Abstract
Juncea canola (Brassica juncea L.) is being developed throughout the worlds canola growing countries as a drought tolerant, shatter resistant and highly blackleg resistant option to canola (Brassica napus L.). Juncea canola was grown commercially in Australia for the first time in 2007. This study determined the incidence and severity of blackleg infection in juncea canola prior to commercial release throughout south-eastern Australia in 2006 and 2007, and then again 5 years after commercialisation (2010–2013) to determine if blackleg severity had increased. Blackleg was found at all 127 sites surveyed throughout Victoria, New South Wales, South Australia and Western Australia. The severity of blackleg infection differed among sites and among the juncea canola cultivars and breeding lines suggesting that differences in resistance may be present. This is the first report that L. maculans isolates virulent on B. juncea are already widespread throughout the Australian canola growing regions and contradicts the widespread opinion that B. juncea is immune to blackleg. This also demonstrates that blackleg infection was already occurring in juncea canola prior to commercialisation of this crop in Australia and that disease management strategies similar to those used in canola cultivation will need to be implemented.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Canola (Brassica napus L.) production ranks third behind wheat and barley in Australia. The area sown to canola has increased from 1.4 million ha in 2009 to 2.4 million ha in 2013 (http://www.australianoilseeds.com). High canola prices compared to cereals are driving the increase in cultivation with prices reaching 550–600 AUD/tonne. Low rainfall regions offer further growth to the Australian canola industry, however current canola cultivars are not reliable in the low rainfall zone.
Brassica juncea L. is known to have increased heat and drought tolerance compared to B. napus as well as other advantages such as early vigour and increased shatter tolerance (Oram et al. 2005). For these reasons B. juncea has been bred to meet canola quality oil and meal standards. The improved crop has been termed juncea canola, and was commercialised in Australia in 2007 (Burton et al. 2008). Prior to the commercialisation of juncea canola only small areas of B. juncea for condiment use were grown in Australia, however not typically in the regions where juncea canola is now being grown (Van de Wouw et al. 2008, 2009). Juncea canola has the potential to become a significant break crop for low rainfall areas of Australia such as the Mallee and areas of northern New South Wales, parts of Queensland and the Geraldton area of Western Australia (Oram et al. 2005). In 2012/2013 up to 10,000 ha of juncea canola was sown in Australia (Burton pers. comm.).
In addition to the above mentioned agronomic traits research has shown B. juncea has a greater level of resistance to the fungal disease blackleg than B. napus (Kirk and Oram 1978, Marcroft et al. 2002). Blackleg disease is the most significant disease of canola (Brassica napus L.) in Australia (Murray and Brennan 2012) with the ability to cause widespread plant death, with yield losses as high as 80 % (Colton and Potter 1999). A species complex of two Dothideomycete species, Leptosphaeria maculans (Desm.) Ces. et de Not and L. biglobosa, is responsible for blackleg disease in Australia (Mendes-Piereira et al. 2003). The L. maculans species has been divided into two subclades, but only one subclade ‘brassicae’ is present in Australia (Mendes-Piereira et al. 2003). Leptosphaeria biglobosa has been separated into six subclades, with isolates from three of these subclades reported in Australia (Plummer et al. 1994; Van de Wouw et al. 2008; Vincenot et al. 2008). Leptosphaeria isolates cultured from B. juncea stubble collected in northern New South Wales were L. biglobosa ‘canadensis’, however isolates cultured from B. juncea stubble collected in Victoria were L. maculans (Van de Wouw et al. 2008).
Blackleg in canola is managed using cultural and chemical practices, such as maintaining a minimum 500 m buffer between the last season’s residues and the current crop, fungicide seed dressings and foliar applications, and host plant resistance. In B. napus there are at least 18 qualitative resistance genes (Rlm1, Rlm2, Rlm3, Rlm4, Rlm5, Rlm6, Rlm7, Rlm8, Rlm9, Rlm10, Rlm11, RlmS, LepR1, LepR2, LepR3, LepR4, BLMR1 and BLMR2) conferring resistance to L. maculans (Balesdent et al. 2013; Delourme et al. 2006; Eber et al. 2011; Long et al. 2011; Van de Wouw et al. 2009; Yu et al. 2005, 2008). Rlm5 and Rlm6 are reported to be present in B. juncea (Chèvre et al. 1997; Barret et al. 1998; Balesdent et al. 2002, 2005), as well as a recessive resistance gene (LMJR2) (Saal et al. 2004). The presence of these resistance genes in the commercialised juncea canola cultivars is unknown.
