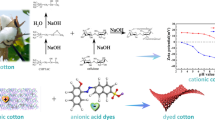
Abstract
Anionic dyes are often used for conventional cotton dyeing. This process, however, has a moderate affinity and it is estimated that less than 70% of the dye interacts with the cotton fiber. Cationization of cellulose is a chemical treatment that modifies the cellulose molecule, making it strongly cationic. This pretreatment increases the affinity between cotton and anionic dyes. Therefore, cationic dyeing reduces water, time, energy and chemical consumption. In this scenario, there is a growing demand to develop new cleaner products, as well as to elucidate the reaction mechanism aiming to create a clean and low-cost process for cotton cationization. In the last decades, more than 800 documents were published, and this number continues to rise. Among the cationic agents, 3-chloro-2-hydroxypropyl trimethylammonium chloride is the most researched and has achieved niche markets. However, poly-diallyldimethylammonium chloride combines effectiveness with a cleaner process. These characteristics make this cationic agent promising for future research. This review reports the state of the art on the techniques used for cationization, with a brief description of the market available for cationic cotton and a critical evaluation of the future perspectives for cationization.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The conventional method of dyeing cotton generally uses besides the dyes, the following chemical inputs: sulfate or sodium chloride, sodium hydroxide, sodium carbonate, acetic acid, surfactant, and chelating agent. In terms of chemical quantity, a conventional cotton dyeing process result in a consumption of 300 to 1200 g of chemical inputs (Ghaly et al. 2013) and 80 L of water per kilogram of fabric (Rosa et al. 2014). The conventional process has a moderate affinity between the fiber and the dyes and it is estimated that only 60–70% of the dye is exhausted (Ma et al. 2015). Therefore, it generates a considerable amount of effluent composed of non-reacted chemical inputs and hydrolyzed dye (Acharya et al. 2014; Arivithamani and Giri Dev 2017a).
The growing need to reduce water consumption and the use of chemicals in textile substrate processing has led to new technologies that increase dyeing effectiveness. One is the cationization of cellulose that chemically modifies the cellulose molecule, making it strongly cationic (Hauser and Tabba 2001; Roy Choudhury 2014; Aktek and Millat 2017; Li et al. 2019). Cotton fiber, which is originally negatively charged after this functionalization, acquire positive surface charges, which facilitates interaction with anionic dyes (Arivithamani and Giri Dev 2018). Using this type of dyeing can bring to cotton fiber more intense and brighter colors. Besides, it brings benefits to the dyeing process mainly by eliminating the use of alkali, soda ash (Na2CO3) and salt, essential inputs for cellulose fibers dyeing with reactive dyes class.
Anionic dyes, such as reactive and direct dyes are often used for cotton dyeing. Reactive dyes are predominantly used for dyeing of cotton due to their high color fastness, brilliancy and wide range of shades (Wang et al. 2009). However, these two dye classes often have a low affinity for cotton fabrics (Helmy et al. 2017). Thus, conventional dyeing of cotton with these classes of dyes requires the presence of electrolytes, which can neutralize negative charges on the cotton surface and thus increases dye-uptake in cellulose (Liu and Yao 2011). The most common electrolytes used for industrial dyeing of cotton are the salts NaCl or Na2SO4 (Liu and Yao 2011; Arivithamani and Dev 2017), in concentrations of 30–100 g L−1 depending on the dye concentration applied in the dyeing process (Wang et al. 2009).
One of the great triumphs of the cationization is the elimination of the salt feed from conventional processes. Dyeing of cationic cotton with reactive dyes can reduce by 70% the quantity of salt in textile effluent (Arivithamani and Giri Dev 2018). Therefore, many authors name the cationization process as salt-free or low-salt dyeing (Chattopadhyay et al. 2007; Arivithamani and Giri Dev 2016; Dong et al. 2020). A high concentration of salts elevates the water density, reduces the solubility of oxygen gases, affects the microorganisms, and is thus undesirable in effluents (Salmanikhas et al. 2016; Ahsan et al. 2019). The conventional effluent treatment is inefficient for salt removal and water desalination processes are unfeasible because of the high cost (Ni et al. 2018). Separation of salt from effluents increases the dyeing cost by 10% (Arivithamani and Giri Dev 2018). Therefore, a viable alternative for companies is the reduction of salt in dyeing processes.
Considering the competitiveness of the market, the greatest benefit of this technology is to achieve a reduction in water, energy, and chemicals in the dyeing process (Farrell and Hauser 2013; Bessa et al. 2019). Cationic cotton helps address market demands and improve inventory and cost efficiencies. It enables to dye fabrics utilizing one-third to one-half the typical amount of dye required in traditional dyeing (Cotton Incorporated 2018).
Several authors have studied cotton cationization as an alternative for lower cost or more sustainable dyeing over the years. In Fig. 1 is shown the historical growth in the number of publications in the Scopus database (up to April 2020), searched with keywords “cationic”, or “cationization”, and “cotton”. There has been a considerable amount of publications for over 50 years. Even after extensive research, cotton cationization still faces structural problems that make it difficult to use. Cationic cotton has still not seen a considerable industrial recognition aside from niche markets. According to Farrell et al. (2015), this happens because of the concerns about the safety of cationic agents, cost of cationic agents and cationization process, lack of results in large scale procedures and lack of methodology to transition from a conventional to a cationic cotton dyeing. The cationization process still needs improvement before achieving industrial-scale use.
Publication in cotton cationization has grown especially in the last 10 years. In this period, two review papers were published. Roy Choudhury (2014) published a paper focused on cationization and implication on the dyeing process. Aktek and Millat (2017) published a critical review regarding cationic agents and cationization procedure. The present review paper not only adds updated information but also add a unique contribution to the literature by focusing on indicators, processes variables, and market. It brings updated information regarding the mechanisms for cationization and application in the textile dyeing and printing. Based on the critical evaluation of the research papers, this work presents tendencies for the future of cationization.
This review article presents an overview of the current state of the art regarding cotton cationization. It provides knowledge about the cationic agents and the cationization process variables used by many authors. This paper also presents indicators to evaluate cationization and dyeing efficacy. Furthermore, it contains a brief discussion about the state of the art of printing of cationic cotton. Finally, the paper presents the market for cationic cotton, challenges, and prospects in this field.
Cationization of cellulose fibers
Cationization is a chemical process that provides cationic sites to the cellulose fibers. After the treatment, the fiber becomes positively charged. Therefore, this modification increases the electrostatic attraction between the fibers and the anionic dyes. Most dyes used for the dyeing of cellulose fibers are anionic in nature (Ibrahim et al. 2010; Arivithamani et al. 2014).
Cellulose is the major component of cotton, as shown in Table 1. While in contact with water, negative charges accumulate on the cotton surface due to the partial ionization of hydroxyl groups of cellulose (Acharya et al. 2014). Thus, in the conventional dyeing process, cotton fibers have electrostatic repulsion to most commercially available dyes, such as reactive and direct dyes, which are negatively charged (Aktek and Millat 2017). The efficiency of the process is increased by using high concentrations of electrolytes in the dyebath and extended dyeing times at elevated temperatures. Therefore, several washing cycles are necessary after dyeing to eliminate the dye that did not react with the fiber. For this reason, dyeing cotton fabrics with direct and reactive dyes consume a large amount of energy and natural resources (Arivithamani and Giri Dev 2017a; Farrell et al. 2017).
By introducing cationic groups into the cotton fibers, the ionic attractions between cationized cotton and anionic dyes result in increased dye uptake, due to the electrostatic attraction. The most common methodology for cationization is to introduce amino groups into the fiber (Roy Choudhury 2014). Quaternary or tertiary amino groups bind to cellulose to provide nucleophilic groups, which show greater attraction for anionic dye resulting in the interaction of dye-fiber without the addition of salt (Aktek and Millat 2017).
Cationic fiber also enables anionic dyeing at neutral pH. Dyeing cationic cotton results in greater exhaustion of dye and higher color values. The strong dye-fiber interaction, resulting from cationic cellulose, allows dyeing with minimal rinsing and after-washing. Furthermore, a fiber with good dye-attracting affinity may continue to exhibit those strong colors and brightness in later use, after consecutive washes (Roy Choudhury 2014).
Cationic agents
Several authors researched the introduction of amine quaternary groups into the cellulose structure for the cationization of cotton. A typical form of the cationic agent is a molecule containing quaternary ammonium and a reactive group that interacts with cellulose. Cationic agents can be grouped into polymer or monomeric based agents. A list of reagents studied for fiber cationization is shown in Table 2.
Hauser and Tabba (2001) studied the effect of commercially CHPTAC (3-chloro-2-hydroxypropyl trimethylammonium chloride), available as a 65% solution in water, in cationization of cotton fabrics with direct and reactive dyes. In comparison with conventional dyeing, color strength values (K/S) of cationic fabric dyed with reactive and direct dyes were increased by approximately 50%. The color fastness of the cationic dyeing was equal or superior in comparison with untreated cotton.
