Abstract
The paper presented a new, non-pollution macromolecule of adsorbing acid dyes, and developed the adsorption mechanism on the basis of effect between the modified groups of hyperbranched hemicellulose polymer and acid dyes. The modified hyperbranched hemicellulose polymer (HHP) with a large number of terminal carboxyl groups and hydroxyl groups was synthesized by hemicellulose maleate and dihydroxymethyl propionic acid. The degree of substitution, kinematic viscosity and thermal stability of HHP was determined. In SEM images, the HHP exhibited the pores and cross-linked structure which were good help for dye adsorption. When the HHP was applied in adsorbing Methyl red dye, Bromocresol green dye and Bromophenol blue dye, it exhibited higher adsorption capacity. The adsorption capacity changed with different adsorbent dosage, pH, temperature and ionic strength, and the optimal adsorption capacity for Methyl red dye, Bromocresol green dye and Bromophenol blue dye could reach 825 mg/g, 675 mg/g and 912 mg/g respectively. The adsorption mechanism, adsorption isotherm and adsorption diffusion mode were demonstrated by Pseudo-order model, Langmuir model, Intraparticle diffusion model and Memberance diffusion model respectively. The adsorption data possessed better correlation with Pseudo-second-order model, Langmuir model and Intraparticle diffusion model. The adsorption isotherms and thermodynamic date indicated the processes of HPP2 adsorbing three acid dyes were endothermic. The N2-adsorption/desorption isotherms of HPP2 revealed the hyperbranched hemicellulose polymer filled the pore in clusters, and its adsorption process met with monolayer adsorption.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
During the past decade various adsorption techniques employed for removing organic dyes from water, such as flocculation sedimentation method, chemical oxidation method, electrochemical method (Peng et al. 2017), membrane separation method (Zhao and Wang 2017) and adsorption method (Fan et al. 2012; Zhou et al. 2013). Among them, the use of adsorbent was an effective and convenient method, which had strong selective adsorption capacity, good mechanical strength and easy to regenerate (Zheng et al. 2015; Voit and Lederer 2009). Those adsorbents such as activated carbon, silica gel, zeolite molecular sieve, active oxidizing recruit and biomass adsorbent had been used commonly (Chen et al. 2012). Different kinds of adsorbents acted in different dye systems exhibited different application effects. Recently, the natural macromolecule with a large number of free hydroxyl, amine or other active groups had been studied for adsorbing organic dyes. This polymer could be modified to improve its chemical inactivation, poor solubility and pH sensitivity which exhibited efficient adsorbing ability (Zhu et al. 2016). For example, Hu studied the chitosan was modified by 3-chloro-2-hydroxypropyl trimethylammonium chloride. The hygroscopicity and decolorization of modifified chitosan could be improved obviously, and the decolorization rate of printing wastewater reached more than 98 % in 45 min (Hu 2008). In 2011, researchers studied the chitosan was modified by ethylenediamine to perpare the cross-linked magnetic chitosan resin. When it was applied to the adsorption of chromium, the maximum adsorption capacities at 293 K, 303 K and 313 K were 51.813 mg/g, 48.780 mg/g and 45.872 mg/g respectively (Hu et al. 2011).
Some scientists have noticed hemicellulose, a renewable natural macromolecule, which was abundant in plants accounting for about one-third of plant resources. Hemicellulose contained a lot of hydroxyl groups which could be modified by the method of hyperbranching and introducing the different active groups to gain some appropriate characteristics such as hydrophobicity, thermoplasticity, water solubility, surface activity and biological functionality (Esposito and Antonietti 2015; Lu et al. 2015). Ferrari studied the hemicellulose modified by polyacrylic acid to gain the hydrogels. The hydrogels had strong hydrophilicity and swelling ability, and showed remarkable adsorption ability for Cu2+, Cd2+, Pb2+, Zn2+, Ni2+ and Co2+ (Ferrari et al. 2015). Rahman had modified the hemicellulose with methyl methacrylate. The modified hemicellulose adsorbent had super adsorption effect on Cu2+, and the adsorption capacity reached to 305.3 mg/g (Rahman et al. 2017). Due to the favorable biocompatibility (Tirtom et al. 2012; Tripathi et al. 2016; Wang et al. 2013), the modified hemicellulose had drawn extraordinary attention in field of adsorbing organic dyes (Guijarro-Aldaco et al. 2011).
Hyperbranched polymer was a kind of macromolecule with high branching structure and a large number of active end groups, which could be synthesized and modified easy by one step reaction (Sun et al. 2017; Li et al. 2012; Ragauskas 2006). The hyperbranched polymer could take on some advantages such as simple design, easy operation, low cost and wide suitability. Some researchers had reported the hyperbranched polymers in adsorbing dyes (Luo et al. 2010; Kanekiyo et al. 2008). For example, Liu and his colleagues studied the hyperbranched polymeric ionic liquid with imidazolium backbones, and it was used to remove the anionic dyes from water (Schutyser et al. 2018). Delidovich and his group reported that the hyperbranched polymer could express the excellent selectivity, and they prepared the multi-responsive microgel to adsorb the hydrophilic dyes (Ning et al. 2016).
