Abstract
Electrostatic self-assembly layer by layer technique was used to immobilize ZnO/SiO2 nanocompsite on cationized cotton fabric. This occurs via the sequential dipping of cotton fabric in dilute solutions of poly (diallyldimethylammonium chloride) (PDDA) and ZnO/SiO2 colloidal suspension nanocomposite of different concentration ratios 1:0, 0:1, 1:1, 2:1, 1:2, and 2:2. The formation of multilayer thin film on cotton fabric creates different functional properties. UV protection properties were monitored at the ratio of (Zn/Si) as well as the number of layers. In the case of 1(Bilayer)BL and 5(Bilayer)BL, increasing the ratio of (ZnO/SiO2) within the nano composite (ZnO/SiO2) ratio, the UPF increases and the results indicate that the best ultraviolet protection factor is obtained when the Zn/Si ratio is 2. Additionally, dyeing the treated fabric often enhanced protection against ultra violet rays. FTIR spectra were utilized to distinguish the existence of effective groups on the surface of the treated cotton. Scanning electron microscopy studies confirmed successful deposition of the PDDA/(ZnO/SiO2) nanocomposite. Moreover, cotton fibers connected together because of the increased coating density and their surface become rougher. Post treatment by stearic acid rendered the fabric water repellent property. Other physical properties such as tensile strength as well as breathability of the cotton fabric were investigated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Surface modification has a significant influence in textile production. The final characteristics of a textile material are essential for defining the performance for different uses. Nanotechnology has real commercial potential for the textile industry (Sharaf and Barakat 2018). Various studies were concerned with the impact of the application of nanoparticles to textile materials These studies aimed at producing finished fabrics with different characteristics such as UV blocking, antibacterial and self-cleaning. Nanotechnology not only provides high durability for fabrics but also has no effect on fabric breath ability or hand feel. As a result, nano-particles have a large surface area-to-volume ratio and high surface energy (Gulrajani and Deepti 2011).
Layer by layer electrostatic self-essembly (ESA) is a novel technique for surface modification of textile fabric via fabrication of multilayer thin films on cruel surfaces. The mechanism of this technique includes the successive adsorption of oppositely charged poly anions and poly cations. At last, composite film consisting of polyelectrolytes is created. The consecutive deposition treatment can be accomplished by substrate charging followed by dipping it into the oppositely charged solutions. This oppositely-charged polyelectrolyte is absorbed on the charged substrate via electrostatic interaction. After deposition of each layer, the substrate is washed via immersing into the water. The succession of this technique can be up to the twenty thin double layers deposited (Tian et al. 2016a; Uğur et al. 2010). Decher et al. (1998) fabricated this technique in 1990. Two methods of electrostatic interaction can be described for the creation of multi-layer films. First, polyelectrolytes and their countercharged ions are combined together in aqueous solution. Second, formation of layer-by-layer films occurred via the deposition of the complex with counter-polyelectrolyte (Shirvan et al. 2014).
It ought to be noticed that LBL method is not restricted to polyelectrolytes. Some positively or negatively charged organic molecules have also been incorporated into multilayer through LbL self-assembled deposition. In perspective on this, it is reasonable that incorporating different effective materials into ESA multilayer assemblies on fiber surfaces could support fabrication of functional textile. However, self-assembly functionalization of textile substrates have been scarcely reported (Dubas et al. 2006). Polyelectrolytes that frequently utilized such as poly (sodium 4-styrenesulfonate) (PSS), poly (dimethyldiammonium chloride) (PDDA), poly (allylamine hydrochloride) (PAH) and polyethyleneimine (PEI) have no effect on textile functionality. Meanwhile, non-polyelectrolytes with negative charges such as nanoparticles and dyestuffs were incorporated into the LbL self-assembled multilayer with polyelectrolytes to alter antibacterial and dyeability properties of the surface (Shi et al. 2017; Khan et al. 2017).