Acid dyes are typically applied to fibers with positive charges, such as wool, silk and polyamide fibers in an acidic bath. Although, it has a low affinity to cellulosic fibers due to repulsions forces between the negative charges of the cellulosic fibers and the acid dye molecules (Rehan et al. 2020). However, according to Hauser and Tabba (2001), cationization with CHPTAC also allowed the dyeing of cellulosic fabric with acid dyes. The authors compared cationic cotton and nylon dyed with different acid dyes. The results showed that K/S values were almost the same for both types of fabric. However, color fastness considerably decreased for acid-dyed cationic cotton. Acid dyes are widely used by the textile market for producing fabrics with intense and bright shades. Acid dyes are also considered easier to dye than other classes (Patil et al. 2019).
A fabric composed of 50% polyester and 50% cotton was cationized using poly diallyldimethylammonium chloride (PDDACl) and chitosan by Oliveira et al. (2017). The cationic fabric was dyed using acid dyes. The best K/S value was 43 for the sample treated by PDDACl, this value was approximately 20% higher than cationic fabric treated with chitosan and nearly 280% higher than the untreated sample. Comparing treated with non-treated samples, no significant variation on the whiteness degree was found; this means that the cationization process does not change the base color of the fabric.
According to Farrell and Hauser (2013), most of the chemicals used for the cationization of cotton have some toxicity issues. Therefore, chitosan has been studied in the textile dyeing process because it represents an environmentally sound and non-toxic practice to increase dye uptake. Chatha et al. (2016) measured the properties of dyeing cotton cationized by chitosan. The authors concluded that pretreatment increased color strength along with a significant improvement in the washing fastness.
Dong et al. (2020) grafted 2-(N,N-dimethylamino)ethyl methacrylate (PDMAEMA) onto the cotton fabric. The results showed that the cotton fabric was successfully grafted. The dyeing with reactive dye presented excellent dye uptake, fixation yield, K/S value, dyeing levelness, and color fastness.
Hasani et al. (2009) prepared two epoxy reagents, N-oxiranylmethyl-N-methylmorpolinium chloride (NMM) and 2-oxiranylpyridine, for cationization of the cotton linter. CHPTAC was also used in cationization tests as reference. The authors observed the dependence between the nitrogen content in cellulose and water retention. The higher the amount of nitrogen, the greater the water retention into the cotton. Better water retention, consequently, resulted in raised adsorption of the acid dye methyl orange to the fiber. NMM exhibited higher reactivity toward cellulose than CHPTAC and 2-oxiranylpyridine.
Wang and Lewis (2002) researched cationic cotton fabrics treated with 1-acrylamido-2-hydroxy-3-trimethylammoniumpropane chloride (AAHTAPC) using a pad–bake process. The treated fiber was dyed with several reactive dyes without the addition of salt or alkali. The cationization increased dye-fiber affinity. The reactive dyes were almost completely exhausted when used with cationic cotton. The dyeings were uniform on the surface, but some dyeings exhibited ring dyeing (a fault in which the dye did not diffuse completely to the interior of fiber). Ring dyeing is associated with the molecular weight of the cationic agent. High molecular cationic agents cannot penetrate cellulose pores, therefore the cationization is restricted to the external surface of the fiber.
Ma et al. (2016) treated cotton fabric with betaine (N,N,N-trimethyl glycine) as a cationic agent, using a pad–dry–bake pretreatment process. The cationic fabrics were applied in salt-free dyeing of reactive dyes. The increase in dye fixation of Reactive Red 195, Reactive Yellow 145 and Reactive Blue 19 on cationic fabrics were 6.7%, 9.5%, and 7.7%, respectively. Dye fixation on the cationic fabrics in the absence of salt improved with satisfactory light fastness property. However, some wash and wet rub fastness could not remain as good as that of the conventional dyeing.
Cotton fabrics were cationized using 2-methacryloyloxyethyltrimethyl ammonium chloride (DMC). Modified and unmodified cotton was dyed with Reactive Blue 19, Reactive Red 195 Reactive Yellow 145. The fixation of dye in modified cotton was 23–24% higher than those obtained using the conventional dyeing method in the presence of inorganic salt. The wastewater parameters, chemical oxygen demand, color and ammonia–nitrogen content of the cationic cotton dyeing method were considerably lower than those of the conventional dyeing (Ma et al. 2015).
Liu and Yao (2011) prepared cationic cotton with two pretreatment solutions. Initially, epichlorohydrin was used for epoxidation of the cotton fibers. Then the fabric was treated with a solution containing thiourea to bind amino groups to the cellulose. The epichlorohydrin acted as a crosslinking agent between the thiourea and the cotton fibers. The cationic samples were dyed using reactive dyes. The cationic dyed fabrics presented higher K/S, levelness, washing and rubbing fastness.
Farrell et al. (2015) cationized cotton using various alkyl chlorohydrin quaternary ammonium compounds, in conjunction with the traditional reagent CHPTAC. The cationic samples were dyed using reactive and disperse dyes. The K/S of reactive dyeings were higher for samples pretreated by molecules with low molecular weights, BCHDAC and CHPTAC. This happens due to the higher nitrogen ratio of these molecules. The authors also found a possibility to dye cotton with disperse dyes. Cationic cotton treated by long-chain compounds (CHPDDAC, CHPCDAC, and CHPDODAC) readily exhausted almost all disperse dye. This is related to the increased hydrophobicity of cotton treated by long-chain compounds. Disperse dyes are often used for dyeing of hydrophobic fibers, such as polyester and polyamide (Matthews 2018).
3-Chloro-2-hydroxypropyl trimethylammonium chloride (CHPTAC)
Among all the cationic agents, CHPTAC has been the most preferred for cotton cationization and has shown potential for industrial scale-up (Arivithamani and Giri Dev 2018). The synthesis procedure for CHPTAC is published by Seong and Ko (1998). CHPTAC is also known by their commercial names Glytac A, Quat-188, CA200, and CR2000.
A great advantage of the CHPTAC is the size of the molecule. It is easier to penetrate the fiber pores if the molecule has low molecular weight. Therefore, dyeings with cotton treated with CHPTAC tends to present a uniform dyeing. The cross-section optical microscope images performed by Arivithamani and Giri Dev (2017b) and Ma et al. (2017) indicated that the dyes penetrated satisfactory in the cotton when treated with CHPTAC.
CHPTAC itself does not react with cellulose. Firstly, it converts into 2,3-epoxypropyl trimethylammonium chloride (EPTAC), also identified as glycidyl trimethylammonium chloride, by reacting with alkali. Then EPTAC reacts with the hydroxyl groups on cotton fiber under alkaline conditions to form cationized cotton. In the presence of alkali, CHPTAC is converted to EPTAC together with partial dissociation of the cellulose hydroxyl group (Farrell and Hauser 2013). The reaction is shown in Scheme 1 (Hauser and Tabba 2001; Wang et al. 2009; Farrell et al. 2015; Arivithamani and Giri Dev 2016).
The produced EPTAC reacts with the primary hydroxyl group of ionized cellulose under alkaline conditions to form the cationized fiber. The reaction mechanism is presented in Scheme 2 (Arivithamani and Giri Dev 2016). Each repeating unit of the cellulose molecule has one primary and two secondary hydroxyl groups, which can undergo chemical reactions. However, the primary hydroxyl groups are more accessible and reactive than the secondary groups (Wakelyn et al. 2006).
CHPTAC is a moderately nontoxic chemical reagent, but to proceed with cellulose reaction, it must be first converted into EPTAC, which presents carcinogen properties (Roy Choudhury 2014). Therefore, to minimize exposure, EPTAC solutions should never be handled or transported. Cationization processes should be designed to introduce CHPTAC and the alkali required for EPTAC conversion, followed by its subsequent reaction with the cellulose. The overall cationization process should be designed in a quantity that EPTAC is consumed to the maximum by fiber, to ensure minimal EPTAC release to the environment (Farrell and Hauser 2013).
Poly diallyldimethylammonium chloride (PDDACl)
Poly diallyldimethylammonium chloride (PDDACl), usually abbreviated as PDDA, PDADMAC, and Poly-DADMAC is a well-known cationic polyelectrolyte. Research interest for PDDACl is related to applications in different fields, such as papermaking, wastewater treatment, mineral processing, food and medical industry (Zhang et al. 2003). It does not have the same acceptance as CHPTAC for cationization of fibers, but recent research indicates that this polyelectrolyte has potential as an environmentally-safe cationic agent (Kim et al. 2016; Helmy et al. 2017; Oliveira et al. 2017; Bessa et al. 2019; Jareansin et al. 2019).