Based on the above characteristics of hemicellulose and hyperbranched polymer, our efforts focused on integrating the advantages of two polymers to be suitable as a new, non-pollution acid dyes absorbent. In our study, the modified hyperbranched hemicellulose polymer HPP1 and HPP2 with terminal carboxyl groups and hydroxyl groups were synthesized by hemicellulose maleate and dihydroxymethyl propionic acid step by step. The acid dyes such as Methyl red dye, Bromocresol green dye and Bromophenol blue dye with anionic groups were chose to study the adsorption process of the modified hyperbranched hemicellulose polymer (Scheme 1). The adsorption between the modified hyperbranched hemicellulose polymer and acid dye depended mainly on dipole bonding and H bonding forces. The carboxyl group of the modified hyperbranched hemicellulose polymer and the sulfonate or azo group of acid dye formed the dipole bonding, while the hydroxyl of the modified hyperbranched hemicellulose polymer and hydroxyl or benzene of acid dye formed the H bonding (Scheme 2). The adsorption capacity of the modified hyperbranched hemicellulose polymer was discussed by the dosage, pH, temperature and ionic strength. Moreover, the adsorption mechanism, adsorption isotherm and adsorption diffusion mode were evaluated by Pseudo-order model, Langmuir model, Intraparticle diffusion model and Memberance diffusion model.
Hyperbranched hemicellulose polymer possess a large number of active end groups, and it can be easily prepared. The different end groups can take the adsorption targeted and the absorbent dose reduced. Also, the hyperbranched hemicellulose polymer can be easily degraded. These advantages indicate that hyperbranched hemicellulose polymer can be applied better as an ideal dye absorbent.
Experimental
Material
Hemicellulose maleate (HCMA, Molecular weight 24,372, DS 0.97) was synthesized from hemicellulose and Maleic anhydride. Hemicellulose (Xylose 80.63%, mannose 11.16%, glucose 6.26%, galactose l.22%, glucuronic acid < 0.72%, Molecular weight 11,665–14,268) was obtained from Sanyou co. (Tangshan, China). Maleic anhydride (AR), N,N-Dimethylformamide (DMF, AR), sodium hydroxide (NaOH, AR), P-toluenesulfonic acid (P-TSA, AR) and Sulfuric acid (H2SO4, AR) were purchased from Xiandai reagent Co. (Shijiazhang, China). 2,2-Bis(hydroxymethyl)propionic acid (DMPA, AR) was purchased from Aladdin Co. (Shanghai, China). Methyl red dye (AR), Bromocresol green dye (AR) and Bromophenol blue dye (AR) were purchased from yongfa Co. (Tianjin, China).
Synthesis of the modified hyperbranched hemicellulose polymer
Hyperbranched hemicellulose polymer HHP1 was synthesized from HCMA and DMPA (Scheme 3). Typically, HCMA (2.4 g), DMPA (3.88 g), P-TSA (0.25 g) and DMF (35 mL) were mixed in a flask. The mixture was stirred at 120 °C under nitrogen atmosphere. The reaction was ended at a constant acid value. After reaction, the resulted HHP1 was thoroughly washed with 95% ethanol, and then dried in a vacuum oven at 40 °C for 24 h.
The modified hyperbranched hemicellulose polymer HHP2 was synthesized from HHP1 (3.3 g), DMPA (7.88 g) and P-TSA (0.45 g) on the same synthetic route as HPP1.
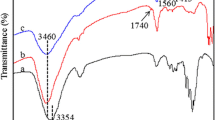
IR (KBr): 3450 cm−1 (–OH), 2920 cm−1 (–CH3), 1750 cm−1 (–COO–), 1720 cm−1 (–COOH), 1690 cm−1 (–C = C–).
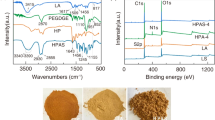
13C-NMR (400 MHz, D2O, δ): 178 ppm (CH3–CHOH–COOH), 172 ppm (CH3–CHOH–COO), 166 ppm (O = C–CH = CH–COOH), 135.6 ppm (O = C–CH = CH–COO), 112.3 ppm (CH3–CHOH–O), 102.4 ppm (O–CH2–O), 85 ppm (–CH2–O–CH2–), 76 ppm (CH3–CH2–CH2OH), 73.6 ppm (CH3–CHOH–CH2OH), 73.1 ppm (OHCH2–CHOH–CH3), 63.2 ppm (–CH2–CH2–O–CH2–), 58 ppm (–CH–CH3–O–), 23.3 ppm (–CH–COOH) and 22.3 ppm (CH3–CHOH–COOH). The spectral peaks were signed as r, o, k, m, p, a, f, d, c, b, e, g, h and q separately in the molecular formula (Fig. 3a–c).
Measurements
In followed measurements, the data from all the experiments were built on measuring three times to get the accurate value.
