leptosphaeria biglobosa isolates and L. maculans isolate LEROY (IBCN80) based a combined analysis of the nucleotide sequences of actin, β-tubulin and internal transcribed spacer (ITS) regions of the rDNA from mycelial cultures of these isolates. The evolutionary history was inferred using the Neighbor-Joining method. The bootstrap consensus tree inferred from 100,000 replicates is taken to represent the evolutionary history of the taxa analyzed. Branches corresponding to partitions reproduced in less than 50 % of bootstrap replicates were collapsed. The percentages of replicate trees in which the associated taxa clustered together in the bootstrap test (100,000 replicates) are shown next to the branches. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Maximum Composite Likelihood method and are in the units of the number of base substitutions per site. Codon positions included were 1st + 2nd + 3rd + Noncoding. All positions containing gaps and missing data were eliminated from the dataset (Complete deletion option). There were a total of 870 positions in the final dataset. Phylogenetic analyses were done in MEGA4
Discussion
These results suggest that phoma stem canker on oilseed rape in China is caused by the less aggressive L. biglobosa and that L. maculans is not currently present in China. In this study, only L. biglobosa was isolated from the 113 stems with phoma stem canker symptoms collected from Inner Mongolia, Anhui and Hubei provinces of China in 2005 and 2006 and no L. maculans was isolated from any of these provinces. Therefore, there is no evidence that the aggressive phoma stem canker pathogen L. maculans is present in different regions in China, including both winter (Anhui and Hubei) and spring (Inner Mongolia) oilseed rape producing regions. This is consistent with the isolation of only L. biglobosa, both from winter oilseed rape samples collected from Anhui and Guizhou provinces by West et al. (2000) and from samples taken from crops in 14 provinces in the period 2005–2012 (Zhang et al. 2014) and the observation that phoma stem canker does not generally cause serious yield losses in China (Li et al. 2013).
The AFLP results showed that the genetic diversity of L. biglobosa isolates collected in China for this study was comparable to that of isolates from European countries; Canadian isolates were the most genetically diverse (Table 2). The Chinese L. biglobosa isolates were collected from four different provinces separated in distance by more than 1000 km and from hosts comprising two different oilseed rape types, but were not less heterogeneous than those from the UK, France or Poland. The low genetic diversity found in the Chinese L. biglobosa population from this study and close genetic relatedness to sub-populations in Europe conform to earlier suggestions that phoma stem canker may be a relatively new disease in China. L. biglobosa may originally have been introduced by a very small pathogen source, for example a few contaminated pieces of crop debris or seed (Chen et al. 2010), and then spread across China through transport of debris or seed from one region to another and by air-borne ascospores (Dawidziuk et al. 2012; Kaczmarek et al. 2012; Zhang et al. 2014) .
Analyses of the molecular data obtained reveal that Chinese L. biglobosa isolates had a genetic diversity that was comparable to those of the isolates from the UK, Poland and France. Isolates from Austria and, particularly, from Canada were genetically more diverse with the greatest scores for Nei’s Genetic Diversity and Shannon’s Information Index amongst the countries compared. It was equally instructive that the L. biglobosa isolates from Canada were the least phenotypically diverse in this study. The phylogenetic data from a concatenation of nucleotide sequences of the ITS, actin and β-tubulin gene fragments confirm that Chinese L. biglobosa isolates are more closely related to L. biglobosa isolates from Europe than to those from Canada or Australia. The multilocus approach to phylogeny that was used in this study has been adjudged (Crouch et al. 2006; Latunde-Dada and Lucas 2007) to be more reliable for ascribing taxonomic similarity than dependence on one gene locus only. The current naming of clades in the phylogeny of L. biglobosa was initiated by the French group led by Thiery Rouxel (Mendes-Pereira et al. 2003) that established ‘brassicae’, ‘canadensis’, ‘australensis’, ‘erysimii’, ‘thalspii’ and later ‘occiaustralensis’ (Vincenot et al. 2008) as the six infraspecific taxa of this ascomycete fungal pathogen. The geographical delineations and specializations of isolates within these clades were clearly obvious and whilst ‘canadensis’, ‘australensis’ and ‘occiaustralensis’ are eponymous, the clade ‘brassicae’ comprised L. biglobosa isolates infecting Brassica juncea, B. oleracea and B. napus hosts from Europe. Our study confirms earlier reports (Mendes-Pereira et al. 2003; Vincenot et al. 2008) that placed two Chinese L. biglobosa isolates within the ‘brassicae’ subclade. Both the AFLP analysis and phylogeny results strongly indicate the close genetic similarity between L. biglobosa populations in China and Europe and suggest that isolates from both regions belong to the L. biglobosa ‘brassicae’ subclade instead of the ‘canadensis’ subclade (of Canadian and Australian isolates) or ‘australensis’ and ‘occiaustalensis’ subclades (of Australian isolates). It is possible that L. biglobosa may have been introduced into China from Europe; similar conclusions were made about the spread of L. maculans into North America (Pongam et al. 1999) and Mexico (Moreno-Rico et al. 2002). The conclusion that European L. biglobosa (‘brassicae’) is distinct from Canadian L. biglobosa (‘canadensis’) agrees with that of previous studies (Mendes-Pereira et al. 2003; Dilmaghani et al. 2009). While the small isolate population sizes used in the current study provide no evidence for a new, distinct or separate subclade for L. biglobosa from China, we propose the use of larger population sizes in future work.