PDDACl is considered as an environmentally friendly and low-cost polyelectrolyte (Wang et al. 2013; Liu et al. 2018). It is the first polymer approved by United States Food and Drug Administration for use in potable water treatment (Helmy et al. 2017). Environmental and human health studies destined for the United States Environmental Protection Agency testified that diallyldimethylammonium chloride (DADMAC), the monomer of PDDACl, is nontoxic to environmental organisms and is readily biodegradable (DADMAC HPV Committee 2004). This report was issued for the High Production Volume Challenge Program, which provides hazard information for the chemicals produced or imported into the United States (Bishop et al. 2012).
The cellulose mechanism for cationization with DADMAC was published by Jareansin et al. (2019). Potassium persulfate was used as an initiator to create cellulose radicals. The initiator creates a radical in the hydroxyl bonded to the primary carbon of cellulose. The cationization mechanism is explained in Scheme 3. The initiator has the same function as the epoxy group in the CHPTAC cationization process shown in Scheme 1. The formation of PDDACl and binding to cellulose occurs from the interaction with hydroxyl radicals.
Considering the polymer structure of PDDACl, its fiber penetration is limited by the size of the molecule. Thus, the degree of polymerization is a particularly important property for determining the effectiveness of cellulose cationization. Zhang et al. (2016) used PDDACl with different molecular weights for the accessibility evaluation of cellulose fiber charges. Fiber charges strongly depended on the molecular weight of polyelectrolyte. A higher fiber charge was detected using PDDACl with low molecular weight. However, the charge was nearly unchanged when the molecular weight varied from 100 to 600 kg mol−1. This suggests that above 100 kg mol−1, PDDACl is not filled into the cellulose pores and the interaction is restricted to the external surface, as illustrated in Fig. 2.
Methods for fiber cationization
The method used by each author for the pretreatment of cotton is available in Table 2. Among them, exhaustion is a discontinuous process considered the conventional system of dyeing as most of the industries follow this process (Arivithamani and Giri Dev 2018). However, continuous and semi-continuous processes are used mainly in the treatment before dyeing. Therefore, various processes are used for the cationization of fibers. A brief explanation of each method is also given in Fig. 3. Pad-batch is a sequence of operations involving padding and batching without intermediate drying. The padded fabric is rolled in a batch, wrapped by plastic sheets, and stored with slow rotation to allow the dye to fix at room temperature. In pad-steam, the fabric is padded and steamed for fixation of the chemicals. Pad-dry is like the pad-steam method, the difference consists in use dry air instead of steam. Pad-bake is like pad-dry but involves chemical fixation at high temperatures. Pad-dry-steam and pad-dry-bake, as the names suggest, are associations between pad-dry, pad-steam and pad-bake. Pad-dry-cure, which is also presented in Table 2, is reciprocal to the pad-dry-bake method (Hashem et al. 2003; Roy Choudhury 2014; Matthews 2018).
Houshyar and Amirshahi (2002) treated cotton fabric with chitosan using five different techniques, consisting of exhaustion, pad-dry, pad-batch, pad-steam, and pad-dry-steam methods. The exhaustion method was carried out in a laboratory dyeing machine at 60 °C during 5 min. The pad-dry method was carried out by padding with 110% pick up and dried at 150 °C for 3 min. In the pad-batch method, cotton was padded with 110% pick up and batched for 30 min, while in pad-steam method samples where padded with 110% pickup and steamed during 30 min at 110 °C. The pad-dry-steam method padded samples with 110% pick up, dried at 150 °C for 3 min and steamed at 100 °C for 30 min. The authors concluded that the pad-dry method ensured the highest dye uptake with a slight reduction of light and wash fastness.
Hashem (2006) developed a one-stage process of scouring, bleaching or desizing with cationization. CHPTAC was the cationic agent used. Three methods were used: exhaustion, cold pad-batch and pad-steam. The authors have found that the cationic agent was compatible with enzymatic desizing agent diastase. However, the CHPTAC was incompatible with the chemical desizing agents, specifically ammonium persulfate and potassium peroxydiphosphate. Besides, CHPTAC was also incompatible with hydrogen peroxide. The combination of CHPTAC and hydrogen peroxide hindered the effect of cationization and bleaching. However, cationization and scouring with NaOH was performed without incompatibility.
Arivithamani et al. (2014) fixed KH into cotton fabric by an esterification reaction. In a round-bottom flask was prepared a solution with cotton, KH, and toluene as solvent. The solution was heated to boiling. During this process, the esterification reaction takes place, water is eliminated from the reaction and it is evaporated from the flask. To avoid reverse esterification reaction, the evaporated water continuously removed from the process was condensed and collected. The process ended when it stopped the formation of water.
Ma et al. (2016) cationized cotton fabric with betaine through the pad-dry-bake method. It was prepared a solution containing 8 wt% of anhydrous betaine, hydrochloric acid (1 mol of betaine per mol of acid) and 5 wt% of dicyandiamide. The fabric was dipped in the solution at material to liquor ratio of 1:10 and padded to 90% pickup. Then the fabrics were dried at 80 °C during 3 min and baked at 150 °C during 40 s. The cationic treatment improved the fixation of the dye, maintaining the washing and rubbing fastness at satisfactory levels.
According to some authors (Fu et al. 2013; Mandal 2017), among the higher temperature application methods, however, the cold pad-batch is possibly the most efficient method to provide uniform cationization of cotton because heat may cause the migration of reactants, which results in non-uniform cationization.
Cationization process variables
The cationization variables must be optimized for achieving higher dye fixation. Understanding all the parameters involved, the treatment can be evaluated comprehensively. Table 3 contains a list and range of values for the variables used in various cationization studies carried out by the exhaustion method.
Although some authors prefer to represent the concentration of the cationic agent in percentage over weight of fiber (owf), according to Table 3 the concentration evaluated varies from 5 to 80 g per liter of solution. It was observed a strong correlation between cationic agent dosage and reactive dye fixation. An addition of a high concentration of cationic agent could achieve a higher cationization degree of cotton, as well as high dye exhaustion and the percentage of the total dye fixed on the cationized cotton (Chatha et al. 2016; Ma et al. 2017).
The alkali concentration is a parameter that needs to be optimized for the cationization process. For example, a low mole ratio of NaOH to CHPTAC result in a low formation of EPTAC, consequently, it decreases the fiber cationization. On the contrary, an increase in the mole ratio led to the hydrolyzed form of EPTAC, a diol namely as 2,3-dihydroxypropyl trimethylammonium chloride, as shown in Scheme 4. The diol form is stable, therefore it does not react with the cotton fabrics and results as an effluent at the end of the cationization process (Hauser and Tabba 2001; Farrell and Hauser 2013).
Ma et al. (2017) studied the effect of NaOH concentration in the fixation of Reactive Red 195 dye on cationic cotton. When the concentration of NaOH was 1, 2, 3, and 4 g L−1, the dye fixation was 45.94, 70.53, 96.74 and 96.18%, respectively. The fixation of the reactive dye did not increase when the NaOH dosage rose from 3 to 4 g L−1. Therefore, they found 3 g L−1 as the optimal concentration for NaOH. According to Hashem, Hauser and Smith (2003), the optimal NaOH:CHPTAC molar ratio for the cationization process is 1.8.
According to Table 3, the temperature used in cationization by the exhaustion method can vary from 30 to 90 °C. Cationization of cotton by CHPTAC is an etherification reaction, dehydration of an alcohol group to form ether, so that increasing the temperature is beneficial for the retention of cationic groups on cotton (Ma et al. 2017).
The material-to-liquor ratio was 1:20, except for the research developed by Oliveira et al. (2017), which used a considerably higher amount of liquor. The nitrogen fixation efficiency is observed to decrease with increasing liquor ratio because the hydrolysis reaction of the cationic agent is more favorable with higher concentrations of water (Mandal 2017). Besides, a higher bath ratio rises the energy costs for temperature elevation and increases the amount of effluent.
Exhaustion is a discontinuous process for fiber cationization. However, there are also some continuous or semi-continuous processes. For example, Hauser and Tabba (2001) used a foulard to pad cotton fabric with a solution consisting of 50 g L−1 of a 65% CHPTAC solution and 36 g L−1 of a 50% sodium hydroxide solution, at 100% wet pick-up. Wang and Lewis (2002) used a pad–bake process with 50 g L−1 AAHTAPC, 30 g L−1 sodium hydroxide, 90% wet pick-up and baked at 125 °C for 6 min.
Nakpathom et al. (2017) used CHPTAC to prepare cationic cotton and dye using purple corncob. The concentration of CHPTAC varied between 5 and 150 g L−1 and NaOH was added to achieve the molar ratio of 1:2.6 (CHPTAC:NaOH). The fabric samples were padded to 100% pick up. The addition of NaOH occurred immediately before proceeding with the padding bath to avoid the hydrolysis of the CHPTAC. Then, the cotton fabric was neutralized using 1.5 g L−1 of acetic acid during 30 min, rinsed with water and air-dried. The authors obtained optimum K/S values for cotton pretreated with 125 g L−1 CHPTAC and dyed at 100 °C and pH 9, during 30 min.