The degree of substitution (DS) was measured by pH Meter to characterize the modified degree of sample. The HHP (0.1 g) was added in water (40 mL), and the pH was adjusted to 8.2 by adding NaOH (0.1 mol/L). Then adding H2SO4 (0.1 mol/L) to titrate it until the pH reached to 3.7. The DS was calculated from the following Equations (Moran et al. 2012):
where the M represented molality of H2SO4 (0.1 mol/L), V represented the volume of H2SO4 consumed, m represented the weight of sample and B represented the content of Sodium acetate per gram of sample.
The kinematic viscosity was measured by kinematic viscosity tester SC-265B. The time t and t0 of the sample (0.01 g/mL) and blank sample flowing through the capillary viscometer at room temperature were recorded respectively. The data of the kinematic viscosity were calculated from the following Equations (Bodnar et al. 2007):
where Γ was the kinematic viscosity, the time t (s) and t0(s) represented the time of sample (0.01 g/mL) and blank sample flowing through the capillary viscometer respectively.
Fourier transform infrared (FT-IR) spectra were taken on a Thermo Nicolet Nexus 670 spectrometer by scanning 20 times with a resolution of 4 cm−1 within a spectral range of 450–4000 cm−1.
13C-NMR spectra were obtained by a Bruker-400 MHz spectrometer using D2O as solvent, and Me 4Si was used as internal standard at room temperature.
The morphology of the samples were studied with SEM S-4800-1. The samples were adhered to conductive adhesive and sprayed with gold before fractographic examination.
The thermal decomposition profiles of the samples were studied with TG/DTA6200 at a constant rate of 10 °C/min in nitrogen atmosphere from 30 to 600 °C.
The N2-adsorption/desorption isotherms were obtained by Micromeritics TriStar volumetric adsorption analyzer 3020 at − 196 C. The amount adsorbed was measured with the change of relative pressure P/P0, where the P was the true pressure of N2 and P0 was the saturated vapor pressure at the measured temperature.
The adsorption capacity was measured by Ultraviolet spectro photo meter TU-1810. First, the standard curves of Methyl red dye, Bromocresol green dye and Bromophenol blue dye were obtained by measuring the absorbance of standard solution at different initial concentration. Second, adding the sample (0.01 g) to Methyl red dye (100 ppm), Bromocresol green dye (100 ppm) and Bromophenol blue dye (100 ppm) respectively, the mixture was stirred for 60 min at 30 °C, 40 °C, 50 °C, 60 °C and 70 °C respectively. The adsorption capacity was determined by the residual concentration of dye derived from absorbance and standard curve.
The adsorption kinetics was ascertained by Pseudo-first-order model and Pseudo-second-order model. The data on Pseudo-first-order model were calculated from the following Equation (Zhao and Wang 2017):
The data on Pseudo-second-order model were calculated from the following Equation (Zhao and Wang 2017):
where qe (mg/g) was the equilibrium adsorption amount, qt (mg/g) was the adsorption amount at a time, k1 (min−1) and k2 (g/mg.min) represented the coefficients of models, and t (min) represented the time of adsorption.
The adsorption isotherm curve was ascertained by Langmuir model. The data on Langmuir model were calculated from the following Equation (Zhao and Wang 2017):
where Ce (mg/L) was the residual concentration of dye at equilibrium, qmax (mg/g) was the largest adsorption amount, qe (mg/g) was the equilibrium adsorption amount, and b (L/mg) represented the constant of Langmuir model.
The data on thermodynamic analysis were calculated from the following Equations (Fan et al. 2008; Lima et al. 2019):
where K 0e was the dimensionless hermodynamic equilibrium constant, γ was the coefficient of activity (dimensionless), [Adsorbate]° (1 mol/L) was the standard concentration of the adsorbate, KL was the Langmuir equilibrium constant (given initially in L/mg converted into L/mol), the molecular weight of the adsorbate HPP2 (g/mol) was 39,892, R (8.314 J/mol.K) was the universal gas constant, ΔH0 (KJ/mol) was the changes of enthalpy, ΔS0 (KJ/mol.K) was the changes in the entropy, and ΔG0 (KJ/mol) was the changes in Gibb’s free energy.
The adsorption diffusion mode was ascertained by Intraparticle diffusion model and Memberance diffusion model. The data on Intraparticle diffusion model were calculated from the following Equation (Zhao and Wang 2017):
The data on Memberance diffusion model were calculated from the following Equations (Zhao and Wang 2017):
where qt (mg/g) was the adsorption amount at a time, qe (mg/g) was the equilibrium adsorption amount, kp (g/mg.min) and kf (cm/s) represented the coefficients of models, C represented the constant of Intraparticle diffusion model, and t (min) represented the time of adsorption.
Results and discussion
DS of the modified hyperbranched hemicellulose polymer
The changes of DS on HCMA polymer and the modified hyperbranched hemicellulose polymer were described in Fig. 1. The DS of HCMA could reach the maximum 0.97, while the maximum DS of HPP1 was 0.89 and the maximum DS of HPP2 was 0.83. Introducing DMPA constantly, more active hydroxyl and carboxyl groups increased the branching degree of main chain. However, the growth of molecular chain and steric effect influenced the increase of DS on the modified hyperbranched hemicellulose polymer. Additionally, more DMPA could cause the breakdown of the glycosylated bonds between hemicellulose groups, and reduce the increase of DS gradually. The prepared HPP2 with maximum DS was selected as adsorbent. The HPP2 with higher DS had more carboxyl groups and hydroxyl groups, which the dipole bond force and hydrogen bond force between the adsorbent HPP2 and the acid dye were stronger and the adsorption effect was better.