Moreover Recent studies are mostly concerned with applying LbL technique for rendering fabric multifunctional properties, such as self-decontaminate (Grandcolas et al. 2011), electroconductive (Tian et al. 2016b), Ultraviolet protection (Huang et al. 2015; Tian et al. 2016a) superhydrophobic(Chen et al. 2018; Huang et al. 2015; Li et al. 2017), and fire retardant (Zhang et al. 2017).
Poly (diallyldimethylammonium chloride) (PDDA) is not only considered a positively charged polyelectrolyte but also a water-soluble quaternary ammonium. It performed as a cationic colloid in aqueous solution. Recently, PDDA has been utilized in stabilization of nanoparticles on different materials such as glass, paper and fabric (Sadeghi and Pourahmad 2011; Wang and Hauser 2010).
The present work focused on fabricating super-hydrophobic and UV protective cotton fabric by deposition of ZnO/SiO2 nanocomposite on cationized cotton fabric using electrostatic self -assembly (ESA) layer by layer technique. The alternating layer by layer build-up was based on PDDA and ZnO/SiO2 nanocomposite. PDDA was used as cationic electrolyte and ZnO/SiO2 nanocomposite was used as negatively charged layer deposited on modified substrate where new features of this fabric were studied.
Experimental
Materials
Mill bleached pure 100% cotton fabric (230 g/m2) was supplied by Misr Company for spinning and weaving Mehalla El-Kobra, Egypt.
Chemicals
Poly (diallyldimethylammonium chloride) (PDDA, 20% w/w, Mw 100,000–200,000, Aldrich), stearic acid, ethanol, acetone, Sodium hydroxide, sodium carbonate, silicon oxide nanoparticles and zinc oxide nanoparticles were supplied by Merck chemical company (Germany).
Preparation of ZnO/SiO2 nanocomposite
Colloidal suspension of ZnO/SiO2 nanocomposite at different concentration ratios (wt/wt) were prepared. Six Powder ratios (1:0, 0:1, 1:1, 2:1, 1:2, and 2:2) are suspended in ethanol solution at room temperature. The mixture was sonicated for 30 min. After sonication time, 40 g/l NaOH drop by drop was added until the solution attained pH 10.
Coating of cotton fabric by PDDA
For the purpose of multilayer film fabrication using the ‘layer-by-layer’ technique fabric should be charged. This could be achieved by cationization of cotton fabrics by firstly treated with PDDA layer. Cotton samples were padded in solution of 20 g/l of PDDA and 8 g/l NaOH at 100% wet pickup. The treated samples then was dried at 80 °C and cured at 120 °C for 3 min. Finally all samples were washed with deionized water several times and dried.
Fabrication of self-assembled multilayers on cotton fabric
Polyelectrolyte/nanocomposite multilayer were fabricated on positively-charged cotton fabrics (cationized fabrics) at room temperature immersed into the following solutions alternately for 5 min according to the following procedures:
(a) the anionic ZnO/SiO2 colloidal solution nanocomposite of different ratios 1:0, 0:1,1:1,2:1,1:2, and 2:2, (wt/wt) (b) The deionized water, (c) the cationic PDDA solution, and (d) the deionized water.This deposition cycles were repeated until 5- and 10-multilayer films were deposited on the surface of cotton fabrics. Multilayer films deposited on cotton fabrics were dried at 80 °C and cured at 120 °C for 3 min.
Hydrophobic treatment
Stearic acid was used to lower the surface energy of the coated fabrics with PDDA and ZnO/SiO2 nanocomposite. The treated fabrics were immersed in 1 wt% stearic acid solution of acetone for 10 min, before it was padded, and cured at 110 °C for 45 min (Farouk et al. 2013). The residual materials not fixed were removed by washing the samples with acetone and were then dried. Finally, the samples were washed for two laundering cycles.
