Abstract
Fast and efficient removal of organic dyes from industrial wastewater by practical and cost-effective adsorption method has been in the spotlight. Herein, lignosulfonate-based hybrid hydrogel adsorbents (HPAS) were one-pot synthesized via cross-linking reaction with lignosulfonate amine, hyperbranched polyamide amine, and polyethylene glycol diglycidyl ether, then were characterized by FTIR, XPS, and SEM measurements. The adsorption capacity of HPAS for separate organic dyes and metallic ion were further studied. HPAS with hierarchical porous structures showed ultra-high capacity for dye removal, especially for Congo red (CR). The actual maximum adsorption capacity of CR can reach 5120 mg/g, when the initial concentration of CR is 6000 mg/L, natural pH, temperature is 298 K, and the amount of adsorbent HPAS-4 is 0.8 g/L. Simultaneously, HPAS-4 also showed a relatively strong adsorption performance for Cu(II); the maximum uptake capacity was obtained at 249 mg/g (initial Cu(II) concentration = 100 mg/L). After 5 adsorption–desorption cycles, HPAS-4 still maintains a high adsorption capacity for CR and Cu(II). The CR and Cu(II) adsorption mechanisms by HPAS were further validated using X-ray photoelectron spectroscopy and Fourier transform infrared spectroscopy. The adsorption of CR and Cu(II) by HPAS was a spontaneous endothermic process, which conforms to the pseudo-second-order kinetic model and Langmuir adsorption model. The key mechanism of CR adsorption process is H-bond interactions due to abundant N and O atoms in HPAS accompanied with electrostatic attraction and π-π stacking interaction. The adsorption of Cu(II) on HPAS is mainly determined by the complexation/chelation accompanied with precipitation of copper derivatives on HPAS. The results of this study provide a new method for the preparation of high-performance biomass-based adsorbents in the field of wastewater treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
With advancement of modern technology and industrial activity, especially textile and printing and dyeing, considerable wastewater containing organic dyes and their derivatives is produced and discharged, which pose a serious threat to human beings and the environment due to their toxicity and accumulation (Bhat et al., 2020; Li et al., 2018). Therefore, numerous physical and chemical methods even combined with biological remediation are applied for the removal of organic dyes from sewage before discharging, including coagulation and flocculation (Moghaddam et al., 2010), membrane separation (Jiet al., 2017), electrochemical oxidation (Dogan et al., 2005), catalytic degradation (Li et al., 2005), and adsorption (Wang et al., 2021). Among these methods, adsorption is a preferential alternative for low cost, simple operation, easy recycling, and non-production of toxic byproducts (Hu et al., 2020). Designing and fabricating high-efficiency and recycled adsorbents for a cleaner and more sustainable wastewater treatment is of great concern for researchers.
Currently, natural biosorbents and biomass-based composites have been widely studied as a suitable and environmentally benign alternative for contaminant removal from wastewater (Sajjadi et al., 2021; Yadav et al., 2021). Natural adsorbents, such as cellulose, lignin, chitosan, starches, galactomannan, and sodium alginate, even bamboo powder, are reported to use for wastewater treatment. Lignosulfonate, a waste by-product from paper industry, is an aromatic three-dimensional polymer structurally and highly water soluble, containing numbers of functional groups such as methoxyl, alcoholic hydroxyl, phenolic hydroxyl, sulfonic acid group, and carbonyl and conjugated double bond, which make it adsorb many kinds of dyes (Xu et al., 2019). Nevertheless, the application of sodium lignosulfonate directly used as an adsorbent was limited for its good water solubility and low uptake capacity (Meng et al., 2020; Teng et al., 2017). By now, various modified lignosulfonate and their composites with other materials were prepared for improving the adsorption of dye. Such as, quaternary ammonium functionalized lignosulfonate can simultaneously adsorb cationic dye methylene blue (MB) and anionic dye Congo red (CR), and the corresponding maximum qe are 153 and 324 mg/g, respectively (Tang et al., 2021). The aminated calcium lignosulfonate also shows relatively higher adsorption performance for Congo red and Titan yellow dyes, the corresponding maximum adsorption capacity reached 258.4 mg/g and 190.1 mg/g, respectively (Wang et al., 2018). Some magnetic lignosulfonate composites combined with Fe3O4 or γ-Fe2O3 were fabricated for separating the organic pollution including dyes and metal ions (Geng et al., 2019; Hao et al., 2021; Hu et al., 2020; Jiang et al., 2019). Sun reported that a hybrid amino-functionalized TiO2/sodium lignosulfonate surface molecularly imprinted polymer was prepared and exhibited high adsorption selectively and quick adsorption of MB (Sun et al., 2022). Other biomass materials, such as chitosan (Gu et al. 2019; Zhang et al., 2021a), tannic acid (Xia et al., 2021), polycatecholamine (Gao et al., 2019), and carboxymethyl cellulose (Fan et al., 2022) were also used to construct composites with lignosulfonate for dye and metal ion