Abstract
A potentially environmentally responsible dyeing procedure for ultra-deep shades on cotton was developed using a cationization method in combination with mercerization. The effects of both treatments on dyeing performance and colorfastness properties of cotton fabrics dyed with reactive dyes were analyzed individually and in combination. Both mercerization and cationization have been proved to be effective in increasing the depth of shade on cotton. The colorfastness properties, except colorfastness to wet crocking, of mercerized–cationized cotton fabrics dyed without salt were much better than untreated cotton dyed using a conventional dyeing procedure. Unlike untreated cotton fabrics, the concentration of Na2CO3 in the dyeing process of mercerized–cationized cotton fabrics was lowered from 20 to 5 g/L without compromising dye fixation and colorfastness properties. With low concentrations of dyes and Na2CO3 and no electrolyte in the dye bath effluent, the dyeing procedure of mercerized–cationized cotton fabrics for ultra-deep shades is potentially a more environmentally benign method than conventional dyeing with reactive dyes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As the most commonly used cellulosic fiber, cotton has been dominant for centuries due to its unique comfort, durability, good dyeability, ease of production, biodegradability, and relatively low cost. To satisfy consumers aesthetically, cotton products must have a large color gamut and satisfactory fastness properties. Black is one of the most commonly used colors for garments and other textile products.
In fact, obtaining ultra-deep shades, especially black, on cotton with good colorfastness in an environmentally responsible way is difficult. Sulfur blacks, such as C.I. Sulfur Black 1, are the most commonly used black dyes for cotton due to their low cost and high washfastness. However, the large amounts of sodium sulfide used in manufacture and application of sulfur dyes can cause serious environmental problems (Broadbent 2001). Other classes of black dyes used for cotton also have their own limitations, such as the poor washfastness of direct dyes and large amount of dye and salt in the dye bath effluent of reactive dyes when applied in conventional dyeing processes. Synthesis of new dyes and modification of cotton dyeing process, while valid for obtaining qualified black cotton dyeings, are likely to involve significant capital investment and development costs (Hauser and Tabba 2001).
Much attention has focused on modification of cotton fiber as another route to obtaining the desired dyeing performance and fastness properties with existing dyes. Cationization of cotton is one of the most widely researched modifications in recent years since both direct and reactive dyes carry anionic charges and they exhibit high affinity for positively-charged cotton. For dyeing with reactive dyes, it can not only increase the dye uptake (Cai et al. 1999; Mouxiou et al. 2008), but also eliminate the use of salt due to the ionic attraction between the dye and cationized cotton (Ma et al. 2005; Montazer et al. 2007; Teng et al. 2011). Thus, cationization can be used to reduce problems associated with dye bath effluent of reactive dyes. Numerous chemicals, including both monomeric and polymeric reagents, have been used to introduce cationic groups into cotton fiber (Lewis and Lei 1989; Khalil-Abad et al. 2009).
Other modifications, such as mercerization and plasma treatments (Patiño et al. 2011) have been used in combination with cationization to further improve the dyeability of cotton. Mercerization permanently changes the fiber crystallinity and increases luster, strength, moisture absorption, dyeability and reactivity. The changes in internal light scattering by mercerization can increase the perceived depth of shade on cotton even with the original dye absorption (Karmakar 1999). Thus, mercerization is effective for obtaining deeper shades on cotton than otherwise would be possible.
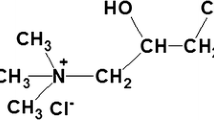
In this study, cationization was used in combination with mercerization to develop a potentially environmentally responsible dyeing procedure for ultra-deep shades on cotton using fiber-reactive dyes that exhibit high exhaustion and fastness properties. The cationic reagent used was 3-chloro-2-hydroxypropyl trimethylammonium chloride (CHPTAC), which is commercially available and has been well studied for its cationization mechanism, application methods, effects in subsequent dyeing and some other properties (Lewis and Mcllroy 1997; Kanik and Hauser 2002; Hashem et al. 2003; Hashem et al. 2005). CHPTAC itself does not react with cellulose. It is first converted into 2,3-epoxypropyl trimethylammonium chloride (EPTAC) by reacting with alkali. Then EPTAC reacts with the hydroxyl groups on cotton fiber under alkaline conditions to form cationized cotton (Rupin et al. 1970; Rupin 1976; Hauser and Tabba 2001). Since heat may cause the migration of reactants and results in nonuniform cationization, cold pad-batch method is possibly the most efficient method to provide uniform cationization of cotton compared with higher temperature application methods such as pad-steam (Tabba 2000).