Characterization of cationic fiber
As exemplified in the mechanism described in Schemes 1, 2 and 3, the cationization process occurs from the incorporation of cationic elements to the fiber, mainly quaternary ammonium compounds, which positively charge the surface of the fiber. Thus, a good indicator of the efficiency of the cationization process is the nitrogen content incorporated in the fiber. Table 4 contains the percentage of nitrogen in cationic cotton researched by some authors, using different methods and reagents concentrations. The nitrogen content analyses were performed using an elemental analyzer by Kanik and Hauser (2002) and Fu et al. (2013), while Wang et al. (2009), Hashem (2006) and Ma et al. (2017) measured with the Kjeldahl method.
For reference purposes, the percentage of nitrogen in a non-cationized sample is 0.01% (Kanik and Hauser 2002). According to Table 4, the percentage of nitrogen increased up to 0.65%, depending on the variables and the process used. It is observed a strong and positive dependence on the concentration of CHPTAC. Considering that is the nitrogen incorporated in the fiber that guarantees the affinity with anionic dyes, the bigger its quantity the better the fixation to the fiber occurs.
Some authors characterized the nitrogen content using X-ray photoelectronic spectroscopy (XPS) (Liu and Yao 2011; Oliveira et al. 2017). The analysis indicated that the atomic percentage of nitrogen for a cationic fabric composed by 50% polyester and 50% cotton treated with PDDACl (concentration of 5%owf and temperature of 50 °C) was 3.7%. Moreover, XPS analysis of cotton fabric cationized by epichlorohydrin and thiourea revealed a nitrogen atomic percentage of 3.35%. For both cases, the nitrogen content in the untreated sample was null.
Kim et al. (Kim et al. 2016) performed a qualitative analysis to validate the deposition of PDDACl and chitosan. The authors assessed the color of dyed fabrics with Coomassie Brilliant Blue dye. The color strength of the fibers regards the quantity of polyelectrolytes attached to the fibers. Therefore, the stronger color after dyeing means more affinity to the fiber surface. Coomassie Brilliant Blue dye is often used for protein detection due to its anionic character and affinity with protonated amino groups (Georgiou et al. 2008). The stronger color was obtained for samples pretreated with cationic agents PDDACl and chitosan.
According to the conventional cationization procedure proposed by Hashem (2006), the exhaustion, pad-steam and pad-batch methods presented a small difference in the nitrogen quantity fixed, although the pad-steam process obtained the best result.
Fourier-transform infrared spectroscopy (FTIR) can also confirm the fixation of the cationic agent into cellulose. A peak between 1000 and 1400 cm−1 may correspond to the presence of quaternary ammonium groups, while a peak at 1200 cm−1 may correspond to C–N stretching vibration (Zhang et al. 2015; Arivithamani and Dev 2017).
As cationization induces a positive charge at the surface of cotton, due to the presence of a quaternary ammonium group, it is expected an increment in the zeta potential of cationic cotton. As observed by Arivithamani and Giri Dev (2017b), the cotton fabric showed zeta potential of − 6 mV and − 4 mV at pH 7 and 6, whereas the cationic cotton fabric showed zeta potential of + 1.3 mV and + 2.2 mV for pH of 7 and 6 respectively. Zhang et al. (2015) obtained similar results: the zeta potential of modified cellulose was + 13.8 mV, in contrast to − 5.2 mV that corresponds to original cellulose.
The surface morphology of cationic cotton fiber was evaluated through scanning electron microscopy (SEM). Based on the micrographs, the surface of the cationic fibers was slightly rougher compared with that of the non-cationic, but the cationization process almost not influenced the structure of the cotton so that it was considered suitable for dyeing application (Wang et al. 2009; Ma et al. 2017).
During the cationization, the intermolecular interactions between the chains are affected due to the penetration of the polyelectrolyte molecules. This is responsible for a slight change of the thermal profiles of cationic fabrics (Arivithamani and Giri Dev 2016). Thermogravimetric analysis (TGA) made by Ma et al. (2017) indicated that the stability of the non-cationic cotton was slightly better, a significant weight loss happened at 320 °C for non-cationic cotton, against 305 °C for the treated sample. Thermal profiles from Arivithamani and Giri Dev (2016) indicated a significant weight loss of around 350 °C 390 °C for cationic and non-cationic cotton, respectively.
X-ray diffraction (XRD) analysis was used to study the effect of cationization on crystal structural changes. The results showed that the X-ray spectra of the cotton before and after cationization are almost equal. It demonstrated that cationization occurred just on the surface of the fiber; it did not affect its crystal structure (Wang et al. 2009).
Dyeing of cationic fibers
Cationization of cotton has been widely researched with the use of direct and reactive dyes because they have a high affinity to materials with positive surface charges. For dyeing with reactive dyes, it can not only increase the dye uptake but also eliminate the use of salt. Thus, cationization can be used to reduce problems associated with dye bath effluent of reactive dyes (Fu et al. 2013).
The cationic cotton fabric contains cationic sites called quaternary ammonium group, which creates a strong positive potential in the dyebath. Thus, anionic dye molecules move from the dyebath solution to the cationic cotton fabric by ionic attraction (Arivithamani and Giri Dev 2018).
For example, in Scheme 5 is shown the reaction mechanism between cationic cotton and a vinyl sulphone based reactive dye (Arivithamani and Giri Dev 2017b). According to the scheme, the dye molecule can bind to the cellulose in two ways. In (a), the reactive group interacts with the ammonium quaternary and causes the breakdown of two N-CH3 bonds. In (b) is presented the conventional cellulose dyeing mechanism, in which the reactive group reacts with the ionized oxygen of the primary carbon of the cellulose. Primary hydroxyl groups are more accessible and reactive than secondary groups (Wakelyn et al. 2006).
Wang and Lewis (2002) investigated the use of salt in a dyeing bath containing a commercial anionic reactive dye (Procion Red H-E3B) and cationic cotton. The exhaustion was almost complete in the absence of salt, but the exhaustion drops to 94.6% after adding 20 g L−1 of sodium sulfate. This unfavorable effect occurs because the sulfate anions have a significant affinity for cationic cellulose, so they compete with dye anions for the quaternary sites in the modified fiber.
Alkalis are often used to obtain the alkaline medium, that is, the pH of the bath more favorable for the exhaustion and fixation (reaction) of the dye to the fiber. Therefore, the use of Na2CO3 is very common in conventional dyeing processes. Fu et al. (2013) investigated the influence of Na2CO3 in the dyeing process with cationic fabrics. With the increase of Na2CO3 concentration, both K/S and dye uptake non-cationic cotton increased gradually from 5 to 20 g L−1. The results also revealed that, for cationic cotton fabrics, eliminating the use of Na2CO3 decreased both the K/S and dye uptake, but increasing the concentration did not influence the K/S and dye uptake values significantly. Thus, 5 g L−1 Na2CO3 appears to be an effective concentration for dyeing cationic cotton fabrics.
Various properties of dyed fibers are presented in Table 5. Through the comparison between noncationic and cationic fiber, it is observed that the cationization increased the color intensity, expressed by color strength values (K/S). The increase in color intensity in dyed fabrics occurred due to the greater interaction between the dye and the cationic fiber, resulting in higher percentages of exhaustion and fixation. Exhaustion (E) refers to the amount of dye migrating from the bath to the fiber surface. The fixation (F) is related to strong dye-fiber interactions, measured by the color difference in the fabric before and after soaping. It is observed in Table 5 that the cationization increased both percentages of exhaustion and dye fixation.
Washing and rubbing fastness area measured on a scale of 1 to 5, where 5 represents the maximum fastness (International Organization for Standardization 2010, 2016), that is, no color changes were detected after washing or rubbing (dry and wet). In the case of light fastness, the scale ranges from 1 to 7, which 7 being the maximum fastness, according to ISO 105 B2 (International Organization for Standardization 2013).
There was little interference of cationization in the fastness properties of cotton dyed with reactive dyes. For wash fastness, a slight improvement was observed for four dyes (Wang and Lewis 2002; Wang et al. 2009; Liu and Yao 2011): Sumifix Supra Yellow 3RF, Reactive Gold Yellow B-3RD, Reactive Blue 19 and Reactive Yellow 145. Cationization altered light fastness for Reactive Yellow 145, Reactive Blue 19 and Reactive Red 195 (Wang et al. 2009; Ma et al. 2020).