Dyeing procedure
Treated cotton samples were added to an aqueous solution containing 2.5% of CI Reactive blue 21 with continuous stirring, the temperature of which was raised to 60 °C and dyeing was continued at this temperature for 30 min. The samples were then washed in an aqueous solution containing soap (5 g/l) boiling for 15 min. Finally, the samples were washed with cold water and dried under ambient conditions. Unexhausted dye was extracted by treating the samples with an aqueous solution of dimethyl formamide (50:50) at 100 °C for 15 min, then washed with water and dried.
Characterization
Fourier-transformed infrared spectroscopy (FT-IR)
FTIR spectroscopy has been extensively used in cellulose research, since it presents a relatively easy method of obtaining direct information on chemical changes that occur during various chemical treatments. FTIR instrument (JASCO, Model IR 4700 Japan) scanned from 4000 to 400 cm−1 in ATR mode using KBr as supporting material.
Scanning electron micrograph SEM/EDX analysis
Samples of SEM/EDX were taken using FEI INSPECTS Company, Philips, Holland environmental scanning without coating. Elemental micro-probe and elemental distribution mapping techniques were used for analyzing the elemental constitution of solid samples. An elemental analysis of the particles was implemented by a SEM equipped with an energy dispersive spectroscope (EDX), to get rapid quantitative and qualitative analysis of the elemental composition.
UV protection factor
Ultraviolet protection factor (UPF) was measured using ultraviolet JASCO model V-750 UV/VIS Spectrophotometer apparatus. UV Protection and classification according to AS/NZS 4399:1996 were evaluated.
Contact angle
Contact angles of cotton coated fabrics were measured using OCA 15EC Contact angle model produced by company of Data Physics Instrument Gmbh to determine hydrophobicity of the treated fabrics.
Air permeability
Air permeability was tested using air permeability test model (MO21A). Air permeability is an important feature in the performance of textile materials used to provide an indication of the breathability of the coated fabrics. In this test a circle sample of the fabric was fixed into the tester and by using high air pressure through the fabric, then the rate of air flow was used to determine the air permeability of the tested fabric.
Evaluation of color strength
The color strength of the dyed samples was expressed as K/S value which is obtained from the Kubelka–Munk equation (Kubelka 1931).
Tensile strength
The tensile strength of the fabric samples was determined by the ASTM Test Method D-1682-94 (1994). Two specimens for each treated fabric were tested in the warp direction and the average value was recorded to represent the fabric breaking load (Lb).
Results and discussion
Mechanism of nanocomposite deposited on cotton fabric fabricated with PDDA
Cotton fabrics have no charge. Because of their characteristic hydroxyl and carbonyl groups, they acquire slightly negatively charged with a zeta-potential of 211 mV when dry in aqueous medium. As shown in scheme 1, the fixation of positively charged polyelectrolyte, such as PDDA is very appropriate for surface modulation of cotton fabric using self-assembly technique. This may be due to its quaternary ammonium group which makes it act as PH independent polycation. Electrostatic interactions between ZnO/SiO2 nanocomposite with negative charges and the positively charged modified surfaces of fibers permit the deposition of multiple layers on the fibers in a simple and easy manner (Kim et al. 2016).

Dyeing of cotton fabric with reactive dye
Compared to the blank sample, the data in Table 1 signify that an enhancement in K/S values is observed for all dyed fabric samples. This enhancement is due to ionic interaction of PDDA cationic group and the anionic group of the reactive dye (Ho et al. 2011). Moreover, high color uniformity is seen which could be attributed to the increase in the number of cationic groups in cotton surface towards reactive dye ions and molecules. Increase in the number of layers improves the dyability due to presence of multi-cationic site. This result is in agreement with several reports involving self-assembly system (Wang and Hauser 2010). Additionally, the smaller particle size of nanoparticles which gives bigger surface area also affected K/S values positivitly.