Experimental
Materials
Bleached desized cotton print (style # 400, 102 g/m2, 45″) and bleached desized mercerized cotton print cloth (style # 400 M, 107 g/m2, 44″) were obtained from Testfabrics, Inc., USA. Two commercial reactive dyes, Remazol Black B 133 (C. I. Reactive Black 5) and Remazol Yellow RR were supplied by DyStar, Germany. The cationic reagent used was CR2000, 65 % solution of 3-chloro-2-hydroxy propyltrimethylammonium chloride (CHPTAC), obtained from Dow Chemical Co., USA. All other chemicals, including sodium hydroxide (50 % solution), citric acid, sodium carbonate, and sodium sulfate were supplied by Fisher Scientific, USA.
Application of cationic reagent
A solution consisting of certain concentration of 65 % CHPTAC solution and 50 % sodium hydroxide was pad applied on both unmercerized and mercerized cotton print cloth at 100 % wet pick up (speed: 1.5 m/min, pressure: 1 bar). The NaOH and CHPTAC solutions were mixed just prior to application to minimize hydrolysis of the cationic reagent. The padded samples were rolled onto hard paper tubes and wrapped in plastic to minimize air contact. The fabrics were batched at room temperature for 20 h. After removing the plastic, the samples were rinsed with copious amounts of water to remove unfixed and hydrolyzed cationic reagent and then neutralized with citric acid (~0.5 g/L). Finally, the treated fabrics were extracted and dried in a tumble dryer.
Nitrogen content analysis
The percentage of nitrogen present in the cotton fabric was measured in duplicate and used as an indicator of the amount of CHPTAC reacted with cellulose. The analysis was conducted using a PE 400 CHE Elemental Analyzer using the classical Pregal and Dumas methods. After been cut into 2 mm squares, the cotton samples were combusted in a pure oxygen environment before elemental analysis (Yeomans and Bremner 1991).
Dyeing
Dyeing of both uncationized and cationized cotton samples were carried out using an Ahiba Texomat laboratory dyeing machine at the liquor ratio of 20:1. A conventional dyeing procedure (Fig. 1, 80 g/L Na2SO4, 20 g/L Na2CO3) was used for uncationized cotton fabrics and the no salt dyeing procedure (Fig. 2, 20 g/L Na2CO3) was used for the cationized cotton samples. After dyeing, both uncationized and cationized cotton fabrics were rinsed thoroughly, then neutralized with citric acid and rinsed again.
Color measurement
After dyeing, reflectance spectra of each sample were measured using a Datacolor Spectraflash 600X spectrophotometer equipped with iMatch software from X-Rite. L*, a*, b*, C*, h° and K/S values of the dyed samples were calculated using the following instrument settings (illuminant D65, 10° supplemental standard observer, specular included, UV included). Since the objective of this research is to obtain ultra-deep shades, the effective K/S value was calculated by summation of the K/S values at 10 nm intervals from the wavelength of 360–750 nm. While testing, the sample was folded two times. Each sample was measured ten times by changing the measuring point randomly to calculate the average value.
Evaluation of dye uptake
At the end of dyeing, exhausted dye solutions were measured using an Agilent 8453 UV–VIS spectrophotometer. First, a calibration plot of concentration vs. absorbance was produced at the wavelength of max absorption by measuring the absorbance of dye solutions with certain concentrations. Then the residual dye solution was diluted and measured at λmax and the concentration of the solution was calculated based on the calibration plot. The percentage of dye uptake was calculated from 100 × (1 − A0/A) where A0 and A are the absorbance of the dye solution before and after dyeing, respectively.