The dry rubbing fastness slightly increased for cationic cotton dyed with Reactive Yellow 145 (Wang et al. 2009) and decreased with Reactive Blue B-RV dyes (Liu and Yao 2011). Wet rubbing fastness increased for cationic cotton dyed with Reactive Gold Yellow B-3RD (Liu and Yao 2011). However, a slight loss of wet rubbing fastness occurred for cationic fabrics dyed with Reactive Blue B-RV, Reactive Red 195 and Reactive Yellow 145 (Wang et al. 2009; Liu and Yao 2011; Ma et al. 2020). The loss may be related to the hydrophilic character of the cationic agent. In the wet rubbing fastness, the water acts as a lubricant and removes the cationic sites at the surface of the fabric (Naikwade et al. 2017).
The decrease in the fastness properties is also related to the tendency to the ring dyeing in cationic fabrics. Due to strong attraction forces, both fixed and unfixed dye can be present on the surface of the cationic fiber. This effect is more evident when dyeing deep shade fabrics (Naikwade et al. 2017). According to Wang and Lewis (2002), ring dyeing can be decreased with anionic surfactants added in the dyebath. The anionic surfactants interact with surface cationic sites so that the dye would penetrate deeper inside the fiber.
Considerable improvements in washing fastness were observed using direct dyes with cationic cotton, increasing from 2 to 4–5 for both dyes tested (Hauser and Tabba 2001). The improvement also occurred for light fastness in cationic fabric dyed with Direct Red 80 dye. The rubbing fastness, however, did not change with cationization.
Cationization is also effective for other natural cellulosic fibers. Tests made by Naikwade et al. (2017) resulted in improved dye exhaustion and fixation on ramie fiber, with no loss of fastness. Cationic treatment improved performance, with 89% and 98% for exhaustion and fixation, respectively. Besides that, K/S values were higher for cationic fibers, with good color uniformity and similar for the untreated fiber.
Printing of cationic fabric
Differently which occur with the cotton cationized dyeing process, so far only a few research papers have been published on the printing of cationic textile substrates. However, the growing use of reactive dyes in printing cotton process, it makes believe in the potential of cationization to reduce problems of dye loss by hydrolysis and to improve its affinity with the cellulose, and consequently reduction of water for washing to remove the unfixed dye. The printing process on textile substrates involves the transfer of a colored paste to a specific location of the substrate (Shang 2013). It can be through several techniques, the most common in use are screen (flatbed and rotary) and digital (inkjet) printing in Fig. 4 (Jurič et al. 2015).
The flatbed screen printing consists of the transfer of the ink through the mesh apertures of a printing plate with the aid of the movement of a squeegee. In rotary printing the screen and the printing plate are cylindrical, the substrate and the pressure roller move synchronously, the ink is transferred from the inside of the printing cylinder to the substrate (Novaković et al. 2015). Digital printing is different from other methods, the application is through a spray. The ink jets spray the drop-shaped ink through a piezoelectric head. The drop is charged by an electrostatic field, passes through a deflection field that determines where the drop falls on the substrate (Soleimani-Gorgani 2015).
Kanik and Hauser (2002) evaluated the properties of cotton fabrics cationized with EPTAC in the process of localized printing with reactive dye. Cationization showed a significant increase in color yield, especially at medium concentrations of dye, the vaporization time can be reduced by half and the washing process has also been significantly reduced.
Kanik and Hauser (2003) investigated the possibility of improving inkjet printing with reactive dye, using cotton fabrics cationized with CHPTAC. Cationization increases the color intensity of samples printing with reactive dyes, the vaporization time for untreated cotton was 10 min and 6 min for cationic cotton. Besides, the washing procedure for cationic cotton reduced from five to three washes.
Kanik and Hauser (2004) modified the properties of cotton substrates with cationic agent CHTAC in the printing process. Printing with direct dye presents some problems in practice, such as low washing strength, migration of dye to unprinted areas. The cationic agent improved these properties of the prints with direct dyes.
Chen, Zhao and Wang (2004) demonstrated that the color yield in the digital printing process with reactive inks on the cotton substrate modified with polycyclohydrin-dimethylamine was higher than the non-cationic substrate. The reason for this is that the introduction of positive charges increases the uptake and fixation of the dye in the cationic substrate. Besides, the cationic modification with PECH amine decreases the frictional strength, however, it increases the washing strength of the treated cotton.
Wang and Zhang (2007) studied the behavior of localized printing using pigment, on a cotton substrate, modified with a cationic agent Cibafix Eco. The curing method (pre-setting) is more suitable for printing pigments than the vaporization process. The fastness properties are acceptable when the printed fabric is treated with only 4% of the cationic agent. However, cationic pretreatments exhibit slightly yellowish phenomena.
Wang, Hu and Yan (2018) studied two applications in the digital printing process; first they evaluated the application of a cationic ink with CHPTAC and a reactive ink stamped simultaneously on the cotton substrate; then they evaluated the application of the cationic agent on the cotton substrate by printing with reactive ink. The reactive dye and the dye with CHPTAC showed a competitive relationship, both react with the cotton substrate in the inkjet printing process. As the reaction progresses between the reactive dye and cotton cationized with CHPTAC the zeta potential becomes positive, which in turn contributes to the adsorption of reactive dyes to the cotton substrate.
Application of cationic agents in functional fabrics
The application of cationic agents is not restricted to the interaction with dyes. Cationization can also be used to increase functional properties of the fabric, such as antimicrobial activity. Other applications are related to UV protection, water repellency and production of conductive fabrics.
Antimicrobial activity
The textile substrates are considered as possible vectors of transmission of diseases (Pachiayappan et al. 2020). Cellulosic fibers have a high retain moisture rate, which creates an environment that enables microorganism growth onto fabric (Aly et al. 2007). For this reason, antimicrobial behavior can be an important property desired mainly for textiles applied in the health area (Kim et al. 2016). Quaternary ammonium compounds, often used for cationization, are also widely used in antimicrobial applications (Liu et al. 2014). In general, they are bacteriostatic, fungistatic, at very low concentrations (< 500 μg ml−1), with Gram-positive bacteria being particularly sensitive (< 10 μg ml−1) (McDonnell 2017).
Chitosan has been studied by many researchers as a natural polymer for antimicrobial treatment of cellulose fibers (Aly et al. 2007; El-Shafei et al. 2008; Kim et al. 2016; Rehan et al. 2017; Rahman Bhuiyan et al. 2017). Chitosan is considered a potential antimicrobial substance and still has very interesting properties such as good biocompatibility, biodegradability, non-toxicity, and availability in abundance (Qin et al. 2020).
Kim et al. (Kim et al. 2016) assessed the antimicrobial activity of cotton pretreated with chitosan and PDDACl by layer-by-layer deposition technique. The gram-negative bacteria Escherichia coli and gram-positive bacteria Staphylococcus aureus were tested. PDDACl on cotton fibers presented an excellent effect of the inhibition rate of microorganisms growing (almost 100%) and the inhibition rate of chitosan was close to 50%. The combination of PDDACl and chitosan layers presented a synergistic effect and enhanced antimicrobial activity.
Farrell et al. (2017) compared fabrics treated with different hydrophobic alkyl quaternary ammonium compounds, reported in Table 2. All pretreatment resulted in antimicrobial activity. The control sample, nontreated cotton, presented an increment in inoculum concentration after 24 h. All pretreatment resulted in a 99% reduction in inoculum concentration, except for pretreatment with CHPTAC which obtained 89% reduction.
Rehan et al. (2017) prepared antimicrobial cotton gauze by reacting it with CHPTAC and impregnation of silver nanoparticles. The pretreated samples presented an inhibition effect above 95%. The results also indicated that pretreatment with CHPTAC (without silver nanoparticles) enabled 73% and 67% inhibition effect on samples, respectively for Escherichia coli and Staphylococcus aureus. A similar methodology was proposed by Shateri Khalil-Abad et al. (2009), who used CHPTAC to enhance the uptake of silver nanoparticles. It was observed that the silver content on cationic cotton was up to three times higher than the conventional cotton.
Other functional properties
Kamal Alebeid and Zhao (2015) used CHPTAC and TiO2 nanoparticles to prepare functional fabrics with ultraviolet (UV) protection. The CHPTAC can increase the interaction with TiO2, which has negative charges. The pretreated fabrics presented enhanced UV protection. The fabrics were dyed using Reactive Yellow 176. The K/S of the pretreated and dyed samples did not change significantly. These results may be related to the opposite effect of CHPTAC and TiO2. While CHPTAC increases the charge of the fabric, the TiO2 nanoparticles tend to decrease due to the anionic character.
Sahito et al. (2015) investigated used the cationization as a way to increase the conductivity of electronic textiles (e-textiles). Bovine serum albumin (BSA) was used as cationic agent. The cationic cotton was then treated with graphene oxide to increase conductivity. The cationization increased 67% of the graphene oxide uptake in the cotton fabric.
Abd El-Hady et al. (2020) functionalized cotton fabric for hydrophobic and UV protective properties. The fabrics were pretreated with a solution containing 20 g L−1 of PDDACl, using the pad-dry-cure method. Then the cationic fabrics were immersed in an anionic solution containing ZnO/SiO2 nanocomposites. The proposed method successfully increased the UV protection. Besides, the coated surface became hydrophobic due to the deposition of ZnO/SiO2 nanocomposites.