removal from wastewater. Polyaniline/lignosulfonate composites were prepared as an adsorbent for the removal of acid red 94, it was found that the maximum adsorption capacity can reach 10.56 g/g according to the Langmuir model. It was suggested that the adsorbate and adsorbent formed a gel-like composite responsible for the superior adsorption efficiency after adsorption (Xu et al., 2019). Serials of polypyrrole-chitosan-lignosulfonate (PPY-CS-LS) composites were prepared via an in situ polymerization of pyrrole monomers with chitosan and/or lignosulfonate (LS) as dispersants for adsorption of dye. It was found that PPY-CS-LS and PPY-CS composites showed very high selectivity for acid (anion) dyes, especially for Congo red (CR); the removal efficiencies of PPY-CS-LS and PPY-CS composites for CR were up to 99.3% and 95.4%, respectively, and the adsorption equilibrium time for CR was only 30 min. The high adsorption performance of the PPY-CS-LS composite for dyes stems from the synergistic effects of amino/hydroxyl-containing functional groups from CS, sulfonic groups from LS, and nitrogen-containing functional groups from PPY chains (Zhou et al., 2017). It is obvious that the abundantly available adsorption groups are the necessary condition for the high adsorption capacity of the adsorbent.
As a kind of hydrophilic polymer with a 3D network structure, hydrogels are widely used in wastewater treatment. The hydrogel adsorbent with high performance prepared by waste biomass as a low-cost raw material has received widespread attention. For example, a starch-based absorptive hydrogel (STAH) prepared by grafting polyacrylic acid onto starch and then cross-linked with N,N′-methylene-bisacrylamide exhibited maximum adsorption capacity (2967.66 mg/g) in optimal conditions for MB. The number of –COOH/–COO– groups seriously affected the hydrogen-bonding interaction and electrostatic interaction between STAH and MB, further affecting the adsorption capacity (Chen et al., 2021). Chatterjee et al. (2018) reported a kind of chitosan-based hydrogel beads used for the removal of anionic dye from wastewater. It was found that the hydrogel capsules treated by alkali treatment process exhibited excellent adsorption rate and capacity of Congo red, and the corresponding adsorption capacity can reach 2592 mg/g compared with the pristine hydrogel capsules (563 mg/g) and beads (183 mg/g), which could be derived from the rearrangement of polymeric networks after the alkali treatment process (Chatterjee et al., 2018). Driven by the concept of sustainable development, the lignosulfonate-based hydrogel was fabricated by simple cross-linking with poly (ethylene glycol) diglycidyl ether. The hydrogels show a high adsorption capacity for methylene blue cationic dye which reached 211 mg/g, but the anionic dye methyl orange (MO) cannot be removed by contrast (Mondal et al., 2021). It deems that the porous and interconnecting networks in the hydrogels are favorable for dye adsorption. A kind lignin and lignosulfonate hybrid hydrogel modified with poly(ethylene-alt-maleic anhydride) [P(E-alt-MA)] was synthesized by an esterification reaction. The adsorbent shows excellent adsorption performance with methylene blue as the model pollutant; the adsorption efficiency can reach 100% under the condition, and exhibited remarkable adsorption speed (in the order of minutes) (pH 7, 1 g/L of adsorbent, 50 mg/L of MB, adsorption time 30 min, 250 rpm, 25 °C) (Panzarasa et al., 2018). Lignosulfonate-graft-poly[acrylamide-co-(acrylic acid)] (LS-g-P(AAm-co-AA)) microgels are prepared by inverse suspension polymerization. LS in microgels promote thermal stability and render faster and stronger MB adsorption, and the corresponding adsorption capacity can reach 154 mg/g (Yiamsawas et al., 2021). A functional biomass terpolymer lignin-based hydrogel adsorbent (LAD) was synthesized through free radical graft copolymerization with sodium lignosulfonate, acrylamide, and acryloxyethyltrimethylammonium chloride as materials. The maximum adsorption capacity of LAD towards acid red 73 can reach 409.84 mg·g−1, and the adsorption mechanism was from electrostatic attraction, and comprehensive effects of physical and chemical adsorption and hydrogen bond (Wei et al., 2021). Jiang et al. (2022) prepared a bentonite-doped lignin hydrogel sphere super adsorbent (LHS-BT) by template method for the removal of cationic dyes from wastewater. The adsorbent LHS-BT showed an ultra-high-adsorption capacity for malachite green (2541.76 mg/g) and MB (1047.22 mg/g), and a faster adsorption rate with 85 min. The electrostatic attraction, π-π interaction, and hydrogen bonding are deemed to be the main reasons for the ultra-high-adsorption performance (Jiang et al., 2022). Simultaneously, the above adsorbed lignin-based hydrogels can be easily separated from the liquid phase, which are a benefit to the following separation work. The design of lignosulfonate-based hydrogels with ultra-strong-adsorption performance for organic dye comes down to how to fabricate the special structure with the unique 3D network and a large number of active groups which can produce stronger electrostatic attraction, π-π interaction, or n-π interaction, and hydrogen bonding between adsorbent with dye (Zhang et al., 2019).