In canola qualitative resistance is effective when first released, however, it has not proven effective in the long-term, being quickly overcome by the pathogen (Sprague et al. 2006). Similar situations have occurred in France (Rouxel et al. 2003; Brun et al. 2000). The large amount of sexual crossing and the ability of sexual spores to travel long distances gives this pathogen the ability to overcome resistance (Howlett 2004; Sprague et al. 2006). A new qualitative resistance for commercial B. napus cultivars was introgressed from B. rapa ssp. sylvestris and released in Australia in 2000. This resistance displayed a hypersensitive response when inoculated with L. maculans isolates (Li and Sivasithamparam 2003), but became ineffective only 3 years after the first cultivar was released (Sprague et al. 2006), which is similar to what happened in France where a single dominant resistance gene, Rlm1, became ineffective in only 3 years when it was exposed to its own inoculum (Brun et al. 2000).
As juncea canola is a new crop in Australia it is unknown how severe blackleg infection will be, if blackleg infection will increase over time as more juncea canola is grown and if current juncea canola resistance will be overcome as has occurred in canola. It is also uncertain if the same management practices will be required for juncea canola cultivation as for canola.
This study determined the level of blackleg disease present in juncea canola cultivars at their commercialisation to set a benchmark for comparison by future studies. At this early stage of juncea canola cultivation it is also important to know whether virulent Leptosphaeria populations are already present within the growing regions and if differences in resistance are evident within the juncea canola cultivars.
Materials and methods
Regional blackleg survey sites
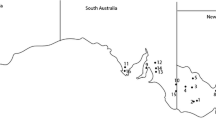
In 2006, 2007, 2010, 2011, 2012 and 2013 a number of sites were surveyed throughout Victoria, South Australia, New South Wales and Western Australian (Fig. 1, Table 1). At each location standard regional agronomic practices for growing canola and juncea canola were used. The trial sites were provided by the Victorian Department of Environment and Primary Industries, New South Wales Department of Primary Industries, the South Australian Research and Development Institute, Department of Agriculture and Food, Western Australia and the National Variety Trials. These sites are used to assess new breeding lines and commercial cultivars for their agronomic potential and are located throughout the canola growing regions of south eastern and western Australia.
Locations of survey sites across Australia for all years. Numbers represent individual field sites (see Table 1 for details)
At each site, each breeding line and cultivar (Table 2) was replicated in three plots, generally eight metres long and six rows wide. During 2006 nine breeding lines and cultivars (six B. juncea and three B. napus) were sampled at each site. During 2007 five breeding lines and cultivars (three B. juncea and two B. napus) were sampled at each site. During 2010–2013 two breeding lines and cultivars (one B. juncea and one B. napus) were sampled. The B. juncea breeding lines were selected based on their blackleg resistance response from previous blackleg nurseries. The B. napus cultivars were chosen as a representative of the current commercial canola cultivars.
Blackleg severity assessment
Each cultivar / breeding line was sampled by selecting the second row into the experimental plot, 1 m from the end. Twenty consecutive plants (60 plants in total from the 3 reps) were pulled from the soil, the plants were cut at the crown and the cross sectional area was visually assessed for blackleg by the percentage of internal infection (0–100 %) (Marcroft et al. 2004). Dead plants were recorded as 100 % internal infection.
Leptosphaeria isolate collection
Single spore isolates of Leptosphaeria spp. were collected from the scored stubble at a minimum of three locations each year (stems were over summered in Dimboola, Victoria) (Elliott et al. 2011) and the spores grown on V8 agar to determine that symptoms scored in the field were due to blackleg infection. The pycnidiospores produced in culture were then used to inoculate B. juncea and B. napus at a concentration of 10 million spores / ml. Tween 20 was added to the spore solution and it was sprayed onto 4 week old cotyledons using a Preval® Power Unit. Plants were placed in high humidity for 48 h and the subsequent symptoms were identified as those caused by Leptosphaeria spp.