In a similar work, Farouk et al. (2013) proposed a method to prepare multifunctional fabrics. The fabric was prior treated with cationic agents CHPTAC and DADMAC. The cationic fabrics were treated with TiO2 and SiO2 nanoparticles. The pretreated fabrics presented antimicrobial activity for gram-negative (Pseudomonas aeruginosa and Escherichia coli) and gram-positive bacteria (Staphylococcus aureus and Bacillus subtilis). The pretreatment with TiO2 and SiO2 nanoparticles enhanced the UV protection. Besides, stearic acid was coated in cotton fabric to achieve water repellency.
Market
There is a significant demand for cationic agents from the dyeing industry. This market is mainly controlled by the use of CHPTAC, thanks to the advances of researchers over the years to enable the development of a reliable product in a robust process. This factor maintained the sales of CHPTAC as a popular cationic agent in the dyeing industry (Arivithamani and Giri Dev 2018). The popularity of the cationic agent is driven by its cost. The purchase cost of the two main cationic agents, CHPTAC and PDDACl, is shown in Table 6. The values were obtained from three random Chinese suppliers on April 3, 2020. The CHPTAC reagent had the highest average cost per solution. However, it represents a 22–28% discount when the percentage of pure CHPTAC is considered.
The market for cationic cotton has seen a resurgence in industrial interest because of the rise in the cost of reactive dyes and the push of more economical and sustainable cotton coloration processes (Farrell et al. 2015). Cationic cotton attracts attention in niche markets because it improves the K/S using the same amount of dye and produces less effluent (Arivithamani and Giri Dev 2016; Dong et al. 2020). Cotton Incorporated surveyed cationic cotton suppliers (Cotton Incorporated 2018). Cotton Incorporated is a not-for-profit company that provides resources to help companies develop cotton products. The list is presented in Table 7. The major players in the market are in China and the USA.
Driven by competition, industrial protection for cationization processes has been growing especially in the last 10 years. In Fig. 5 is shown the number of patent publications available in Espacenet, searched with keywords “cationic cotton” or “cotton cationization” and “cotton” in textile classification. Espacenet is a patent database maintained by the European Patent Office that offers free access to over 110 million documents (European Patent Office 2020).
The USA has 31 published patents and is the country with most publications, followed by China with 17. Both are responsible for 73% of the entire number of patents. The expressiveness of these countries can also be seen in the list of suppliers. According to Table 7, six companies are from China and five from America, corresponding to 64% of suppliers.
Challenges of cationization and future perspectives
According to Farrell and Hauser (2013), three main concerns hinder the expansion of cotton cationization on an industrial scale: the safety of the predominant cationization reagent CHPTAC, lack of large scale cationization demonstrations, and environmental hazards. For Arivithamani and Dev (2017), the major limitation is because cationization was commonly applied by batch processes, but most of the industries adopt the exhaustion method of dyeing. Thus, the authors propose the application of cationic agents by exhaustion technique followed by reactive dyeing. This is more like the usual practices already adopted by the industry and would enable it to use this process without modification of the existing setup.
Increasing concerns have been expressed over how the industry can adapt to this trend and still achieve energy savings (Hashem 2006). Cationization requires an extra pretreatment step which may lead to extra cost (Aktek and Millat 2017). In this sense, Ma et al. (2017) proposed a combinative methodology for scouring, bleaching, and cationization of cotton. All these processes are all under alkaline conditions. If combined into one, alkali dosage, treatment time, consumed energy and water, and the overall cost will be greatly reduced. Cationization is shown to be incompatible together with scouring and bleaching. However, the results were satisfactory when scouring and bleaching were first carried out in one bath, and then followed by cationization.
A great concern about industrial cationization is the nuisance odor that remains in the cationic fabric after treatment. This reduces market acceptance of the product. According to Farrell et al. (2015), when alkali and CHPTAC are mixed in solution to form the reactive EPTAC, trimethyl amine is liberated. It is presumed that trimethyl amine is already present in the commercial product and is released upon pH increase by the addition of alkali. Volatile trimethyl amine can be easily detected in the processes and is highly undesirable because of the resemblance to dead fish odor.
The most common cationic agent faces some safety criticism, CHPTAC is currently classified as a Carcinogen Category 3, while EPTAC is classified as a Carcinogen Category 2. This classification is given by IARC (International Agency for Research on Cancer) that classifies into four groups based on the existing scientific evidence for carcinogenicity. Category 3 means that there is no evidence at present that it causes cancer in humans. Category 2 means it is probably or possibly carcinogenic to humans (International Agency for Research on Cancer 2015). Besides, in all CHPTAC use scenarios, the principal concern is the conversion of CHPTAC to EPTAC. EPTAC is a known genotoxic carcinogen (Farrell and Hauser 2013). It is important to understand that EPTAC is an intermediate and unstable product that rapidly reacts with cotton cellulose (Schemes 1 and 2). The EPTAC not fixed to cellulose tends to hydrolyze and converts to a stable form of diol (Scheme 4). EPTAC is commercially available by the name glycidyl trimethylammonium chloride, but due to instability and safety problems, its industrial use is not recommended. To avoid safety concerns, many authors have been researched natural inputs as cationic agents, such as chitosan, ovalbumin, bovine serum albumin and keratin (Ibrahim et al. 2010; Arivithamani et al. 2014; Sahito et al. 2015; Chatha et al. 2016; Oliveira et al. 2017; Sadeghi-Kiakhani and Safapour 2018; Giacomini et al. 2020).
The literature search indicates the existence of more than 800 papers related to cotton cationization, published for eight decades. Even so, some gaps remain. The amount of publications has grown considerably in the last 10 years, due to the need for economical and sustainable alternatives for textile processing processes. The researchers focused mostly on CHPTAC reagent, but even after years of research, this reagent has not achieved global recognition in the textile industry. The cationization process still needs to be optimized, mainly to be operated at safer levels.
In the coming years, research is expected to focus on cleaner processes. In this group are included the research for sustainable cationic agents. Recent research using PDDACl indicates good process efficiency, coupled with the use of a clean reagent and large-scale supply availability. Therefore, PDDACl is considered a cationic agent promising for future research and the market.