It is also observed that the highest dyeing ability is achieved when the ratio (ZnO/SiO2) nanocomposite was 1:1 with ten layers. This indicates the presence of SiO2 nanoparticles enhance dyability up to 10 layers which may be attributed to the distribution of the dye in zinc oxide/silica network. This reduces the tendency for outside forces to extract the dye molecules as the dyes are fixed in the coating (Yin et al. 2012). In other pervious report incorporation of the synthesized SiO2 with other nanoparticles TiO2 in the reactive dyeing process causes negligible impacts on coloration of cotton fabric (Fakin et al. 2012).
UV protection of coated and dyed fabrics
UPF was used to evaluate UV protection of different ratios of (ZnO/SiO2) nanocomposite coated cotton fabrics. UPF of the blank and PDDA modified cotton fabrics are illustrated in Table 2. The calculated UPF of the blank cotton is 4.7, and for PDDA modified cotton fabric is 14.2. In the case of 1BL and 5BL, by increasing the amount of ZnO within the nano composite (ZnO/SiO2) ratio, the UPF increases and the results indicate that better UPF value is for 2:1 (ZnO/SiO2) nanocomposite ratio (AbdElhady 2012). Furthermore, during the coating process, the Si amount within the (ZnO/SiO2) nanocomposite was deposited on the fabric surface as well as in the spaces between fibers. The spaces that are subjected to coat were able to hinder the penetration of the UV radiation among the yarns of the fabric and bring about superior protection.
Dyes often offer a perfect blocking influence of UV light transmittance, so all dyed fabrics provided outstanding protection from UV radiation as shown by UPF values.
Analysis of contact angle
Cotton fabric has a hydrophilic nature, because its structure has abundant hydroxyl groups that make water totally wetted it. Therefore, its surface has a contact angle equal to 0 (Peng et al. 2016). The contact angle measurement was used to check the wettability. Table 3 shows the optical photograph of different LBL treated cotton fabrics. After coating cotton surface with different ratios of (Zn/Si) nanocomposite, the surface becomes hydrophobic with contact angle of about 115 to 136. It could be concluded that, (ZnO/SiO2) nanocomposite coating the spaces between yarns decreases the gap area in the cotton fabric and hinders penetration in coated fabrics.
Additionally, PDDA/nanocomposite coated surface is hydrophobic, since a coated surface becomes rougher than that of a blank fabric. As layer by layer increases, the contact angle increases. This is due to the increase in surface roughness. Therefore, 10 BL PDDA/(ZnO/SiO2) nanocomposite coated surface has a larger contact angle value and better hydrophobicity beside other functionality. It should be noted that earlier reports on the combination of hydrophobic and UV-protective functions on cotton fabrics were based on inorganic UV absorbers (e.g., TiO2, ZnO and CeO2) and hydrophobic treatment with silicon compound. Since these metal oxides could cause degradation of silicon layer and hence lose the hydrophobicity under UVlight (Duan et al. 2011; Wang et al. 2011; Xue et al. 2008).
IR analysis
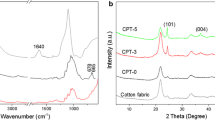
The existence of functional groups on the surface of the treated cotton were identified by Fourier-transform infrared spectra. Figure 1 shows FT-IR spectra of uncoated cotton (blank), 1 BL PDDA/(ZnO/SiO2) nanocomposites treated cotton (1), 5 BL PDDA/(ZnO/SiO2) nanocomposite treated cotton (2), and 10 BL PDDA/(ZnO/SiO2) nanocomposites treated cotton samples (3).
In the case of uncoated cotton fabric (black), a band occurred at 3200–3500 cm−1, which is assigned to O–H stretching. The bands in the range of 1500–800 cm−1 appeared due to the presence of C–H, O–H, C–O, and C–O–C vibrations, representing the cellulose. All the above characteristic peaks of uncoated cotton fabric showed in the spectrum of PDDA/(ZnO/SiO2) nanocomposite. Compared with uncoated cotton (blank), all cationized cotton samples showed shifting at hydrogen bonded O–H stretching peak at 3100–3686 cm−1 and aliphatic C–H stretching at 2917 cm−1, C–N stretching vibration at 1200 cm−1. The characteristic vibrational peaks of nano ZnO at 410 and 580 cm−1 are obviously seen in Fig. 1 (samples 1, 2, and 3) Also multicomponent band in the range of 1000–1200 cm−1 is the same for nano SiO2 IR spectra of (Raevskaya et al. 2014). Finally, it was concluded from Fig. 3 (blank, 1, 2 and 3) that the increase in the number of layers deposited leads to increase band intensities of BL deposited fabrics.