Statistical analysis
Disease severity data were analysed using analysis of variance (ANOVA) tests. Log (base 10) transformations were used prior to analysis in order to normalize the data. Least significant difference tests were used to compare differences between cultivars within sites. All analyses were performed using MINITAB® Release 14.13.
Results
Blackleg disease was present at all sites surveyed in all years. Disease severity was low in 2006 and 2007 due to drought conditions (Table 1). Mean internal infection levels in the juncea canola breeding lines and cultivars were below 9, 15 and 23 % in New South Wales, Victoria and South Australia, respectively (Table 3). Mean internal infection levels in the B. napus cultivars were below 6, 10 and 12 % in New South Wales, Victoria and South Australia, respectively. In 2010–2013 drought conditions had ceased and mean internal infection levels of B. napus were higher, with mean internal infection levels ranging from 9 to 59 %. No plant death due to blackleg was evident at any of the sites in 2006 and 2007, however plant death was evident in 2010, 2011, 2012 and 2013 (data not shown).
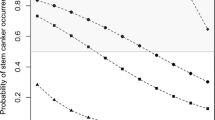
Significant differences in mean internal infection severity between the juncea canola breeding lines and cultivars within some sites in 2006 and 2007 are shown in Table 4. When the internal infection levels of B. juncea breeding lines and cultivars were compared to B. napus cultivars at the same location, nine sites in 2006 displayed significantly higher infection levels in the B. juncea breeding lines and cultivars (Fig. 2). In 2007, eight locations had significantly higher infection levels in the B. juncea breeding lines and cultivars than in the B. napus, four of which were locations known to or likely to have L. biglobosa existing there. Four sites had significantly lower infection levels in the B. juncea breeding lines and cultivars compared to the B. napus cultivars (Fig. 3). In each of 2010 and 2011 all sites except one had lower mean internal infection severity in the B. juncea compared to the B. napus (Figs. 4 and 5). In 2012, 21 sites had lower mean internal infection severity in the B. juncea breeding line compared to the B. napus and one site, Lockhart NSW, had higher mean internal infection severity in the B. juncea breeding line compared to the B. napus (Fig. 6). Ten sites had no significant difference between the internal infection severities of the two species. In 2013, 24 sites had lower mean internal infection severity in the B. juncea breeding line compared to the B. napus and one site, Charlton (Victoria), had higher mean internal infection severity in the B. juncea breeding line compared to the B. napus (Fig. 7). Seven sites had no significant difference between the internal infection severities of the two species.
Discussion
This is the first extensive survey of blackleg disease in juncea canola in its potential growing regions of south-eastern Australia and is also one of the few examples of a “pre-commercialization” assessment of disease potential for any crop. Our study showed that although the Australian juncea canola industry is yet to be established, blackleg infection was found at low levels at all sites that were surveyed during 2006 and 2007. Previous work showed that L. maculans isolates capable of infecting and causing internal infection in B. juncea exist in Australia (Ballinger and Salisbury 1996), however, this is the first study to show that blackleg, capable of causing disease in B. juncea, is already widespread throughout the Australian canola growing regions on juncea canola, even before the crop is widely grown.
In the pre-commercialisation survey (2006–2007), the internal infection levels in the B. juncea breeding lines and cultivars were low, however the severity of internal infection was also very low in the B. napus plots. This may be a consequence of low rainfall in the surveyed areas during 2006 and 2007. The mean internal infection severities were low during 2006 and 2007, and it was probable that the disease would not have resulted in yield loss as internal infection levels greater than 50 % are required to significantly lower yield per plant in canola (Marcroft et al. 2004). Although B. juncea is considered more blackleg resistant than B. napus (Kirk and Oram 1978; Chèvre et al. 1997; Purwantara et al. 1998), at nine sites in 2006 and eight in 2007 the mean level of infection in the juncea canola breeding lines and cultivars was higher than the B. napus cultivars at the same site.