References
Abd El-Hady MM, Sharaf S, Farouk A (2020) Highly hydrophobic and UV protective properties of cotton fabric using layer by layer self-assembly technique. Cellulose 27:1099–1110. https://doi.org/10.1007/s10570-019-02815-0
Abdelileh M, Ben TM, Moussa I, Meksi N (2019) Pretreatment optimization process of cotton to overcome the limits of its dyeability with indigo carmine. Chem Ind Chem Eng Q 25:277–288. https://doi.org/10.2298/CICEQ181115006A
Acharya S, Abidi N, Rajbhandari R, Meulewaeter F (2014) Chemical cationization of cotton fabric for improved dye uptake. Cellulose 21:4693–4706. https://doi.org/10.1007/s10570-014-0457-2
Ahsan MA, Satter F, Siddique MAB et al (2019) Chemical and physicochemical characterization of effluents from the tanning and textile industries in Bangladesh with multivariate statistical approach. Environ Monit Assess 191:1–24. https://doi.org/10.1007/s10661-019-7654-2
Aktek T, Millat AKMM (2017) Salt free dyeing of cotton fiber—a critical review. Int J Text Sci 6:21–33. https://doi.org/10.5923/j.textile.20170602.01
Aly AS, Mostafa ABE, Ramadan MA, Hebeish A (2007) Innovative dual antimicrobial & anticrease finishing of cotton fabric. Polym Plast Technol Eng 46:703–707. https://doi.org/10.1080/15583720701271559
Arivithamani N, Dev VRG (2017) Cationization of cotton for industrial scale salt-free reactive dyeing of garments. Clean Technol Environ Policy 19:2317–2326. https://doi.org/10.1007/s10098-017-1425-y
Arivithamani N, Giri Dev VR (2016) Salt-free reactive dyeing of cotton hosiery fabrics by exhaust application of cationic agent. Carbohydr Polym 152:1–11. https://doi.org/10.1016/j.carbpol.2016.06.087
Arivithamani N, Giri Dev VR (2017a) Sustainable bulk scale cationization of cotton hosiery fabrics for salt-free reactive dyeing process. J Clean Prod 149:1188–1199. https://doi.org/10.1016/j.jclepro.2017.02.162
Arivithamani N, Giri Dev VR (2017b) Industrial scale salt-free reactive dyeing of cationized cotton fabric with different reactive dye chemistry. Carbohydr Polym 174:137–145. https://doi.org/10.1016/j.carbpol.2017.06.045
Arivithamani N, Giri Dev VR (2018) Characterization and comparison of salt-free reactive dyed cationized cotton hosiery fabrics with that of conventional dyed cotton fabrics. J Clean Prod 183:579–589. https://doi.org/10.1016/J.JCLEPRO.2018.02.175
Arivithamani N, Agnes Mary S, Senthil Kumar M, Giri Dev VR (2014) Keratin hydrolysate as an exhausting agent in textile reactive dyeing process. Clean Technol Environ Policy 16:1207–1215. https://doi.org/10.1007/s10098-014-0718-7
Bessa PS, Ladchumanandasivan R, Steffens F, Oliveira FR (2019) Dyeing of meta-aramid fibres previously functionalized with poly(diallyldimethylammonium chloride). Key Eng Mater 812:107–113. https://doi.org/10.4028/www.scientific.net/KEM.812.107
Bishop PL, Manuppello JR, Willett CE, Sandler JT (2012) Animal use and lessons learned in the U.S. high production volume chemicals challenge program. Environ Health Perspect 120:1631–1639. https://doi.org/10.1289/ehp.1104666
Chatha SAS, Hussain AI, Ali S et al (2016) Significance of chitosan to improve the substantivity of reactive dyes. J Chil Chem Soc 61:2895–2897. https://doi.org/10.4067/S0717-97072016000200009
Chattopadhyay DP, Chavan RB, Sharma JK (2007) Salt-free reactive dyeing of cotton. Int J Cloth Sci Technol 19:99–108. https://doi.org/10.1108/09556220710725702
Chen W, Zhao S, Wang X (2004) Improving the color yield of ink-jet printing on cationized cotton. Text Res J 74:68–71. https://doi.org/10.1177/004051750407400112
Cotton Incorporated (2018) Cationic cotton. In: Cottonworks. https://www.cottonworks.com/topics/sourcing-manufacturing/dyeing/cationic-cotton/. Accessed 2 Apr 2020
DADMAC HPV Committee (2004) Test plan for diallyldimethylammonium chloride (DADMAC). Washington, DC
Dong W, Zhou M, Li Y et al (2020) Low-salt dyeing of cotton fabric grafted with pH-responsive cationic polymer of polyelectrolyte 2-(N, N-dimethylamino)ethyl methacrylate. Colloids Surf A Physicochem Eng Asp 594:124573. https://doi.org/10.1016/j.colsurfa.2020.124573
El-Shafei AM, Fouda MMG, Knittel D, Schollmeyer E (2008) Antibacterial activity of cationically modified cotton fabric with carboxymethyl chitosan. J Appl Polym Sci 110:1289–1296. https://doi.org/10.1002/app.28352
European Patent Office (2020) Espacenet—patent search. https://worldwide.espacenet.com/. Accessed 2 Apr 2020
Farouk A, Sharaf S, Abd El-Hady MM (2013) Preparation of multifunctional cationized cotton fabric based on TiO2 nanomaterials. Int J Biol Macromol 61:230–237. https://doi.org/10.1016/j.ijbiomac.2013.06.022
Farrell MJ, Hauser PJ (2013) Cationic cotton, reservations to reality. AATCC Rev 13:56–63
Farrell MJ, Ormond RB, Gabler WJ (2015) Quantitative analysis of trimethyl amine in cotton fabrics cationized with 3-chloro-2-hydroxypropyltrimethylammonium chloride. Cellulose 22:3435–3439. https://doi.org/10.1007/s10570-015-0692-1
Farrell MJ, Fu S, Ankeny MA (2017) Cationic cotton prepared with hydrophobic alkyl chlorohydrin quats: a new fiber with new properties. AATCC 2017—2017 AATCC international conference proceedings, pp 97–125
Fu S, Hinks D, Hauser P, Ankeny M (2013) High efficiency ultra-deep dyeing of cotton via mercerization and cationization. Cellulose 20:3101–3110. https://doi.org/10.1007/s10570-013-0081-6
Georgiou CD, Grintzalis K, Zervoudakis G, Papapostolou I (2008) Mechanism of Coomassie brilliant blue G-250 binding to proteins: a hydrophobic assay for nanogram quantities of proteins. Anal Bioanal Chem 391:391–403. https://doi.org/10.1007/s00216-008-1996-x
Ghaly AE, Ananthashankar R, Alhattab M, Ramakrishnan VV (2013) Production, characterization and treatment of textile effluents: a critical review. J Chem Eng Process Technol 05:1–18. https://doi.org/10.4172/2157-7048.1000182
Giacomini F, de Souza AAU, de Barros MASD (2020) Cationization of cotton with ovalbumin to improve dyeing of modified cotton with cochineal natural dye. Text Res J. https://doi.org/10.1177/0040517519899652
Hasani M, Westman G, Potthast A, Rosenau T (2009) Cationization of cellulose by using N-oxiranylmethyl-N-methylmorpholinium chloride and 2-oxiranylpyridine as etherification agents. J Appl Polym Sci 114:1449–1456. https://doi.org/10.1002/app.30548
Hashem MM (2006) Development of a one-stage process for pretreatment and cationisation of cotton fabric. Color Technol 122:135–144. https://doi.org/10.1111/j.1478-4408.2006.00022.x
Hashem M, Hauser P, Smith B (2003) Reaction efficiency for cellulose cationization using 3-chloro-2-hydroxypropyl trimethyl ammonium chloride. Text Res J 73:1017–1023. https://doi.org/10.1177/004051750307301113
Hauser PJ, Tabba AH (2001) Improving the environmental and economic aspects of cotton dyeing using a cationised cotton+. Color Technol 117:282–288. https://doi.org/10.1111/j.1478-4408.2001.tb00076.x
Helmy HM, Hauser P, El-Shafei A (2017) Influence of atmospheric plasma-induced graft polymerization of DADMAC into cotton on dyeing with acid dyes. J Text Inst 108:1871–1878. https://doi.org/10.1080/00405000.2017.1298206
Houshyar S, Amirshahi SH (2002) Treatment of cotton with chitosan and its effect on dyeability with reactive dyes. Iran Polym J 11:295–302
Ibrahim NA, El-Sayed WA, Ameen NA (2010) A novel technique to minimise energy and pollution in the dyeing of linen fabric. Color Technol 126:289–295. https://doi.org/10.1111/j.1478-4408.2010.00263.x
International Agency for Research on Cancer (2015) IARC monographs questions and answers. In: International Agency for Research on Cancer. https://www.iarc.fr/wp-content/uploads/2018/07/Monographs-QA.pdf. Accessed 8 Oct 2019
International Organization for Standardization (2010) ISO 105. Tests for colour fastness—part C06: colour fastness to domestic and commercial laundering
International Organization for Standardization (2013) ISO 105.Tests for Colour fastness—part B02: colour fastness to artificial light: xenon arc fading lamp test
International Organization for Standardization (2016) ISO 105. Tests for colour fastness—part X12: colour fastness to rubbing
Jareansin S, Sukaam P, Kusuktham B (2019) Preparation and characterization of modified cotton fabrics with responsive pH. Polym Bull 76:4507–4520. https://doi.org/10.1007/s00289-018-2603-8
Jurič I, Kašiković N, Stančić M et al (2015) The influence of heat treatment on print mottle of screen printed textile knitted fabrics. Appl Therm Eng 90:215–220. https://doi.org/10.1016/j.applthermaleng.2015.07.013
Kamal Alebeid O, Zhao T (2015) Anti-ultraviolet treatment by functionalizing cationized cotton with TiO2 nano-sol and reactive dye. Text Res J 85:449–457. https://doi.org/10.1177/0040517514549989
Kanik M, Hauser PJ (2002) Printing of cationised cotton with reactive dyes. Color Technol 118:300–306. https://doi.org/10.1111/j.1478-4408.2002.tb00114.x