Surface morphology analysis of the coated cotton
SEM was carried out to determine the morphological properties of uncoated and coated cotton fabrics; and SEM images of such fabrics are shown in Fig. 2. A detailed view in Fig. 2a,c,e show that, the fabric is covered by multilayer thick films, which confirmed the successful deposition of PDDA/(ZnO/SiO2) nanocomposite on the cationized cotton fabrics. It can be seen that the surface of cotton fabrics has no significant aggregation of ZnO/SiO2 nanocomposite, and this is due to the charge caused by cationization and nanocomposite which stops aggregation. Figure 2 blank cotton fabric has a fiber with smooth surface. Higher magnification indicated that fiber of cotton-1BL still exhibited quite a smooth surface. However, cotton fibers exhibited rougher surface by increasing the number of layers to 5 BL and 10 BL. Moreover, increasing coatings density, the cotton fibers become attached closely. These results suggested that the treatment of PDDA/(ZnO/SiO2) nanocomposite has been successfully deposited by LBL self-assembly technique on the surface of cotton fabrics.
Figures 2b,d, f shows EDX analysis of coated cotton fabric. Presence of silicon and zinc on cotton fabric is detected by EDX spectrum. There are peaks for oxygen (O) as well as carbon (C) elements detected from the cotton fabrics (Fig. 2). After cotton fabric surface has been assembled with 1, 5 and 10 layers of ZnO/SiO2 nanocomposite, Zinc (Zn) and silicon (Si), elements peak can be detected from the EDX spectra. Based on the results, the presence of the zinc and silicon peak indicates that nanocomposite has been successfully assembled onto the cotton fabric. The weight percentage of Zn and Si elements on cotton fabrics after being assembled with multilayers is observed in Fig. 2f. The higher weight percentage of zinc and silicon is attributed to more layers deposited on the surface of treated fabric with PDDA/(ZnO/SiO2) nanocomposite.
Tensile strength
The influence of the cationization and deposited layer of PDDA/(ZnO/SiO2) nanocomposite on the tensile strength of the treated fabrics was totally examined and its values are given in Table 4. Data received from the untreated, cationized, 1, 5, and 10 multilayer deposited fabrics are presented in Table 4. It is noticed that the tensile strength of cationized fabric is marginally decreased. This could be attributed to the treatment of cotton fabrics with cationic modifying agents of PDDA. Additionally, the fiber strength damaged by sodium hydroxide (Cheng et al. 2018). It can be concluded that the structure of the yarns was not exposed to any notable damage due to multilayer coating of the cotton fabrics through LbL process compared to the untreated cotton fabric. Additionally, increasing the number of layer leads to an improvement in tensile strength of the treated fabric. This improvement appears after one layer deposition of ZnO/SiO2 nanocomposite. This may be due to the fact that nanocomposite layers are beneficial to fixing defects on the fiber surface (Li et al. 2018). In a previous report the enhancement in tensile strength for cotton fabric occured after coating by 16 layers of Nano-TiO2 (Uğur et al. 2011).
Air permeability
Air permeability is considered an important factor that indicates the breathability of the treated textile fabrics. Along the data outlined in Fig. 3, it can be seen that the values of air permeability slightly decreased in the case of fabrics coated with 1 BL as well as 5 BL (with the exception of 2:2 nanocomposite ratio) but highly decreased in the case of fabrics coated with 10 BL nanocomposite. This could be attributed to the closing of open interstices caused by layer by layer coating of the finish (Farouk et al. 2012).