The results from the 2010–13 survey show that although disease severity was increased overall, the disease severity was consistently lower in the B. juncea cultivar compared to the B. napus. This suggests that although disease severity was higher in the B. juncea cultivars at some sites in the earlier survey, when conditions are conducive to greater disease the severity did not rise in the B. juncea cultivars to the same extent as it did in the B. napus cultivars. One possible explanation for this is the virulence profile of the Leptosphaeria population. The resistance genes of the juncea canola cultivars are currently unknown, however they are assumed to be different to those of the B. napus cultivars (Marcroft et al. 2012). Due to this, there is potentially a lower frequency of isolates that can attack the juncea canola cultivars compared to the B. napus cultivars, as the fungus has not previously been exposed to large areas of this resistance. This is positive news for the emerging juncea canola industry, however does not mean that disease severity will not rise in the future.
Shifts in the Leptosphaeria population structure occur in response to the resistance genes deployed in commercial cultivars (Sprague et al. 2006; Brun et al. 2000). As acreage of juncea canola increases so too will the selection pressure placed on the pathogen, resulting in a greater number of virulent isolates over time. The resistance genes present in juncea canola can be overcome in the same way that B. napus genes can be, as shown in field trials in France where Rlm6 resistance has been overcome (Brun et al. 2010).
The difference in mean internal infection severity between juncea canola breeding lines and cultivars within sites may reflect differences in resistance. In B. napus there are both qualitative and quantitative genes that play a part in the plants total resistance. Marcroft et al. (2012) showed that B. napus cultivars with the same complement of qualitative resistance genes could display different reactions due to their quantitative resistance.
Three genes, Rlm5, Rlm6 and LMJR2 have been identified in B. juncea (Chèvre et al. 1997; Barret et al. 1998; Balesdent et al. 2002, 2005; Saal et al. 2004). There may also be additional currently unidentified genes present in B. juncea that could confer resistance to L. maculans. There is also the possibility of genes being introgressed from B. napus during crossing for desirable oil quality and agronomic traits. Alternative combinations of these resistance genes are possible within the juncea canola cultivars tested and would explain the differences in internal infection severity seen. In addition, nothing is currently known about quantitative plant resistance in B. juncea and the role it may play. Screening of cultivars using a variety of Leptosphaeria isolates would need to be done to more clearly identify whether differences occur. Differences in resistance sources within juncea canola cultivars would be a positive finding for future breeding of juncea canola in Australia.
In order to maintain the blackleg resistance in B. juncea, and reduce the likelihood of L. maculans overcoming resistance genes, it is essential that the management techniques similar to those used in B. napus production be adopted for juncea canola production. This includes separating crops from the previous years stubble (Marcroft et al. 2004), and the application of a fungicide (seed dressing) if past disease levels deem it necessary. Breeding for resistance to blackleg and monitoring of disease severity in juncea canola should continue to be a high priority within the Australian canola industry. By monitoring disease severity growers can be warned if high disease severity, and hence yield losses are expected to occur.
As the primary locations for juncea canola production will be in low rainfall regions, the disease pressure, and therefore the selection pressure placed onto L. maculans, is likely to be low. Although this study has shown that blackleg isolates capable of attacking juncea canola are widespread, wise management of the resistance should allow juncea canola to be widely grown in low rainfall environments.