Kinematic viscosity of the modified hyperbranched hemicellulose polymer
The kinematic viscosity of HCMA polymer and the modified hyperbranched hemicellulose polymer were shown in Table 1. After HCMA polymer was grafted by DMPA, the long branched chain caused the increase of viscosity. With the increase of carboxyl and hydroxyl groups, the polarity and intermolecular force of the modified hyperbranched hemicellulose polymer were improved. The higher viscosity of the modified hyperbranched hemicellulose polymer was appropriate for absorbing dyes.
Structure analysis
The FT-IR spectra of HCMA polymer and the modified hyperbranched hemicellulose polymer were shown in Fig. 2. The characteristic peaks of HCMA were at 3450 cm−1 (–OH), 1750 cm−1 (–COO), 1720 cm−1 (–COOH) and 1690 cm−1 (–C = C–) [indicated with HCMA in Fig. 2]. The characteristic peaks of HPP were at 3450 cm−1 (–OH), 2920 cm−1 (–CH3), 1750 cm−1 (–COO–), 1720 cm−1 (–COOH), 1690 cm−1 (–C = C–) [indicated with HPP1 and HPP2 in Fig. 2]. Compared to the HCMA, the sample of HPP with more hydroxyl groups indicated a wide hydroxyl peak at 3450 cm−1. When the DMPA with methyl group was grafted onto HCMA, the sample of HPP appeared the methyl peak at 2920 cm−1. The sample of HPP2 with more carboxyl, hydroxyl and ester groups exhibited higher hydroxyl peak at 3450 cm−1, ester peak at 1750 cm−1 and carboxyl peak at 1720 cm−1 than HPP1.
The 13C-NMR spectra of HCMA polymer and the modified hyperbranched hemicellulose polymer were shown in Fig. 3a–c. The sample of HCMA showed characteristic peaks of carbon connected to double bond at 140.5 ppm, ester carbon at 169 ppm and carboxyl carbon at 181 ppm (Fig. 3a). With the introduction of DMPA, the spectrum of HPP increased the characteristic peaks of carboxyl carbon at 178 ppm, ester carbon at 172 ppm and hydroxyl carbon at 112.3 ppm. Compared to the HPP1 (Fig. 3b), the sample of HPP2 showed higher strong peaks of terminal carboxyl carbon at 178 ppm, ester carbon at 172 ppm, hydroxyl carbon at 112.3 ppm and methyl carbon of the branched chain at 22.3 ppm, which could be attributed to more hydroxyl and carboxyl groups introduced from DMPA (Fig. 3c).
SEM analysis of the modified hyperbranched hemicellulose polymer
The SEM images of the hyperbranched hemicellulose HPP1 and HPP2 were shown in Fig. 4a, b. There were many pores in the surface of HPP1 and HPP2. With DMPA grafted gradually to the HPP2, more branched chains linked on the hemicellulose macromolecular. The HPP2 exhibited more distinct cross-linked structure than the HPP1. The pores and cross-linked structure were help for dye adsorption. When the hyperbranched hemicellulose polymer was used for dye adsorption, dye molecules could quickly cross the surface of macromolecules and adsorbed by the hyperbranched hemicellulose polymer. The pores and cross-linked structure increased the adsorption area and improved the adsorption capacity. Its structure showed a good prospect on adsorption application.
Thermal stability of the modified hyperbranched hemicellulose polymer
The Fig. 5 displayed the TGA and DTG curves of HCMA and the modified hyperbranched hemicellulose polymer HPP. All samples showed good thermal stability, and the temperature data were reported in Table 2. The cross-linked network structure produced thermal stability at low temperature. As the branched ester chains were added on the linear units, the onset decomposition temperature of hyperbranched hemicellulose polymer was later than the HCMA polymer. The sample of HPP2 was grafted more branched ester chains, which resulted in the higher thermal stability. Because of the consistency of glycosidic bond fractured in hemicellulose molecular chain, the sample of HCMA, HPP1 and HPP2 exhibited the similar thermal decomposition curve (Fig. 5b). They had a strong degradation at about 268–278 °C, and the final degradation temperature was at about 568–596 °C (Table 2).