The spread of the global invasive species L. maculans into Canada and Eastern Europe suggests that there is a risk that it may spread into China and cause severe phoma stem canker epidemics there. In Canada, before the 1970s only the less aggressive pathogen L. biglobosa was identified on oilseed rape crops and there were no severe stem canker epidemics (Gugel and Petrie 1992; Fitt et al. 2008). In 1975, L. maculans was first isolated from crops in the Saskatchewan province and by the early 1980s it had spread to Alberta and Manitoba provinces. Since then, L. maculans has spread and become endemic so that it causes serious yield losses in all the main oilseed rape producing regions in Canada (West et al. 2001). The social, natural and technical factors contributing to the rapid spread of L. maculans in Canada (Juska et al. 1997) also exist in China (large cropped area and social demand, high density of oilseed rape cropping in various geographic regions, inadequate knowledge about the disease amongst growers, etc.). There is therefore a serious risk that the pathogen L. maculans will spread into China and other Asian countries where only L. biglobosa is present, increasing the worldwide losses it causes. In the context of increasing severity of epidemics with climate change (Evans et al. 2008) and a world-wide shortage of vegetable oil for human consumption, there is therefore an urgent need for strategies to be developed to decrease the risk of L. maculans entry into China and to prevent the spread of the pathogen within China (Fitt et al. 2008; Zhang et al. 2014).
References
Anderson, M. J. (2003). PCO: a FORTRAN computer program for principal coordinate analysis. Department of Statistics, University of Auckland, New Zealand. <http://www.stat.auckland.ac.nz/~mja/Programs.htm>.
Ansan-Melayah, D., Balesdent, M. H., Buée, M., & Rouxel, T. (1995). Genetic characterization of AvrLm1, the first avirulence gene of Leptosphaeria maculans. Phytopathology, 85, 1525–1529.
Balesdent, M. H., Jedryczka, M., Jain, L., Mendes-Pereira, E., Bertrandy, J., & Rouxel, T. (1998). Conidia as a substrate for internal transcribed spacer-based PCR identification of members of the Leptosphaeria maculans species complex. Phytopathology, 88, 1210–1217.
Barrins, J. M., Ades, P. K., Salisbury, P. A., & Howlett, B. J. (2004). Genetic diversity of Australian isolates of Leptosphaeria maculans, the fungus that causes blackleg of canola (Brassica napus). Australasian Plant Pathology, 33, 529–536.
Biddulph, J. E., Fitt, B. D. L., Leech, P. K., Welham, S. J., & Gladders, P. (1999). Effects of temperature and wetness duration on infection of oilseed rape leaves by ascospores of Leptosphaeria maculans (stem canker). European Journal of Plant Pathology, 105, 769–781.
Brun, H., Levivier, S., Eber, F., Renard, M., and Chevre, A.M. (1997). Electrophoretic analysis of natural populations of Leptosphaeria maculans directly from leaf lesions. Plant Pathology, 46, 147–154.
Chen, G. Y., Wu, C. P., Li, B., Su, H., Zhen, S. Z., & An, Y. L. (2010). Detection of Leptosphaeria maculans from imported Canola seeds. Journal of Plant Diseases and Plant Protection, 117, 173–176.
Crouch, J. A., Clarke, B. B., & Hillman, B. L. (2006). Unravelling evolutionary relationships among divergent lineages of Colletotrichum causing anthracnose disease in turfgrass and corn. Phytopathology, 96, 46–60.