References
Balesdent MH, Attard A, Kühn ML, Rouxel T (2002) New avirulence genes in the phytopathogenic fungus Leptosphaeria maculans. Phytopathology 92:1122–1133
Balesdent MH, Barbetti MJ, Li H, Sivasithamparam K, Gout L, Rouxel T (2005) Analysis of Leptosphaeria maculans race structure in a world-wide collection of isolates. Phytopathology 95:1061–1071
Balesdent MH, Fudal I, Ollivier B, Bally P, Grandaubert J, Eber F, Chèvre A, Leflon M, Rouxel T (2013) The dispensable chromosome of Leptosphaeria maculans shelters an effector gene conferring avirulence towards Brassica rapa. New Phytol 198:887–898
Ballinger DJ, Salisbury PA (1996) Seedling and adult plant evaluation of race variability in Leptosphaeria maculans on Brassica species in Australia. Aust J Exp Agric 36:485–488
Barret P, Guérif J, Reynoird JP, Delourme R, Eber F, Renard M, Chèvre AM (1998) Selection of stable Brassica napus-B. juncea recombinant lines resistant to blackleg (Leptosphaeria maculans) 2: a ‘to and fro’ strategy to localise and characterise interspecific introgressions on B. napus genome. Theor Appl Genet 96:1097–1103
Brun H, Levivier S, Somda I, Ruer D, Renard M, Chèvre AM (2000) A field method for evaluating the potential durability of new resistance sources: application to the Leptosphaeria maculans-Brassica napus pathosystem. Phytopathology 90:961–966
Brun H, Chèvre AM, Fitt BDL, Powers S, Besnard AL, Ermel M, Huteau V, Marquer B, Eber F, Renard M, Andrivon D (2010) Quantitative resistance increases the durability of qualitative resistance to Leptosphaeria maculans in Brassica napus. New Phytol 185:285–299
Burton W, Salisbury P, Potts D (2003) The potential of canola quality Brassica juncea as an oilseed crop for Australia. In ‘13th Australian Research Assembly on Brassicas’. Tamworth, New South Wales, Australia pp. 62–64
Burton WA, Flood RF, Norton RM, Field B, Potts DA, Robertson MJ, Salisbury PA (2008) Identification of variability in phenological responses in canola-quality Brassica juncea for utilisation in Australian breeding programs. Aust J Agric Res 59:874–881
Chèvre AM, Barret P, Eber F, Dupuy P, Brun H, Tanguy X, Renard M (1997) Selection of stable Brassica napus-B. juncea recombinant lines resistant to blackleg (Leptosphaeria maculans). 1. Identification of molecular markers, chromosomal and genomic origin of the introgression. Theor Appl Genet 95:1104–1111
Colton B, Potter TD (1999) History. In ‘In: Canola in Australia: the first thirty years’. (Eds PA Salisbury, TD Potter, M G, AG Green). (Published for the 10th International Rapeseed Congress, Canberra, Australia
Delourme R, Chèvre AM, Brun H, Rouxel T, Balesdent MH, Dias JS, Salisbury P, Renard M, Rimmer SR (2006) Major gene and polygenic resistance to Leptosphaeria maculans in oilseed rape (Brassica napus). Eur J Plant Pathol 114:41–52
Eber F, Lourgant K, Brun H, Lode M, Huteau V, Coriton O, Alix K, Balesdent MH, Chèvre AM (2011) Analysis of Brassica nigra chromosomes allows identification of a new effective Leptosphaeria maculans resistance gene introgressed in Brassica napus. 13th International rapeseed congress, Prague 5–9 June 2011
Elliott VL, Marcroft SJ, Norton RM, Salisbury PA (2011) Reaction of Brassica juncea to Australian isolates of Leptosphaeria maculans and Leptosphaeria biglobosa ‘canadensis’. Can J Plant Pathol 33:38–48
Howlett BJ (2004) Current knowledge of the interaction between Brassica napus and Leptosphaeria maculans. Can J Plant Pathol 26:245–252
Kirk JTO, Oram RN (1978) Mustards as possible oil and protein crops for Australia. J Aust Inst Agric Sci 44:143–156
Li H, Sivasithamparam K (2003) Breakdown of a Brassica rapa subsp. sylvestris single dominant blackleg resistance gene in B. napus rapeseed by Leptosphaeria maculans field isolates in Australia. Plant Dis 87:752
Long Y, Wang Z, Sun Z, Fernando DWG, McVetty PBE, Li G (2011) Identification of two blackleg resistance genes and fine mapping of one of these two genes in a Brassica napus cultivar ‘Surpass 400’. Theor Appl Genet 122:1223–1231
Marcroft SJ, Purwantara A, Salisbury PA, Potter TD, Wratten N, Khangura R, Barbetti MJ, Howlett BJ (2002) Reaction of a range of Brassica species under Australian conditions to the fungus, Leptosphaeria maculans, the casual agent of blackleg. Aust J Exp Agric 42:587–594