Conclusion
Coating of cotton fabric by multilayer of poly (diallyldimethylammonium chloride) (PDDA) and ZnO/SiO2 colloidal solution nanocomposite is successfully achieved via electrostatic self-assembly (ESA) layer by layer technique. The deposition of ZnO/SiO2 nanocomposite on the fibers was monitored by the number of the deposition layers. Pre-treatment of cotton fabrics by cationization could possibly improve the deposition process. This method renders the fabric UV protection properties and improves dyability as well. Other properties such as water repellency could be acquired by cotton fabric in post treatment with stearic acid.
References
AbdElhady M (2012) Preparation and characterization of chitosan/zinc oxide nanoparticles for imparting antimicrobial and UV protection to cotton fabric. Int J Carbohydr Chem 2012:1–6
Chen D et al (2018) UV-blocking, superhydrophobic and robust cotton fabrics fabricated using polyvinylsilsesquioxane and nano-TiO2. Cellulose 25:3635–3647
Cheng D, He M, Cai G, Wang X, Ran J, Wu J (2018) Durable UV-protective cotton fabric by deposition of multilayer TiO2 nanoparticles films on the surface. J Coat Technol Res 15(3):603–610
Decher G, Eckle M, Schmitt J, Struth B (1998) Layer-by-layer assembled multicomposite films. Curr Opin Colloid Interface Sci 3:32–39
Duan W, Xie A, Shen Y, Wang X, Wang F, Zhang Y, Li J (2011) Fabrication of superhydrophobic cotton fabrics with UV protection based on CeO2 particles. Ind Eng Chem Res 50:4441–4445
Dubas ST, Kumlangdudsana P, Potiyaraj P (2006) Layer-by-layer deposition of antimicrobial silver nanoparticles on textile fibers. Colloids Surf A Physicochem Eng Asp 289:105–109
Fakin D, Veronovski N, Ojstršek A, Božič M (2012) Synthesis of TiO2–SiO2 colloid and its performance in reactive dyeing of cotton fabrics. Carbohydr Polym 88:992–1001
Farouk A, Moussa S, Ulbricht M, Textor T (2012) ZnO nanoparticles-chitosan composite as antibacterial finish for textiles. Int J Carbohydr Chem 2012:1–8
Farouk A, Sharaf S, El-Hady MA (2013) Preparation of multifunctional cationized cotton fabric based on TiO2 nanomaterials. Int J Biol Macromol 61:230–237
Grandcolas M, Sinault L, Mosset F, Louvet A, Keller N, Keller V (2011) Self-decontaminating layer-by-layer functionalized textiles based on WO3-modified titanate nanotubes. Appl Sol Photocatal Remov Chem Warf Agents Appl Catal A Gen 391:455–467
Gulrajani M, Deepti G (2011) Emerging techniques for functional finishing of textiles. Indian J Fibre Text Res 36:388–397
Ho T, Zimmermann T, Hauert R, Caseri W (2011) Preparation and characterization of cationic nanofibrillated cellulose from etherification and high-shear disintegration processes. Cellulose 18:1391–1406
Huang J et al (2015) Robust superhydrophobic TiO2@ fabrics for UV shielding, self-cleaning and oil–water separation. J Mater Chem A 3:2825–2832
Khan F, Liu P, Yang S, Ma Y, Qiu Y (2017) Concentration-dependent dye aggregation in the LbL-assembly of fluorescein isothicyanate labeled poly (allylamine hydrochloride) and poly (acrylic acid) on cotton fabrics. Dye Pigment 142:358–364
Kim S, Nakamatsu J, Maurtua D, Oliveira F (2016) Formation, antimicrobial activity, and controlled release from cotton fibers with deposited functional polymers. J Appl Polym Sci 133:43054
Kubelka P (1931) Ein Beitrag zur Optik der Farbanstriche (Contribution to the optic of paint). Zeitschrift fur technische Physik 12:593–601