The adsorption capacity on HPP2 adsorbing acid dyes
The sample of HPP2 was applied in adsorbing Methyl red dye, Bromocresol green dye and Bromophenol blue dye. The influences on different dosage of HPP2, temperature and pH for adsorption capacity were shown in Fig. 6a–c. The dosage of HPP2 and temperature expressed the similar influence for three acid dyes, which the adsorption capacity began to increase quickly and then leveled off. When adding 4% HPP2, the adsorption capacity reached the maximum value (Fig. 6a). The optimum temperature was about 60 °C at which the adsorption capacity of Methyl red dye showed stronger (Fig. 6b). The pH had some different influences for adsorption capacity of three acid dyes. For the Bromocresol green dye and Bromophenol blue dye, the adsorption capacity increased with the rise of pH (Fig. 6c). While for Methyl red dye, the adsorption capacity decreased with the rise of pH. The pH could not only affect the surface polarity of adsorbent and dye, but also affect the dissociation degree of terminal functional groups on absorbent (Jayakumar et al. 2013; Zuo et al. 2011). As the pH increased, the carboxyl groups deprotonated gradually. Different functional groups on Methyl red, Bromocresol green and Bromophenol blue resulted unlike dipole bonding and H bonding forces on hyperbranched hemicellulose polymer. The adsorption capacity was affected by the change of adsorption force. The dipole bonding force between HPP2 and Bromocresol green dye or Bromophenol blue dye were strengthened, and the adsorption capacity was improved. But for Methyl red dye, its carboxyl group increased the repulsion between HPP2 and dye, which led to the adsorption capacity reduce.
It could be seen that the dissociation degree of functional groups on Bromocresol green was more affected by the external environment than that of Methyl red and Bromophenol blue, resulting in a weak force between the adsorbent and Bromocresol green and a low adsorption capacity. The optimal adsorption capacity for Methyl red dye, Bromocresol green dye and Bromophenol blue dye could reach 825 mg/g, 675 mg/g and 912 mg/g respectively.
The changes of adsorption capacity caused by different ion strength were shown in Fig. 7a–d. The adsorption capacity at different concentration of Ca2+, Mg2+, NO3− and SO42− showed the similar changes, which the adsorption capacity declined gradually as the increase of concentrations of ion strength. The results revealed that the existence of charged ion would cause the competitive adsorption among hyperbranched hemicellulose polymer, dye and charged ion, which resulted in some negative influence for adsorption between hyperbranched hemicellulose polymer and dyes.
The adsorption mechanism analysis on HPP2 adsorbing acid dyes
The kinetic adsorption curves on HPP2 adsorbing three dyes were shown in Figs. 8a–c and 9a–c, and the related kinetic adsorption data were listed in Table 3. The kinetic adsorption curves could be explained by the related kinetic adsorption data. For Methyl red dye as example, the R2 of the Pseudo-first-order kinetics adsorption data were 0.899, 0.759 and 0.634 respectively. While the R2 of the Pseudo-second-order kinetics adsorption data were 0.995, 0.998 and 0.996 respectively. Obviously, the Pseudo-second-order kinetics model showed better consistency than the Pseudo-first-order kinetics model according to the correlation coefficient of R2 close to 1. Moreover, the data of qe obtained by matching the Pseudo-second-order kinetic adsorption curve were 28.56 mg/g, 113.6 mg/g and 234.9 mg/g respectively, which were close to the data obtained from the experiment. The data of HPP2 adsorbing Bromcresol green dye and Bromophenol blue dye were also met with the Pseudo-second-order kinetics model well.
The adsorption isotherm analysis on HPP2 adsorbing acid dyes
The adsorption isotherms curves on HPP2 adsorbing three dyes based on Langmuir model were shown in Fig. 10a–c, and the related adsorption isotherm data were listed in Table 4. The adsorption capacity was improved with the increase of temperature and then gradually stabilized (Fig. 10a–c). The adsorption isotherms curves could be explained by the related adsorption isotherm data. For HPP2 adsorbing Methyl red dye as example, the data of R2 were 0.962, 0.981 and 0.982 at 30 °C, 40 °C and 50 °C respectively. The date of R2 closing to 1 suggested that the adsorption could well consistent with Langmuir isothermal adsorption model (Table 4). The data of qmax were 287.5 mg/g, 289.9 mg/g and 301.2 mg/g respectively, and their slight change with the increase of temperature indicated that the process of adsorption was endothermic. The data of KLwere changed from 3.521*10−2 L/mg, 3.940*10−2 L/mg to 3.978*10−2 L/mg at 30 °C, 40 °C and 50 °C respectively, which implied that raising temperature could strengthen the HPP2 adsorbing Methyl red dye. According to the same analysis, the process of HPP2 adsorbing Bromcresol green dye and Bromophenol blue dye also met with Langmuir isothermal adsorption model. Above results revealed that the adsorption process of HPP2 on acid dyes was monolayer adsorption. At the same time, it may include physical adsorption process and electrostatic attraction process.
The thermodynamic analysis on HPP2 adsorbing acid dyes
Thermodynamic data on HPP2 adsorbing three dyes were listed in Tables 5 and 6. For HPP2 adsorbing Methyl red dye as example, the data of ΔG0 were − 35.65 kJ/mol, − 37.11 kJ/mol and − 38.23 kJ/mol respectively under 303 K, 313 K and 323 K. The negative ΔG0 indicated that the adsorption process was spontaneous. With the increase of temperature, the functional groups on adsorbent surface possessed more active energy. The temperature affected the thickness of the boundary layer around the adsorbent, which could change the diffusion rate of dyes (Tian et al. 2010). The variation of ΔG0 met the physical adsorption process, which was consistent with the results of adsorption isotherms analysis. The negative ΔH0 indicated the endothermic adsorption process, and increasing temperature would conducive to the adsorption. The date of ΔS0 was 72.65 kJ/mol.K, 72.85 kJ/mol.K and 73.11 kJ/mol.K respectively, which suggested a certain degree of chaotic on solid–liquid interface. The thermodynamic data testified HPP2 had a strong adsorption capacity for acid dyes.