Colorfastness tests
Colorfastness to laundering was measured using AATCC Test Method 61 (AATCC 2012) using an Atlas LEF Launder-Ometer. A multi-fiber test fabric was attached to each sample to evaluate the staining and the test conditions were set according to the Test No. 2A. Colorfastness to crocking was measured according to AATCC Test Method 8 (AATCC 2012). Both dry and wet crocking test were measured using the AATCC automated crockmeter. Colorfastness to light of the dyed samples was measured using test option 3 in AATCC Test Method 16 (AATCC 2012). The color change after 20 and 40 continuous light-on operating hours of each dyed sample were measured separately using an Atlas Ci 3000+ Weather-Ometer. For all colorfastness tests, the evaluation of shade change and staining were made according to AATCC Evaluation Procedure 1 (AATCC 2012) and AATCC Evaluation Procedure 2 (AATCC 2012) using grey scale ratings from 1 to 5 where 5 implies no shade change.
Evaluation of mechanical properties
The mechanical properties of yarns, including breaking load and elongation, were measured using the ASTM Standard Test Method D2256-10 (ASTM 2010). The gauge length was set as 250 mm and the rate of operation was 300 mm/min. Yarns were extracted from different positions of the cotton fabrics randomly.
Results and discussion
The effect of cationization and mercerization
The cotton fabrics were cationized with different concentration of cationization solution while the molar ratio between NaOH and CHPTAC was kept constant based on previous work (Farrell 2011). The concentrations of the cationization solutions used are summarized in Table 1.
Nitrogen content analysis
Figure 3 shows the nitrogen content of both unmercerized and mercerized cotton fabrics cationized with varying concentrations of cationic solutions with the uncationized fabrics as controls. The nitrogen content of the fabrics increased in proportion to the amount of CHPTAC applied. For mercerized cotton fabrics, the increase in nitrogen content was slightly higher than corresponding unmercerized cotton fabrics cationized with the same method. This may be because, after mercerization, the reactivity of cotton was improved due to increased accessibility of primary and secondary hydroxyl groups.
The effect of mercerization and cationization on dyeing performance
To evaluate the dyeing properties of the cationized samples, the samples were dyed with 6 % on weight of fabric (owf) Remazol Black B (C.I. Reactive Black 5). After dyeing, colorimetric values (L*, a*, b*, C* and h°) of all samples were calculated (Table 2). In addition, K/S values were calculated for each sample (Fig. 4). The results of L* and K/S values show that both mercerization and cationization increased the depth of shade of dyed samples relative to the control. Also, the depth of shade increased as a function of CHPTAC concentration which is consistent with the nitrogen content analysis. However, the difference in L* and K/S between MC150 and MC200 was negligible. An indication of the degree of levelness for each sample was obtained by measuring lightness at 10 randomly selected places on the fabric. As shown in Table 2, the standard deviation of lightness (σL*) indicates that the treatments did not influence the levelness of dyeing.
To better evaluate the effect of mercerization and cationization in dyeing performance of cotton fabrics, the dye uptake was measured. As shown in Fig. 5, the dye uptake increased with an increase in the concentration of CHPTAC applied. The dye uptake of all cationized samples is close to or higher than 95 %, indicating that substantially less dye would be released in the effluent compared to conventional dyeing. The effect of cationization in increasing the depth of dyed sample is mostly achieved by attracting and fixing more dye molecules. Fixation may be via covalent bond formation (when alkali is added to the dye bath) or ionic bonding between negatively charged dye and the positively charged fiber. However, different from cationization, mercerization not only increases dye absorption but also changes the shape of the fibers, thus decreasing the light scattering on the surface and inside the fiber. Visual assessment of UC200 and MC200 sample shows that even with similar amount of dyes, the perceived depth of shade of mercerized samples is deeper. Since the effect of increasing color depth by mercerization does not only result from increasing the dye uptake, maximum color depth requires both mercerization and cationization to obtain ultra-deep dyeings even though the dye uptake values of unmercerized–cationized samples are also very high.
The effect of mercerization and cationization on colorfastness properties
The dyed fabrics were tested for colorfastness to laundering, crocking and light and the results are summarized in Table 3. It can be seen that all the samples exhibited good wash fastness and staining properties while both mercerization and cationization produced excellent colorfastness to laundering. All samples have excellent colorfastness to dry crocking. However, the colorfastness to wet crocking of mercerized–cationized samples was not as good as other samples. This may because that the depth of dyeing of mercerized–cationized samples was higher than the untreated ones, which resulted in relatively more dye transferred from the testing fabric during rubbing. By comparing the color change of unmercerized samples with mercerized samples, it is clear that mercerization improves colorfastness to light. The relatively poor colorfastness to light of unmercerized–cationized samples indicated that cationization slightly decreases photo stability of dyed samples. However, this effect appears to be mitigated by the mercerization process since the colorfastness to light of mercerized–cationized samples was close to mercerized–uncationized samples.