Kanik M, Hauser PJ (2003) Ink-jet printing of cationised cotton using reactive inks. Color Technol 119:230–234. https://doi.org/10.1111/j.1478-4408.2003.tb00177.x
Kanik M, Hauser PJ (2004) Printing cationized cotton with direct dyes. Text Res J 74:43–50. https://doi.org/10.1177/004051750407400108
Kim S, Nakamatsu J, Maurtua D, Oliveira F (2016) Formation, antimicrobial activity, and controlled release from cotton fibers with deposited functional polymers. J Appl Polym Sci 133:1–11. https://doi.org/10.1002/app.43054
Li M, Zhang L, Qiu M et al (2019) Dyeing property of fluorescent pigment latex on cationic knitted cotton fabrics. Text Res J 89:422–433. https://doi.org/10.1177/0040517517748494
Liu L, Yao J (2011) Salt-free dyeability of thiourea grafted cotton fabric. Fibers Polym 12:42–49. https://doi.org/10.1007/s12221-011-0042-3
Liu Y, Liu Y, Ren X, Huang TS (2014) Antimicrobial cotton containing N-halamine and quaternary ammonium groups by grafting copolymerization. Appl Surf Sci 296:231–236. https://doi.org/10.1016/J.APSUSC.2014.01.106
Liu Y, Zhang J, Guan H et al (2018) Preparation of bimetallic Cu–Co nanocatalysts on poly (diallyldimethylammonium chloride) functionalized halloysite nanotubes for hydrolytic dehydrogenation of ammonia borane. Appl Surf Sci 427:106–113. https://doi.org/10.1016/j.apsusc.2017.08.171
Ma W, Wang T, Li H, Zhang S (2015) Cotton fabric modification through ceric (IV) ion-initiated graft copolymerisation of 2-methacryloyloxyethyltrimethyl ammonium chloride to enhance the fixation of reactive dyes. Cellulose 22:4035–4047. https://doi.org/10.1007/s10570-015-0713-0
Ma W, Meng M, Yan S, Zhang S (2016) Salt-free reactive dyeing of betaine-modified cationic cotton fabrics with enhanced dye fixation. Chin J Chem Eng 24:175–179. https://doi.org/10.1016/J.CJCHE.2015.07.008
Ma W, Shen K, Xiang N, Zhang S (2017) Combinative scouring, bleaching, and cationization pretreatment of greige knitted cotton fabrics for facilely achieving salt-free reactive dyeing. Molecules 22:2235. https://doi.org/10.3390/molecules22122235
Ma W, Du S, Yan S et al (2020) Salt-free dyeing of modified cotton through graft polymerization with highly enhanced dye fixation and good strength properties. Polymers (Basel). https://doi.org/10.3390/polym12020462
Mandal S (2017) Capping and characterizing dyeing properties of cationized cotton. North Carolina State University
Matthews K (2018) Encyclopaedic dictionary of textile terms. Woodhead Publishing India, New Delhi
McDonnell GE (2017) Antisepsis, disinfection, and sterilization: types, action and resistance, 2nd edn. ASM Press, Washington
Naikwade M, Liu F, Wen S et al (2017) Combined use of cationization and mercerization as pretreatment for the deep dyeing of ramie fibre. Fibers Polym 18:1734–1740. https://doi.org/10.1007/s12221-017-5512-9
Nakpathom M, Somboon B, Narumol N, Mongkholrattanasit R (2017) Dyeing of cationized cotton with natural colorant from purple corncob. J Nat Fibers. https://doi.org/10.1080/15440478.2017.1354742
Ni G, Zandavi SH, Javid SM et al (2018) A salt-rejecting floating solar still for low-cost desalination. Energy Environ Sci 11:1510–1519. https://doi.org/10.1039/C8EE00220G
Novaković D, Kašiković N, Vladić G, Pál M (2015) Screen printing. Print Polym Fundam Appl. https://doi.org/10.1016/B978-0-323-37468-2.00015-4
Oliveira FR, De Oliveira DAJ, Steffens F et al (2017) Dyeing of cotton and polyester blended fabric previously cationized with synthetic and natural polyelectrolytes. Procedia Eng 200:309–316. https://doi.org/10.1016/j.proeng.2017.07.044
Pachiayappan KM, Prakash C, Kumar V (2020) Influence of process variables on antimicrobial properties of cotton knitted fabrics. J Nat Fibers 17:313–325. https://doi.org/10.1080/15440478.2018.1492487
Patil AA, Maiti S, Adivarekar RV (2019) The use of poly(amido)amine dendrimer in modification of cotton for improving dyeing properties of acid dye. Int J Cloth Sci Technol 31:220–231. https://doi.org/10.1108/IJCST-04-2018-0055
Qin Y, Li P, Guo Z (2020) Cationic chitosan derivatives as potential antifungals: a review of structural optimization and applications. Carbohydr Polym 236:116002. https://doi.org/10.1016/j.carbpol.2020.116002
Rahman Bhuiyan MA, Hossain MA, Zakaria M et al (2017) Chitosan coated cotton fiber: physical and antimicrobial properties for apparel use. J Polym Environ 25:334–342. https://doi.org/10.1007/s10924-016-0815-2
Rehan M, Zaghloul S, Mahmoud FA et al (2017) Design of multi-functional cotton gauze with antimicrobial and drug delivery properties. Mater Sci Eng C 80:29–37. https://doi.org/10.1016/j.msec.2017.05.093
Rehan M, Mahmoud SA, Mashaly HM, Youssef BM (2020) β-Cyclodextrin assisted simultaneous preparation and dyeing acid dyes onto cotton fabric. React Funct Polym 151:104573. https://doi.org/10.1016/j.reactfunctpolym.2020.104573
Rosa JM, Tambourgi EB, Santana JCC et al (2014) Development of colors with sustainability: a comparative study between dyeing of cotton with reactive and vat dyestuffs. Text Res J 84:1009–1017. https://doi.org/10.1177/0040517513517962
Roy Choudhury AK (2014) Coloration of cationized cellulosic fibers—a review. AATCC J Res 1:11–19. https://doi.org/10.1450/ajr.1.3.2
Sadeghi-Kiakhani M, Safapour S (2018) Salt-free dyeing of cotton fabric modified with prepared chitosan-poly(propylene) imine dendrimer using direct dyes. Prog Color Color Coat 11:21–32
Sahito IA, Sun KC, Arbab AA et al (2015) Integrating high electrical conductivity and photocatalytic activity in cotton fabric by cationizing for enriched coating of negatively charged graphene oxide. Carbohydr Polym 130:299–306. https://doi.org/10.1016/j.carbpol.2015.05.010
Salmanikhas N, Tizghadam M, Rashidi Mehrabadi A (2016) Treatment of saline municipal wastewater using hybrid growth system. J Biol Eng 10:9. https://doi.org/10.1186/s13036-016-0030-7
Seong HS, Ko SW (1998) Synthesis, application and evaluation of cationising agents for cellulosic fibres. J Soc Dye Colour 114:124–129. https://doi.org/10.1111/j.1478-4408.1998.tb01963.x
Shang SM (2013) Process control in printing of textiles. Woodhead Publishing Limited, New Delhi
Shateri Khalil-Abad M, Yazdanshenas ME, Nateghi MR (2009) Effect of cationization on adsorption of silver nanoparticles on cotton surfaces and its antibacterial activity. Cellulose 16:1147–1157. https://doi.org/10.1007/s10570-009-9351-8
Soleimani-Gorgani A (2015) Inkjet printing. Elsevier, Amsterdam
Wakelyn PJ, Bertoniere NR, French AD et al (2006) Cotton fiber chemistry and technology. Taylor & Francis Group, Boca Raton
Wang H, Lewis DM (2002) Chemical modification of cotton to improve fibre dyeability. Color Technol 118:159–168. https://doi.org/10.1111/j.1478-4408.2002.tb00094.x
Wang C-X, Zhang Y-H (2007) Effect of cationic pretreatment on modified pigment printing of cotton. Mater Res Innov 11:27–30. https://doi.org/10.1179/143307507X196194
Wang L, Ma W, Zhang S et al (2009) Preparation of cationic cotton with two-bath pad-bake process and its application in salt-free dyeing. Carbohydr Polym 78:602–608. https://doi.org/10.1016/j.carbpol.2009.05.022
Wang T, Zhang L, Wang H et al (2013) Controllable synthesis of hierarchical porous Fe3O4 particles mediated by poly(diallyldimethylammonium chloride) and their application in arsenic removal. ACS Appl Mater Interfaces 5:12449–12459. https://doi.org/10.1021/am403533v
Wang L, Hu C, Yan K (2018) A one-step inkjet printing technology with reactive dye ink and cationic compound ink for cotton fabrics. Carbohydr Polym 197:490–496. https://doi.org/10.1016/j.carbpol.2018.05.084
Zhang YL, Yi M, Ren J, Ha HF (2003) Effect of radiation on high-charge-density polydially-dimethyl ammonium chloride in dilute aqueous solution. Chin J Polym Sci 21:459–464
Zhang F, Pang Z, Dong C, Liu Z (2015) Preparing cationic cotton linter cellulose with high substitution degree by ultrasonic treatment. Carbohydr Polym 132:214–220. https://doi.org/10.1016/j.carbpol.2015.06.055
Zhang H, Zhao C, Li Z, Li J (2016) The fiber charge measurement depending on the poly-DADMAC accessibility to cellulose fibers. Cellulose 23:163–173. https://doi.org/10.1007/s10570-015-0793-x
Acknowledgments
The authors are grateful for the financial support from the Brazilian Federal Foundation for Support and Evaluation of Graduate Education (CAPES)—Finance Code 001 and Internationalization Program CAPES-PRINT (Project Number 88887.310560/2018-00).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Correia, J., Rainert, K.T., Oliveira, F.R. et al. Cationization of cotton fiber: an integrated view of cationic agents, processes variables, properties, market and future prospects. Cellulose 27, 8527–8550 (2020). https://doi.org/10.1007/s10570-020-03361-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-020-03361-w