The N2-adsorption/desorption isotherms analysis on HPP2
The N2-adsorption/desorption isotherms of HPP2 were shown in Fig. 11. Above was the adsorption curve and below was the desorption curve. At the low relative pressure, the interaction between HPP2 and N2 was weak resulted the slow increase of amount adsorbed. When the relative pressure was 0.12, the desorption rate was only 35.1% (which was the the ratio of desorption amount and adsorption amount). Then the relative pressure was improved, the amount adsorbed increased rapidly. When the relative pressure was above 0.82, the desorption rate exceeded 80%. At the higher relative pressure, the HPP2 could be successfully regenerated and repeatedly used in N2 adsorption. The N2-adsorption/desorption isotherms revealed that the modified hyperbranched hemicellulose polymer filled the pore in clusters, and its adsorption process met with monolayer adsorption. The analysis of N2-adsorption/desorption isotherms was consistent with the results of adsorption isotherms and thermodynamic analysis.
The diffusion mode analysis on HPP2 adsorbing acid dyes
Generally, the study on diffusion followed the theory of four-step adsorption process. First, the dye moved to the liquid film surface of the adsorbent, which was called Bulk diffusion. Secondly, the dye moved toward the surface of the adsorbent and passed through the liquid film surface of the adsorbent, which was called Memberance diffusion. Thirdly, the dye continued to move into the inside of adsorbent, which was called Interparticular diffusion. Fourthly, the dye was adsorbed by physical or physicochemical processes, which was the Solute sorption. Also, many researches had shown that the first and fourth steps did not generally become rate control steps, and the second and third step were the most likely control steps (Ozcan 2005; Karthik and Meenakshi 2015). It was necessary to build the diffusion curves based on Intraparticle diffusion model and Membrane diffusion model to ascertain the diffusion theory of HPP2 in adsorption process.
The diffusion curves on HPP2 adsorbing three acid dyes based on Intraparticle diffusion model and Membrane diffusion model were shown in Figs. 12a–c and 13a–c, and the related diffusion data were listed in Table 7. The diffusion rate varied with the initial concentration of dye (Figs. 12a–c, 13a–c). The adsorption diffusion theory on HPP2 adsorbing three acid dyes could be discovered by the related diffusion data. For HPP2 adsorbing Bromophenol blue dye as example, the R2 of Intraparticle diffusion model were 0.942, 0.760 and 0.806 at 20 ppm, 50 ppm and 100 ppm respectively, while the R2 of Memberance diffusion model were 0.904, 0.427 and 0.484 at 20 ppm, 50 ppm and 100 ppm respectively. Obviously the adsorption was more conformed to the Intraparticle diffusion model. Moreover, it could be discovered that the intercepts of both diffusion model were not 0 (Table 7), which indicated the adsorption was not controlled by one model but by both models simultaneously. The diffusion modes obtained by HPP2 adsorbing Methyl red dye, Bromocresol green dye and Bromophenol blue dye were consistent with the adsorption diffusion theory of which the adsorption rate was controlled both by Intraparticle diffusion model and Memberance diffusion model, and Intraparticle diffusion model affected more.
Conclusions
The modified hyperbranched hemicellulose polymer (HHP) was synthesized and analyzed by degree of substitution, kinematic viscosity, FT-IR, 13C-NMR, TGA and SEM. The modified hyperbranched hemicellulose polymer had high branched degree and possessed a large number of terminal carboxyl groups and hydroxyl groups. In SEM images, the HHP exhibited the pores and cross-linked structure which were good help for dye adsorption. The adsorption capacity on HPP2 adsorbing ethyl red dye, Bromocresol green dye and Bromophenol blue dye were studied through the adsorbent dosage, pH, temperature and ionic strength. The adsorption capacity was influenced significantly by different adsorbent dosage, pH, temperature and ionic strength. The adsorption mechanism of HPP2 adsorbing three acid dyes were accordance with the Pseudo-second-order kinetic model better. The adsorption isotherms were more matched with Langmuir model, and the data indicated that raising temperature could improve the adsorption capacity. The results of adsorption isotherms and thermodynamic date all indicated the processes of HPP2 adsorbing three acid dyes were endothermic. The N2-adsorption/desorption isotherms of HPP2 revealed the hyperbranched hemicellulose polymer filled the pore in clusters, and its adsorption process met with monolayer adsorption. The adsorption diffusion mode on HPP2 adsorbing three acid dyes were discussed by Intraparticle diffusion model and Membrane diffusion model. The adsorption rate was controlled both by Intraparticle diffusion model and Membrane diffusion model, of which Intraparticle diffusion model affected more. The optimal adsorption capacity of HPP2 on Methyl red, Bromomethyl green and Bromophenol blue could reach 825 mg/g, 675 mg/g and 912 mg/g respectively, which were better than many macromolecules for adsorbing organic dyes. Compared with others’ research works, the HHP have a variety of active groups, and as a new, non-pollution adsorbent it exhibits high efficiency and adaptability in dye adsorption. At the same time, the HHP can well meet the needs of regeneration and reuse.