The effect of mercerization and cationization on mechanical properties
Figure 6 shows that cationization did not influence the peak load of the yarns significantly, but mercerization increased peak load, especially for warp yarns. The elongation at peak load, on the other hand, was found to be similar for both untreated and untreated samples.
Color modification to produce ultra-deep black shades
Binary dyeing recipes
Previous results indicated that even though mercerization and cationization increased the shade depth and decreased the chroma of the dyed sample (Table 2), all mercerized–cationized samples dyed with 6 % owf Remazol Black B were deep blue shades rather than black. To reduce the blueness of the dyed samples, MC100 cotton fabrics were dyed with 6 % Remazol Black B and varying concentrations of Remazol Yellow RR. The a*, b* and L* of dyed samples are shown in Fig. 7 (the coordinate axis on the left is for a* b* and the axis on the right is for L*). The a*, b* values as well as visual assessment indicated that the samples dyed with 2 and 2.5 % Yellow RR were in ultra-deep black.
Dyeing performance of MC samples dyed with two-dye combination
Based on previous results, MC150 and MC200 samples were also dyed after adding 2 and 2.5 % owf Remazol Yellow RR into the previous dyeing recipe. Since the total concentration of dyes may be beyond the saturation point, the mercerized–cationized samples were also dyed with 5 % Remazol Black B plus 2 % Remazol Yellow RR. Table 4 summarizes the L*, C* and K/S of all dyed mercerized–cationized samples. The results confirmed that all dyed samples were in deep neutral black shade range, especially MC150 and MC200 samples dyed with 6 % Remazol Black B plus 2 or 2.5 % Yellow RR. Increasing the concentration of Remazol Black B from 5 to 6 % increased the K/S of all three mercerized–cationized samples. However, further increase in the concentration of Remazol Yellow RR from 2 to 2.5 % only increased the K/S of MC200 sample. This is likely because that MC200 samples have higher levels of cationization and could therefore absorb more dye compared with other samples.
To examine whether the concentrations of dyes applied were beyond a practical saturation point, dye exhaustion was measured (Fig. 8). The trend of dye uptake values agrees with previous results that an increase in the concentration of CHPTAC increases the dye uptake. However, increasing the concentration of Remazol Black B from 5 to 6 % did not influence the dye uptake of mercerized–cationized samples much. Further increasing the concentration of Remazol Yellow RR from 2 to 2.5 % decreased the % exhaustion of all samples. This may be because the dye concentration (6 % Remazol Black plus 2.5 % Remazol Yellow RR) is beyond the saturation point of the fiber. Thus, to maintain relatively high dye uptake, MC150 and MC200 samples should be selected and the concentration of dyes used should not be higher than 6 % Remazol Black B plus 2 % Remazol Yellow RR for this particular application process.
Colorfastness of MC dyed with two-dye combination
The colorfastness to laundering, crocking and light of all dyed mercerized–cationized samples were measured for the two-dye combination (Table 5). By comparing with previous results, colorfastness properties of the samples dyed with two-dye combination were as good as the mercerized–cationized samples dyed with 6 % Remazol Black B only.
The effect of Na2CO3 concentration
The effect of Na2CO3 concentration on dyeing performance
In previous experiments, the dyeing recipe of mercerized–cationized samples included 20 g/L Na2CO3 which is commonly considered the optimum concentration for these fiber-reactive dyeings on untreated cotton. However, the amount of Na2CO3 required for fixation on mercerized–cationized cotton fabrics may be acceptable at lower concentrations. Hence, MC150 and MC200 samples were dyed with 6 % owf Remazol Black B and different concentration of Na2CO3 from 0 to 20 g/L with untreated cotton fabric as a control. With the increase of Na2CO3 concentration, both K/S (Fig. 9) and dye uptake (Fig. 10) of unmercerized–uncationized sample increased gradually from 5 to 20 g/L. The results also reveal that, for mercerized–cationized cotton fabrics, eliminating the use of Na2CO3 decreased both the K/S and dye uptake, but increasing the concentration from 5 to 20 g/L did not influenced the K/S and dye uptake values significantly. Hence, 5 g/L Na2CO3 appears to be an effective concentration for dyeing mercerized–cationized cotton fabrics while considering the depth of shade and dye uptake, i.e., substantially lower than conventional dyeings on untreated cotton.