Dawidziuk, A., Kaczmarek, J., & Jedryczka, M. (2012). The effect of winter weather conditions on the ability of pseudothecia on Leptosphaeria maculans and L. biglobosa to release ascospores. European Journal of Plant Pathology, 134, 329–343.
Dilmaghani, A., Balesdent, M. H., Didier, J. P., Wu, C., Davey, J., Barbetti, M. J., Li, H., Moreno-Rico, O., Phillips, D., Despeghel, J. P., Vincenot, L., Gout, L., & Rouxel, T. (2009). The Leptosphaeria maculans-L. biglobosa species complex in the American continent. Plant Pathology, 58, 1044–1058.
Evans, N., Baierl, A., Semenov, M. A., Gladders, P., & Fitt, B. D. L. (2008). Range and severity of a plant disease increased by global warming. Journal of the Royal Society Interface, 5, 525–531.
Felsenstein, J. (1985). Confidence limits on phylogenies: An approach using the bootstrap. Evolution, 39, 783–791.
Fitt, B. D. L., Brun, H., Barbetti, M. J., & Rimmer, S. R. (2006a). World-wide importance of phoma stem canker (Leptosphaeria maculans and L. biglobosa) on oilseed rape (Brassica napus). European Journal of Plant Pathology, 114, 3–15.
Fitt, B. D. L., Evans, N., Howlett, B. J., & Cooke, B. M. (2006b) (Eds). Sustainable strategies for managing Brassica napus (oilseed rape) resistance to Leptosphaeria maculans (phoma stem canker). Springer, Dordrecht, the Netherlands. 126 pp.
Fitt, B. D. L., Huang, Y. J., van den Bosch, F., & West, J. S. (2006c). Coexistence of related pathogen species on arable crops in space and time. Annual Review of Phytopathology, 44, 163–182.
Fitt, B. D. L., Hu, B. C., Li, Z. Q., Liu, S. Y., Lange, R., Kharbanda, P., Butterworth, M. H., & White, R. P. (2008). Strategies to prevent spread of Leptosphaeria maculans (phoma stem canker) onto oilseed rape crops in China; costs and benefits. Plant Pathology, 57, 652–664.
Gall, C., Balesdent, M. H., Desthieux, I., Robin, P., & Rouxel, T. (1995). Polymorphism of Tox0 Leptosphaeria maculans isolates as revealed by soluble protein and isozyme electrophoresis. Mycological Research, 99, 221–229.
Graham, G. C., Meyers, P., & Henry, R. J. (1994). A simplified method for preparation of fungal DNA for PCR and RAPD analyses. BioTechniques, 16, 48–50.
Gudelj, I., Fitt, B. D. L., & van den Bosch, F. (2004). Evolution of sibling fungal pathogens in relation to host specialisation. Phytopathology, 94, 789–795.
Gugel, R. K., & Petrie, G. A. (1992). History, occurrence, impact, and control of blackleg of rapeseed. Canadian Journal of Plant Pathology, 14, 36–45.
Hao, L., Song, P., Li, Z., Huangpu, H., & Li, Q. (2014). Genetic diversity of phoma stem canker pathogen Leptosphaeria biglobosa by ISSR. Chinese Journal of Oil Crop Sciences, 36, 98–105.
Howlett, B. J. (2004). Current knowledge of the interaction between Brassica napus and Leptosphaeria maculans. Canadian Journal of Plant Pathology, 26, 245–252.
Juska, A., Busch, L., & Tanaka, K. (1997). The blackleg epidemic in Canadian rapeseed as a “normal agricultural accident”. Ecological Applications, 7, 1350–1356.
Kaczmarek, J., Jedryczka, M., Cools, H. J., Fitt, B. D. L., Lucas, J. A., & Latunde-Dada, A. O. (2012). Quantitative PCR analysis of abundance of airborne propagules of Leptosphaeria species in air samples from different regions of Poland. Aerobiologia, 28, 199–212.
Latunde-Dada, A. O., & Lucas, J. A. (2007). Localized hemibiotrophy in Colletotrichum: cytological and molecular taxonomic similarities and C. destructivum, C. linicola and C. truncatum. Plant Pathology, 56, 437–447.