Change history
04 July 2019
In the original publication of the article, the graphical abstract and Scheme 1(a) and Scheme 1(b) were published incorrectly.
References
Bodnar M, Kjoniksen A, Molnar R, Hartmann J, Daroczi L, Nystrom B, Borbely JY (2007) Nanoparticles formed by complexation of poly-gamma-glutamic acid with lead ions. J Hazard Mater 153:1185–1192. https://doi.org/10.1016/j.jhazmat.2007.09.080
Chen Z, Zhou L, Zhang F, Yu C, Wei Z (2012) Multicarboxylic hyperbranched polyglycerol modified SBA-15 for the adsorption of cationic dyes and copper ions from aqueous media. J Appl Surf Sci 258:5291–5298. https://doi.org/10.1016/j.apsusc.2012.02.021
Esposito D, Antonietti M (2015) Redefining biorefinery: the search for unconventional building blocks for materials. J Chem Soc Rev 44:5821–5835. https://doi.org/10.1039/c4cs00368c
Fan T, Liu YG, Feng BY, Zeng GM, Yang CP, Zhou M, Zhou HZ, Tan ZF, Wang X (2008) Biosorption of cadmium(II), zinc(II) and lead(II) by penicillium simplicissimum: isotherms, kinetics and thermodynamics. J Hazard Mater 160:655–661. https://doi.org/10.1016/j.jhazmat.2008.03.038
Fan W, Gao W, Zhang C, Tjium WW, Pan J, Liu T (2012) Hybridization of graphene sheets and carbon-coated Fe3O4 nanoparticles as a synergistic adsorbent of organic dyes. J Mater Chem 22:25108–25116. https://doi.org/10.1039/c2jm35609k
Ferrari E, Ranucci E, Edlund U, Albertsson AC (2015) Design of renewable poly (amidoamine)/hemicellulose hydrogels for heavy metal adsorption. J App Polym Sci 132: 41682–41695. https://doi.org/10.1002/app.41695
Guijarro-Aldaco A, Hernandez-Montoya V, BonillA-Petriciolet A, Montes-Moran MA, Mendoza-Castillo DI (2011) Improving the adsorption of heavy metals from water using commercial carbons modified with egg shell wastes. J Chem Eng 50:9354–9362. https://doi.org/10.1021/ie2006627
Hu QK (2008) Preparation of modified chitosan and discolorationof dyeing and printing wastewater. Text Auxiliaries 25:253–256. https://doi.org/10.3969/j.issn.1004-0439.2008.03.005
Hu XJ, Wang JS, Liu YG et al (2011) Adsorption of chromium (VI) by ethylenediamine-modified cross-linked magnetic chitosan resin: isotherms, kinetics and thermodynamics. J Hazard Mater 185:306–314. https://doi.org/10.1016/j.jhazmat.2010.09.034
Jayakumar S, Gomathi T, Sudha PN (2013) Sorption studies of lead (II) onto crosslinked and non crosslinked biopolymeric blends. J Biol Macro 59:165–169. https://doi.org/10.1016/j.ijbiomac.2013.04.031
Kanekiyo Y, Tao H, Sellergren B (2008) Stimuli-responsive guest binding and releasing by dendritic polymer-based hydrogels. J Polymer 40:684–687. https://doi.org/10.1295/polymj.PJ2008018
Karthik R, Meenakshi S (2015) Removal of Pb(II) and Cd(II) ions from aqueous solution using polyaniline grafted chitosan. J Chem Eng 263:168–177. https://doi.org/10.1016/j.cej.2014.11.015
Li B, Jiang X, Yin J (2012) Multi-responsive microgel of hyperbranched poly(ether amine) (hPEA-mGel) for the selective adsorption and separation of hydrophilic fluoresce in dyes. J Mater Chem 22:17976–17983. https://doi.org/10.1039/C2JM33188H
Lima EC, Hosseini-Bandegharaei A, Moreno-Piraján JC, Anastopoulos I (2019) A critical review of the estimation of the thermodynamic parameters on adsorption equilibria. J Mol Liq 273:425–434. https://doi.org/10.1016/j.molliq.2018.10.048
Lu L, Zhao H, Yan L, Wang G, Mao Y, Wang X, Jiang T (2015) Removal characteristics of Cd(II) ions from aqueous solution on ordered mesoporous carbon. J Chem Eng 32:2161–2167. https://doi.org/10.1007/s11814-015-0150-7
Luo Y, Zhang C, Xu F, Chen Y, Fan L, Wei Q (2010) Synthesis and characterization of novel THTPBA/PEG-derived polyurethane scaffolds for tissue engineering. J Mater Sci 45:1866–1877. https://doi.org/10.1007/s10853-009-4171-7
Moran JI, Cyras VP, Vazquez A (2012) Preparation and characterization of three different derivatized potato starches. J Polym Environ 21:395–404. https://doi.org/10.1007/s10924-012-0539-x
Ning L, Liao S, Sun Y, Yu L, Tong X (2016) The efficient oxidation of biomass-derived 5-hydroxymethyl furfural to produce 2,5-diformylfuran over supported cobalt catalysts. J Chem Rev 9:95–101. https://doi.org/10.1007/s12649-016-9724-9