The effect of Na2CO3 concentration on colorfastness properties
Colorfastness results for both untreated and mercerized–cationized samples are given in Table 6. Both colorfastness to laundering and light indicate that, for unmercerized–uncationized cotton fabric, at least 10 g/L Na2CO3 ensures adequate fastness properties. However, the results of mercerized–cationized samples show that adding 5 g/L Na2CO3 produced relatively good colorfastness to laundering and light and further increase in the concentration produced identical results. While both the colorfastness to dry and wet crocking were the highest for the untreated sample dyed without Na2CO3, this was due to the very low shade depth. For other samples, the colorfastness to both dry and wet crocking all performed similarly.
Therefore, when dyeing untreated cotton fabrics, adding 20 g/L Na2CO3 is necessary for obtaining deep shades with relatively good colorfastness. However, the depth of shade and colorfastness properties of mercerized–cationized cotton fabrics dyed with 5 g/L Na2CO3 were as good as those dyed with higher concentrations. The same results were achieved by dyeing MC150 samples with two dye combination (6 % Remazol Black B + 2 % Remazol Yellow RR).
Reductions in chemical release by mercerization–cationization
Previous results show that compared with untreated cotton, mercerized–cationized cotton can be dyed with reduced amounts of Na2CO3 and no other electrolyte, without negatively impacting the dyeing performance and fastness properties. Also, mercerization–cationization increased the percentage of dye exhausted on the fiber relative to untreated cotton and thereby reduced the amount of dye in the effluent. As an example of the effect of mercerization–cationization on reducing the dye and other chemicals released in the dye bath effluent, consider 1,000 kg UU and MC150 cotton fabrics dyed with 6 % Remazol Black B, respectively. From the data shown in Fig. 10, the theoretical amount of dye, Na2SO4, and Na2CO3 released in the effluent was calculated and shown in Table 7. Clearly, the mercerization–cationization pretreatment enables substantial reductions in chemical release at the dyeing stage.
Conclusions
In this paper, ultra-deep black shades on cotton dyeings were produced using a mercerization–cationization method before dyeing with fiber-reactive dyes. Both mercerization and cationization were effective in increasing the depth of shade on cotton fabrics dyed with Remazol Black B and Remazol Yellow RR. With the increase in concentration of cationizing agent (CHPTAC), dyeing performance of cationized cotton fabrics was improved, with higher than 95 % dye uptake being achieved. Another advantage of cationization is that the extensive use of salt for exhaustion of reactive dyes was avoided. With respect to the low concentration of dyes and zero Na2SO4 in the dye bath effluent, dyeing of mercerized–cationized cotton fabrics for deep black shades was shown to be more environmentally benign than conventional dyeing procedures using fiber-reactive dyes. It has also been proved that compared with untreated cotton dyed with a conventional dyeing recipe, mercerized–cationized cotton dyed without salt have better colorfastness properties, except colorfastness to wet crocking. Physical testing of peak load and elongation of yarns showed that neither mercerization nor cationization had a negative effect on the mechanical properties of the pretreated cotton fabrics.
To obtain deep shades with relatively good colorfastness, adding 20 g/L Na2CO3 is necessary for the conventional dyeing of untreated cotton fabrics. For mercerized–cationized cotton fabrics, the optimum concentration of Na2CO3 was 5 g/L. Lowering the concentration of Na2CO3 from 20 g/L to 5 g/L did not have negative influence in dyeing performance or colorfastness properties on mercerized–cationized cotton fabrics.
The data presented demonstrated the potential for obtaining high performance ultra-deep shades on cotton while at the same time reducing environmental impact of the dyeing phase by reducing the amounts of unfixed dye and electrolyte in dye bath effluent. Further work is required to elucidate the full scope and limitations of fiber-reactive dyeing of cotton pretreated by mercerization–cationization including assessment of the environmental impact of the pretreatment processes as part of a life cycle assessment.