Polyamine (branched polyethyleneimine (PEI), hyperbranched polyamide (HP)) with abundant amine groups were used in the preparation of adsorbents for the removal of anionic dyes. For example, the polyethyleneimine (PEI) modified waste bamboo powder was used for removing Congo red from an aqueous solution, and the corresponding maximum adsorption capacity can reach 992.94 mg/g at 298 K, and the removal efficiency was over 98% (Zhang et al., 2021b ). The microcrystalline cellulose modified with Fe3O4 and polyethyleneimine was prepared and applied to Congo red adsorption, the adsorption equilibrium of CR can reach within 100 min at 298K, the maximum adsorption capacity was 862.25 mg/g (Zhang et al., 2021c). Cellulose functionalized with hyperbranched polyethyleneimine showed very high adsorption capacity for the anionic dye Congo red and cationic basic yellow 28 in an aqueous solution; the corresponding maximum adsorption capacity was 2100 mg/g and 1860 mg/g, respectively (Zhu et al., 2016). Especially, dendrimers and hyperbranched polymers, such as polyamide amine dendrimer (PAMAM) and amino-terminated hyperbranched polyamide, which have numerous interior cavities (subnano-3D structure) and abundant active sites, also have been used for enhancing the adsorption of anionic dye. He et al. (2021) prepared a polyamide amine dendrimer aerogel based on aramid nanofiber and used for Congo red removal from an aqueous solution. The composite aerogels exhibited an ultra-high capacity for CR; the maximum CR adsorption capacity is 1957.881 mg/g (He et al., 2021). Cellulose-based adsorbents by surface functionalized with hyperbranched polyamide (HP) were synthesized to the removal of organic dyes and metal ions; the results showed that the adsorption rate and capacity were obviously enhanced, and the maximum adsorption capacity of golden orange OII could reach 935 mg/g, much larger than that of most reported adsorbents (Yu et al., 2019). Hence, the easily prepared and costless hyperbranched polyamide with well-developed subnano-3D cavities and a large number of N-containing groups provide the possibility to fabricate high-performance hydrogel adsorbents with other biomass-based materials for removal dyes from aqueous solution.
Congo red (CR) as one of the most common discharge dyes is intensively used in many industries such as textiles, solar cells, paper, printing, cosmetics, and plastics. However, CR is a benzidine-based anionic dye; its decomposition results in carcinogenic products. It is a mutagen and affects the reproductive systems of living things, and acts as a skin, eye, and gastrointestinal irritant. It influences blood clotting and induces lethargy and respiratory disorders (Kumari et al., 2016). Herein, we developed ultra-high-capacity lignosulfonate-based hybrid hydrogel adsorbents (HPAS) synthesized via a one-pot cross-linking reaction with a lignosulfonate amine, hyperbranched polyamide amine, and polyethylene glycol diglycidyl ether, and studied the adsorption performance of Congo red and Cu(II) ions. In this work, sodium lignosulfonate was first amino-functionalized to improve the number of active sites; then, the hyperbranched polyamide amine/lignosulfonate hybrid hydrogel was synthesized with polyethylene glycol diglycidyl ether (PEGDGE) as a cross-linking agent. A hierarchical network could be formed cross-linked from hyperbranched polyamide amine with the branched 3D network and three-dimensional lignosulfonate chain, and abundant amino groups were introduced into the hybrid hydrogels; the special 3D network and increased active sites would improve the mass transfer of dye and affinity between dye and adsorbents, so this special kind of hybrid hydrogel showed a super high capacity for CR compared with most other reports. Therefore, the lignosulfonate-based hybrid hydrogel adsorbents have great application potential in the field of CR removal.