Ozcan A (2005) Adsorption of acid red 57 from aqueous solutions onto surfactant-modified sepiolite. J Hazard Mater 125:252–259. https://doi.org/10.1016/j.jhazmat.2005.05.039
Peng Y, Wang KK, Liu T, Xu J, Xu BG (2017) Synthesis of one-dimensional Bi2O3-Bi2O2 heterojunctions with high interface quality for enhanced visible light photocatalysis in degradation of high-concentration phenol and MO dyes. J Appl Catal Environ 203:946–954. https://doi.org/10.1039/c8ce00101d
Ragauskas AJ (2006) The path forward for biofuels and biomaterials. J Sci 311:484–489. https://doi.org/10.1126/science.1114736
Rahman ML, Sarkax SM, Yusoff MM et al (2017) Optical detection and efficient removal of transition metal ions from water using poly (hydroxamic acid) ligand. J Sens Actuators B Chem 242:595–608. https://doi.org/10.1016/j.snb.2016.11.007
Schutyser W, Renders T, Vanden BS, Koelewijn SF (2018) Chemicals from lignin: an interplay of lignocellulose fractionation, depolymerisation and upgrading. J Chem Soc Rev 47:852–908. https://doi.org/10.1039/C7CS00566K
Sun K, Shi Y, Xu W, Potter N, Li Z, Zhu J (2017) Modification of clays and zeolites by ionic liquids for the uptake of chlorampheicol from water. J Chem Eng 313:336–344. https://doi.org/10.1016/j.cej.2016.12.083
Tian Y, Ji C, Zhao M (2010) Preparation and characterization of baker’s yeast modified by nano-Fe3O4: application of biosorption of methyl violet in aqueous solution. J Chem Eng 165:474–481. https://doi.org/10.1016/j.cej.2010.09.037
Tirtom VN, Dincer A, Becerik S, Aydemir T, Celik A (2012) Comparative adsorption of Ni(II) and Cd(II) ions on epichlorohydrin crosslinked chitosan-clay composite beads in aqueous solutions. J Environ Sci 22:379–386. https://doi.org/10.1016/j.cej.2012.05.059
Tripathi N, Choppala G, Singh RS, Srivastava P, Seshadri B (2016) Adsorption kinetics of zinc and nickel on modified chitosan. J Hazard Mater 188:507–520. https://doi.org/10.1007/s10661-016-5499-5
Voit BI, Lederer A (2009) Hyperbranched and highly branched polymer architectures-synthetic strategies and major characterization aspects. J Chem Rev 109:5924–5973. https://doi.org/10.1021/cr900068q
Wang J, Wang D, Zhang G, Guo Y, Liu J (2013) Adsorption of rhodamine B from aqueous solution onto heat-activated sepiolite. J Hazard Mater 18:219–225. https://doi.org/10.1007/s11859-013-0918-8
Zhao S, Wang Z (2017) A loose nano-filtration membrane prepared by coating HPAN UF membrane with modified PEI for dye reuse and desalination. J Membr Sci 524:214–224. https://doi.org/10.1016/j.apcatb.2016.11.011
Zheng Y, Li S, Weng Z, Gao C (2015) Hyperbranched polymers: advances from synthesis to applications chemical. J Soc Rev 44:4091–4130. https://doi.org/10.1039/c4cs00528g
Zhou L, He B, Huang J (2013) One-step synthesis of robust amine- and vinyl-capped magnetic iron oxide nanoparticles for polymer grafting and in dye adsorption and catalysis. J Appl Mater Interface 5:8678–8685. https://doi.org/10.1021/am402334f
Zhu W, Liu L, Liao Q, Chen X, Qian Z, Shen J, Liang J, Yao J (2016) Functionalization of cellulose with hyperbranched polyethylenimine for selective dye adsorption and separation. J Cellul 23:3785–3797. https://doi.org/10.1007/s10570-016-1045-4
Zuo L, Yu S, Zhou H, Jiang J, Tian X (2011) Adsorption of Eu(III) from aqueous solution using mesoporous molecular sieve. J Radioanal Nucl Chem 288:579–586. https://doi.org/10.1007/s10967-010-0972-z
Acknowledgments
Valuable cooperation of Prof. Hanjiang Yu from Tangshan Sanyou Group Xingda Chemical Fibre Co. Ltd. is greatly appreciated by the authors.
Funding
This study was funded by Bureau of Science and Technology of Hebei Province (16211223D).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, L., Xue, W. & Gu, L. Modification of hyperbranched hemicellulose polymer and its application in adsorbing acid dyes. Cellulose 26, 5583–5601 (2019). https://doi.org/10.1007/s10570-019-02483-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-019-02483-0