References
AATCC (2012) Technical manual of the American Association of Textile Chemists and Colorists. American Association of Textile Chemists and Colorists, Research Triangle Park
ASTM (2010) Standard test method for tensile properties of yarns by the single-strand method. ASTM International, West Conshohocken
Broadbent AD (2001) Basic principles of textile coloration. Society of Dyers and Colourists, Bradford
Cai Y, Pailthorpe MT, David SK (1999) A new method for improving the dyeability of cotton with reactive dyes. Text Res J 69:400–446. doi:10.1177/004051759906900608
Farrell MJ (2011) Sustainable cotton dyeing. Dissertation, North Carolina State University
Hashem M, Hauser PJ, Smith B (2003) Reaction efficiency for cellulose cationization using 3-chloro-2- hydroxypropyl trimethyl ammonium chloride. Text Res J 73:1017–1023. doi:10.1177/004051750307301113
Hashem M, Refaie R, Hebeish A (2005) Crosslinking of partially carboxymethylated cotton fabric via cationization. J Clean Prod 13:947–954. doi:10.1016/j.jclepro.2004.05.002
Hauser PJ, Tabba AH (2001) Improving the environmental and economic aspects of cotton dyeing using a cationised cotton. Color Technol 117:282–288. doi:10.1111/j.1478-4408.2001.tb00076.x
Kanik M, Hauser PJ (2002) Printing of cationised cotton with reactive dyes. Color Technol 118:300–306. doi:10.1111/j.1478-4408.2002.tb00114.x
Karmakar SR (1999) Chemical technology in the pre-treatment processes of textiles. Elsevier, New York
Khalil-Abad MS, Yazdanshenas ME, Nateghi MR (2009) Effect of cationization on adsorption of silver nanoparticles on cotton surfaces and its antibacterial activity. Cellulose 16:1147–1157. doi:10.1007/s10570-009-9351-8
Lewis DM, Lei X (1989) Improved cellulose dyeability by chemical modification of the fiber. JSCD 21:23–39
Lewis DM, Mcllroy KA (1997) Modification of cotton with nicotinoyl thioglycollate to improve its dyeability. Dyes Pigments 35:69–86. doi:10.1016/S0143-7208(96)00087-3
Ma W, Zhang S, Tang B, Yang J (2005) Pretreatment of cotton with poly(vinylamine chloride) for salt-free dyeing with reactive dyes. Color Technol 121:193–197. doi:10.1111/j.1478-4408.2005.tb00272.x
Montazer M, Malek RMA, Rahimi A (2007) Salt free reactive dyeing of cationized cotton. Fiber Polym 8:608–612. doi:10.1007/BF02875997
Mouxiou E, Eleftheriadis I, Nikolaidis N, Tsatsaroni E (2008) Reactive dyeing of cellulosic fibers: use of cationic surfactants and their interaction with reactive dyes. J Appl Polym Sci 108:1209–1215. doi:10.1002/app.27705
Patiño A, Canal C, Rodríguez C, Caballero G, Navarro A, Canal JM (2011) Surface and bulk cotton fibre modifications: plasma and cationization. Influence on dyeing with reactive dye. Cellulose 18:1073–1083. doi:10.1007/s10570-011-9554-7
Rupin M (1976) Dyeing with direct and fibre reactive dyes. Text Chem Colorist 8:54–58
Rupin M, Veaute G, Balland J (1970) Utilisation de composes reactifs epoxy ammonium quatinairs dans la cellulose encolorante direct et reactifs. Textilveredlung 5:829–838
Tabba AH (2000) Cationization of cotton with 2, 3-epoxypropyltrimethylammonium chloride. North Carolina State University, Thesis
Teng X, Zhang S, Ma W (2011) Application of a hydrolyzable cationic agent, poly(acryloxyethyl trimethylammonium chloride), in salt-free reactive dyeing for good dyeing properties. J Appl Polym Sci 122:2741–2748. doi:10.1002/app.34023
Yeomans JC, Bremner JM (1991) Carbon and nitrogen analysis of soils by automated combustion techniques. Commun Soil Sci Plant Anal 22(9–10):843–850. doi:10.1080/00103629109368458
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fu, S., Hinks, D., Hauser, P. et al. High efficiency ultra-deep dyeing of cotton via mercerization and cationization. Cellulose 20, 3101–3110 (2013). https://doi.org/10.1007/s10570-013-0081-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-013-0081-6