Li, C. X., Wratten, N., Salisbury, P. A., Burton, W. A., Potter, T. D., Walton, G., Li, H., Sivasithamparam, K., Banga, S. S., Banga, S., Singh, D., Liu, S. Y., Fu, T. D., & Barbetti, M. J. (2008). Response of Brassica napus and B. juncea germplasm from Australia, China and India to Australian populations of Leptosphaeria maculans. Australasian Plant Pathology, 37, 162–170.
Li, Q. S., Rong, S. B., Hu, B. C., Jiang, Y. F., Hou, S. M., Fei, W. X., Chen, F. X., Wu, X. J., Fan, Z. X., & Lei, W. X. (2013). Distribution of blackleg disease on oilseed rape in China and its pathogen identification. Chinese Journal of Oil Crop Sciences, 35, 415–423. in Chinese.
Liu, S. Y., Liu, Z., Fitt, B. D. L., Evans, N., Foster, S. J., Huang, Y. J., Latunde-Dada, A. O., & Lucas, J. A. (2006). Resistance of Leptosphaeria maculans (phoma stem canker) in Brassica napus (oilseed rape) induced by L. biglobosa and chemical defence activators in field and controlled environments. Plant Pathology, 55, 401–412.
Mahuku, G. S., Hall, R., & Goodwin, P. H. (1996). Distribution of Leptosphaeria maculans in two fields in southern Ontario as determined by the polymerase chain reaction. European Journal of Plant Pathology, 102, 569–576.
Mendes-Pereira, E., Balesdent, M. H., Brun, H., & Rouxel, T. (2003). Molecular phylogeny of the Leptosphaeria maculans-L. biglobosa species complex. Mycological Research, 107, 1287–1304.
Moreno-Rico, O., Séguin-Swartz, G., Nettleton, J. A., Luna-Ruiz, J. J., Frias-Treviño, A. G., & Romero-Cova, S. (2002). Mexican isolates of Leptosphaeria maculans belong to the aggressive strain of the fungus. Canadian Journal of Plant Pathology, 24, 69–73.
Nei, M. (1972). Genetic distance between populations. American Naturalist, 106, 283–296.
Nei, M. (1978). Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics, 89, 583–590.
Nei, M. (1987). Molecular evolutionary genetics. New York: Columbia University Press.
Payne, R. W., Harding, S. A., Murray, D. A., Soutar, D. M., Baird, D. B., Glaser, A. I., Welham, S. J., Gilmour, A. R., Thompson, R., & Webster, R. (2011). The Guide to GenStat Release 14, Part 2: Statistics (p. 997). Hemel Hempstead: VSN International Ltd.
Pongam, P., Osborn, T. C., & Williams, P. H. (1999). Assessment of genetic variation among Leptosphaeria maculans isolates using pathogenicity data and AFLP analysis. Plant Disease, 83, 149–154.
Purwantara, A., Barrins, J. M., Cozijnsen, A. J., Ades, P. K., & Howlett, B. J. (2000). Genetic diversity of isolates of the Leptosphaeria maculans species complex from Australia, Europe and North America using amplified fragment length polymorphism analysis. Mycological Research, 104, 772–781.
Rouxel, T., & Balesdent, M. H. (2005). The stem canker (blackleg) fungus, Leptosphaeria maculans, enters the genomic era. Molecular Plant Pathology, 6, 225–241.
Saitou, N., & Nei, M. (1987). The neighbor-joining method: A new method for reconstructing phylogenetic trees. Molecular Biology and Evolution, 4, 406–425.
Tamura, K., Nei, M., & Kumar, S. (2004). Prospects for inferring very large phylogenies by using the neighbor-joining method. Proceedings of the National Academy of Sciences (USA), 101, 11030–11035.
Tamura, K., Dudley, J., Nei, M., & Kumar, S. (2007). MEGA4: Molecular evolutionary genetics analysis (MEGA) software version 4.0. Molecular Biology and Evolution, 24, 1596–1599.
Toscano-Underwood, C., West, J. S., Fitt, B. D. L., Todd, A. D., & Jedryczka, M. (2001). Development of phoma lesions on oilseed rape leaves inoculated with ascospores of A‐group or B‐group Leptosphaeria maculans (stem canker) at different temperatures and wetness durations. Plant Pathology, 50, 28–41.
Van de Wouw, A. P., Thomas, V. L., Cozijnsen, A. J., Marcroft, S. J., Salisbury, P. A., & Howlett, B. J. (2008). Identification of Leptosphaeria biglobosa ‘canadensis’ on Brassica juncea stubble from northern New South Wales, Australia. Australasian Plant Disease Notes, 3, 124–128.