2 Materials and Methods
2.1 Materials
Sodium lignosulfonate (SLS, indeterminate molecular weight), methyl acrylate, and diethylenetriamine were obtained from Aladdin Co. Ltd., China. Formaldehyde, isopropanol, methanol, ethanol, sodium hydroxide, hydrochloric acid, and poly(ethylene glycol) diglycidylether (PEGDGE) are purchased from Gaojing Chemical Agent Co. Ltd. Congo red (CR), methyleneblue (MB), and copper sulfate were supplied by Innochem Co. Ltd., China. Deionized water (DW) was used for all experiments.
2.2 Preparation of Lignosulfonate-Based Hybrid Hydrogel Adsorbents
Lignosulfonate amine (LA) was prepared according to the report via the Mannich reaction by reacting SLS with formaldehyde and diethylenetriamine (DETA) (Teng et al., 2017).
Hyperbranched polyamide amine (HP) was prepared according to the previous report via an easy polycondensation method (Yu et al., 2019).
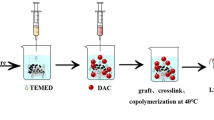
The lignosulfonate-based hybrid hydrogel adsorbents (HPAS) were prepared by cross-linking reaction between lignosulfonate amine and hyperbranched polyamide amine with poly(ethylene glycol) diglycidylether (PEGDGE) (as shown in Scheme 1). Typically, 0.125g lignosulfonate amine was firstly dissolved in 5 mL water, and then 0.5g hyperbranched polyamide amine was added and stirred to obtain a homogeneous solution at room temperature. Then, poly(ethylene glycol) diglycidylether (2 mL) was added to the mixture, stirred well, and then heated in a water bath at 70°C for 1h to obtain hybrid hydrogel. The hydrogel was extracted alternatively with warm and cold water six times, and then the sample was freeze-dried to obtain lignosulfonate-based hybrid hydrogel adsorbents (HPAS). Various mount PEGDGE cross-link agents were added to the mixture to obtain different adsorbents with the same procedures. The resulting adsorbents were denoted by HPAS-x, where x=2, 3, 4, and 5, and x is the volume of PEGDGE. Similarly, hyperbranched polyamide amine–based hydrogel adsorbents (HPA) were synthesized from HP and PEGDGE without lignosulfonate amine; the resulting adsorbents were denoted by HPA-x, where x=2, 3, 4, and 5, and x is the volume of PEGDGE.
2.3 Characterization
Fourier transform infrared (FTIR) spectroscopy of samples was conducted by an infrared spectrometer (Nicolet Nexus 670, American Thermoelectric Company). The X-ray photoelectron spectroscopy (XPS) spectra of samples were obtained using an X-ray photoelectron spectrometer (K Alpha, USA). The surface morphology of the materials was examined using a scanning electron microscope (Hitachi S-4800). The surface charge of samples at different pH values was measured by a zeta potential analyzer (Malvern-Nano-ZS90, UK). The absorbency of the CR and MB solutions was measured on a UV-vis spectrophotometer (SPECORD S6000).
2.4 Batch Adsorption Experiment
Congo red (anion dye), methylene blue (cation dye), and Cu(II) were chosen as the representative of dyes and metal ions, respectively. Serial batch adsorption experiments were conducted to obtain adsorption kinetics and isotherms. Shortly, a certain amount of hybrid hydrogels was added into dyes or metal ion water solution with a certain concentration; after adsorption, the concentration of solutions was determined by using a UV-vis spectrometer or inductively coupled plasma emission spectrometer. The adsorption capacities of HPA and HPAS adsorbents were estimated for dye and affecting parameters such as temperature, contact time, initial dye concentration, pH, and adsorbent doses were studied. A dye solution of 50 mL (at specific pH, dye concentration, adsorbent dose) was poured into a 250-mL conical flask and placed at 120 rpm (in a shaker) for 24 h. The HCl/NaOH solution was employed for pH setting. At specific intervals, the sample (3 mL) was taken and centrifuged. The centrifuged solution was used for absorbance measurement at 510 nm and adsorption capacity was estimated as depicted in Eq. (1).
where qe, Co, Ce, V, and m are the adsorption capacity, initial concentration of dye, dye concentration at time “t” (mg/L), volume (mL), and adsorbent mass (g), respectively. All data reported are at least an average of triplicate experiments.