Marcroft SJ, Sprague SJ, Pymer SJ, Salisbury PA, Howlett BJ (2004) Crop isolation, not extended rotation length, reduces blackleg (Leptosphaeria maculans) severity of canola (Brassica napus) in south-eastern Australia. Aust J Exp Agric 44:601–606
Marcroft SJ, Elliott VL, Cozijnsen AJ, Salisbury PA, Howlett BJ, Van de Wouw AP (2012) Identifying resistance genes to Leptosphaeria maculans in Australian Brassica napus cultivars based on reactions to isolates with known avirulence genotypes. Crop Pasture Sci 63:338–350
Mendes-Piereira E, Balesdent MH, Brun H, Rouxel T (2003) Molecular phylogeny of the Leptosphaeria maculans-L. biglobosa species complex. Mycol Res 107:1287–1304
Murray GM, Brennan JP (2012) The current and potential costs from diseases of oilseed crops in Australia. Grains Research and Development Corporation, Australia
Oram RN, Kirk JTO, Veness PE, Hurlstone CJ, Edlington JP, Halsall DM (2005) Breeding Indian mustard [Brassica juncea (L.) Czern.] for cold-pressed, edible oil production — a review. Aust J Agric Res 56:581–596
Plummer KM, Dunse K, Howlett BJ (1994) Non-aggressive strains of the blackleg fungus, Leptosphaeria maculans, are present in Australia and can be distinguished from aggressive strains by molecular analysis. Aust J Bot 42:1–8
Purwantara A, Salisbury PA, Burton WA, Howlett BJ (1998) Reaction of Brassica juncea (Indian mustard) lines to Australian isolates of Leptosphaeria maculans under glasshouse and field conditions. Eur J Plant Pathol 104:895–902
Rouxel T, Penaud A, Pinochet X, Brun H, Goutl D, Schmit J, Balesdent MH (2003) A 10-year survey of populations of Leptosphaeria maculans in France indicates a rapid adaptation towards the Rlm1 resistance gene of oilseed rape. Eur J Plant Pathol 104:871–881
Saal B, Brun H, Glais I, Struss D (2004) Identification of a Brassica juncea derived recessive gene conferring resistance to Leptosphaeria maculans in oilseed rape. Plant Breed 123:505–511
Sprague SJ, Marcroft SJ, Hayden HL, Howlett BJ (2006) Major gene resistance to blackleg in Brassica napus overcome within three years of commercial production in southeastern Australia. Plant Dis 90:190–198
Van de Wouw AP, Thomas VL, Cozijnsen AJ, Marcroft SJ, Salisbury PA, Howlett BJ (2008) Identification of Leptosphaeria biglobosa ‘canadensis’ on Brassica juncea stubble from northern New South Wales, Australia. Australas Plant Dis Notes 3:124–128
Van de Wouw AP, Marcroft SJ, Barbetti MJ, Li H, Salisbury PA, Gout L, Rouxel T, Howlett BJ, Balesdent MH (2009) Dual control of avirulence in Leptosphaeria maculans towards a Brassica napus cultivar with ‘sylvestris-derived’ resistance suggests involvement on two resistance genes. Plant Pathol 58:305–313
Vincenot L, Balesdent MH, Li H, Barbetti MJ, Sivasithamparam K, Gout L, Rouxel T (2008) Occurrence of a new subclade of Leptosphaeria biglobosa in Western Australia. Phytopathology 98:321–329
Yu F, Lydiate DJ, Rimmer SR (2005) Identification of two novel genes for blackleg resistance in Brassica napus. Theor Appl Genet 110:969–979
Yu F, Lydiate DJ, Rimmer SR (2008) Identification and mapping of a third blackleg resistance locus in Brassica napus derived from B. rapa subsp. sylvestris. Genome 51:64–72
Acknowledgments
We thank the Grains Research and Development Corporation, Australia for funding. We also thank the Department of Environment and Primary Industries Victoria, the Department of Primary Industries New South Wales and the South Australian Research and Development Institute for allowing the survey to take place within their yield trial sites. The authors thank Professor Barbara Howlett, Dr Grant Hollaway and Dr Angela Van de Wouw for critical comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Elliott, V.L., Norton, R.M., Khangura, R.K. et al. Incidence and severity of blackleg caused by Leptosphaeria spp. in juncea canola (Brassica juncea L.) in Australia. Australasian Plant Pathol. 44, 149–159 (2015). https://doi.org/10.1007/s13313-014-0337-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13313-014-0337-0