Li S, Huang J, Chen Z, Chen G, Lai Y (2017) A review on special wettability textiles: theoretical models, fabrication technologies and multifunctional applications. J Mater Chem A 5:31–55
Li Z, Liu B, Kong H, Yu M, Qin M, Teng C (2018) Layer-by-layer self-assembly strategy for surface modification of aramid fibers to enhance interfacial adhesion to epoxy resin. Polymers 10:820
Peng L, Guo R, Lan J, Jiang S, Wang X (2016) Microwave-assisted coating of silver nanoparticles on bamboo rayon fabrics modified with poly (diallyldimethylammonium chloride). Cellulose 23:2677–2688
Raevskaya A et al (2014) Spectral and luminescent properties of ZnO–SiO 2 core–shell nanoparticles with size-selected ZnO cores. RSC Adv 4:63393–63401
Sadeghi B, Pourahmad A (2011) Synthesis of silver/poly (diallyldimethylammonium chloride) hybride nanocomposite. Adv Powder Technol 22:669–673
Sharaf S, Barakat OAS (2018) Use of nanotechnology to achieve the best functional characteristics of the fabrics sheets used in hospitals. Egypt J Chem 61:705–715
Shi Q, Qian Z, Liu D, Liu H (2017) Surface modification of dental titanium implant by layer-by-layer electrostatic self-assembly. Front Physiol 8:574
Shirvan AR, Nejad NH, Bashari A (2014) Antibacterial finishing of cotton fabric via the chitosan/TPP self-assembled nano layers. Fibers Polym 15:1908–1914
Tian M, Hu X, Qu L, Du M, Zhu S, Sun Y, Han G (2016a) Ultraviolet protection cotton fabric achieved via layer-by-layer self-assembly of graphene oxide and chitosan. Appl Surf Sci 377:141–148
Tian M, Hu X, Qu L, Zhu S, Sun Y, Han G (2016b) Versatile and ductile cotton fabric achieved via layer-by-layer self-assembly by consecutive adsorption of graphene doped PEDOT: PSS and chitosan. Carbon 96:1166–1174
Uğur ŞS, Sarıışık M, Aktaş AH, Uçar MÇ, Erden E (2010) Modifying of cotton fabric surface with nano-ZnO multilayer films by layer-by-layer deposition method. Nanoscale Res Lett 5:1204
Uğur ŞS, Sarııšık M, Aktaş AH (2011) Nano-TiO2 based multilayer film deposition on cotton fabrics for UV-protection. Fibers Polym 12:190–196
Wang Q, Hauser P (2010) Developing a novel UV protection process for cotton based on layer-by-layer self-assembly. Carbohydr Polym 81:491–496
Wang L, Zhang X, Li B, Sun P, Yang J, Xu H, Liu Y (2011) Superhydrophobic and ultraviolet-blocking cotton textiles. ACS Appl Mater Interfaces 3:1277–1281
Xue C-H, Jia S-T, Chen H-Z, Wang M (2008) Superhydrophobic cotton fabrics prepared by sol–gel coating of TiO2 and surface hydrophobization. Sci Technol Adv Mater 9:035001
Yin Y, Wang C, Wang Y (2012) Fabrication and characterization of self-assembled multifunctional coating deposition on a cellulose substrate. Colloids Surf A Physicochem Eng Asp 399:92–99
Zhang D et al (2017) Flame retardant and hydrophobic coatings on cotton fabrics via sol–gel and self-assembly techniques. J Colloid Interface Sci 505:892–899
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abd El-Hady, M.M., Sharaf, S. & Farouk, A. Highly hydrophobic and UV protective properties of cotton fabric using layer by layer self-assembly technique. Cellulose 27, 1099–1110 (2020). https://doi.org/10.1007/s10570-019-02815-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-019-02815-0



 PDDA,
PDDA,
 nano composite (ZnO/SiO2),
nano composite (ZnO/SiO2),
 cotton fabric
cotton fabric