Vincenot, L., Balesdent, M. H., Li, H., Barbetti, M. J., Sivasithamparam, K., Gout, L., & Rouxel, T. (2008). Occurrence of a new subclade of Leptosphaeria biglobosa in Western Australia. Phytopathology, 98, 321–329.
Voigt, K., Cozijnsen, A. J., Kroymann, J., Pöggeler, S., & Howlett, B. J. (2005). Phylogenetic relationships between members of the crucifer pathogenic Leptosphaeria maculans species complex as shown by mating type (MAT1-2), actin, and β-tubulin sequences. Molecular Phylogenetics and Evolution, 37, 541–557.
West, J. S., Evans, N., Liu, S., Hu, B., & Peng, L. (2000). Leptosphaeria maculans causing stem canker of oilseed rape in China. Plant Pathology, 49, 800.
West, J. S., Kharbanda, P. D., Barbetti, M. J., & Fitt, B. D. L. (2001). Epidemiology and management of Leptosphaeria maculans (phoma stem canker) on oilseed rape in Australia, Canada and Europe. Plant Pathology, 50, 10–27.
West, J. S., Balesdent, M. H., Rouxel, T., Nancy, J. P., Huang, Y. J., Roux, J., Steed, J. M., Fitt, B. D. L., & Schmit, J. (2002). Colonisation of winter oilseed rape tissues by A/Tox+ and B/Tox0 Leptosphaeria maculans (phoma stem canker) in France and England. Plant Pathology, 51, 311–321.
Williams, R. H., & Fitt, B. D. L. (1999). Differentiating A and B groups of Leptosphaeria maculans, causal agent of stem canker of winter oilseed rape in the UK. Plant Pathology, 46, 161–175.
Yeh, F. C., Yang, R. C., Boyle, T., Ye, Z. H., & Mao, J. X. (1997). POPGENE: the user-friendly shareware for population genetic analysis. Molecular Biology and Biotechnology Centre, University of Alberta, Canada. (Available at http://www.ualberta.ca/~fyeh/).
Zhang, X., White, R. P., Demir, E., Jedryczka, M., Lauge, R. M., Islam, M., Li, Z. Q., Huang, Y. J., Hall, A. M., Zhou, G., Wang, Z., Cai, X., Skelsey, P., & Fitt, B. D. L. (2014). Leptosphaeria spp., phoma stem canker and potential spread of L. maculans on oilseed rape crops in China. Plant Pathology, 63, 598–612.
Zhou, Y., Fitt, B. D. L., Welham, S. J., Gladders, P., Sansford, C. E., & West, J. S. (1999). Effects of severity and timing of stem canker (Leptosphaeria maculans) symptoms on yield of winter oilseed rape (Brassica napus) in the UK. European Journal of Plant Pathology, 105, 715–728.
Acknowledgments
This work was supported by the China Scholarship Council, Perry Foundation, Henry Lester Trust and Great Britain-China Education Trust, and the University of Hertfordshire. Rothamsted Research receives funding from the UK Biotechnology and Biological Sciences Research Council. The authors thank Aiming Qi for assistance with the analysis of the AFLP data, Jonathan West, Maria Eckert, Malgorzata Jedryczka, Hortense Brun, Marie-Hélène Balesdent, Randy Kutcher and Dilantha Fernando for providing isolates of L. biglobosa, Yongju Huang and John Hood for assistance with controlled environmental experiments, Georgia Mitrousia and Kevin King for assistance with the figures and molecular biological work, QiangSheng Li for Fig. 2a and b, and with Ziqin Li and many others for collecting diseased oilseed rape stems from China and other countries.
Author information
Authors and Affiliations
Corresponding author
Additional information
Ze Liu and Akinwumi O. Latunde-Dada to be regarded as joint first authors of this paper
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(GIF 49 kb)
Supplementary Table 1
(DOC 120 kb)
ESM 2
(PDF 66 kb)
Rights and permissions
About this article
Cite this article
Liu, Z., Latunde-Dada, A.O., Hall, A.M. et al. Phoma stem canker disease on oilseed rape (Brassica napus) in China is caused by Leptosphaeria biglobosa ‘brassicae’. Eur J Plant Pathol 140, 841–857 (2014). https://doi.org/10.1007/s10658-014-0513-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-014-0513-7