2.5 Stability and Reusability Test
The stability and reusability of hybrid hydrogels were explored through five consecutive adsorption-desorption cycles. After adsorption, the hydrogel is successively desorbed by ethanediamine, ethanol, and water to thoroughly clean the re-collected adsorbents. The hydrogel after desorption was directly used, and the pollutant concentration was measured after adsorbing CR again. The adsorption cycle efficiently was calculated according to Eq. (2).
Here, ɸ refers to the cycle adsorption efficiency.
3 Results and Discussion
3.1 Synthesis and Characterization of HPAS
Sodium lignosulfonate does not exhibit insufficient affinity to dyes while containing a large amount of hydroxyl and sulfonic acid groups, let alone water solubility. Although lignosulfonate ionic hydrogels could be prepared by simple cross-linking with poly (ethylene glycol) diglycidyl ether, the maximum capacity of the hydrogel for cationic dye methylene blue is only 211 mg/g less than other lignosulfonate-based adsorbents (Mondal et al., 2021). In addition, the hydrogel with a high-strength 3D network and good swelling capacity could not be easily formed by a cross-linking reaction due to the low reactivity of hydroxyl groups in SLS (Teng et al., 2017). Therefore, in this work, lignosulfonate amine (LA) was firstly synthesized by introducing alkyl amino groups to the aromatic ring of lignin by the Mannich reaction for improving the reactivity of cross-link reaction and affinity to dyes (Teng et al., 2017). The efficiency of high hydrogel formation could be greatly enhanced by LA with an increased primary amino content. Then lignosulfonate-based hybrid hydrogel adsorbents HPAS were synthesized with polyethylene glycol diglycidyl ether (PEGDGE) as a cross-linking agent. Scheme 1 gives a schematic illustration of the reaction and the possible structure of the formed HPAS.
The comparison of Fourier transform infrared (FTIR) spectra between LA, PEGDGE, HP, and HPAS is shown in Fig. 1a. The IR spectrum of lignosulfonate amine showed the characteristics peaks at 1617, 1509, and 1456 cm−1 which were assigned to aromatic ring skeleton vibrations (Teng et al., 2017). The strong peak at 1036 cm−1 was assigned to C–O–S stretching vibration and the peak at 1113 cm−1 was dominated by O=S=O antisymmetric stretching vibration (Yao et al., 2014). The peaks at 3420 and 2932 cm−1 were attributed to the stretching vibration of O-H and N-H in the sodium lignosulfonate amine, and the peak at 3415 cm−1 became wider, which may stem from the introduction of amino groups. The peak at ∼817 cm−1 assigned to aromatic C-H stretching vibrations in LA became much wider compared to that of LS, indicating the successful introduction of amino groups via the Mannich substitution reaction on the aromatic ring of LS (Teng et al., 2017). The cross-linking agent PEGDGE showed the characteristic peaks at 1114 and 951cm−1, which were assigned to the C-O-C stretching vibration, while the peak at 852 cm−1 was attributed to C-O-C oxirane group (Mondal et al., 2021). The hyperbranched polyamide amine has characteristic peaks at 1643 cm−1 attributed to the amide bond (O=C-N), and the stretching and bending vibration peaks of C-N at 1260 cm−1 and 1552 cm−1, respectively (Yu et al., 2019). For the lignosulfonate-based hybrid hydrogel adsorbents (HPAS), the characteristic peaks attributable to epoxy groups clearly disappeared, and the peak intensities at 2930 and 2855 cm−1 attributed to C-H stretching of methylene in PEGDGE increased, indicating the presence of PEGDGE segments in the hydrogel. Furthermore, the absorbance at ~3400 cm−1 in the HAPS spectrum assigned to the stretching vibration of the hydroxyl groups and amino groups showed little change compared with LA due to the formation of a new hydroxyl group from cross-link reaction between the epoxy group and amino group (Teng et al., 2017). In addition, it was found that the color of sodium lignosulfonate becomes lighter after introducing the amino group and the obtained HAPS adsorbents were obviously porous and loose.
The active groups on the surface of the adsorbent seriously affected the adsorption performance, and the surface element compositions of LS, LA, HPA, and HPAS were determined by XPS measurement. The main active groups of LS are –SO3− and -OH; as can be seen in Fig. 1b, the peak assigned to S2p at ~170 eV was found in all spectra of materials. A characteristic N1s peak at 399.5 eV was found in LA, HPS, and HPAS spectra except LS which would directly affect the interaction between dye and adsorbents. Figure S1 shows high-resolution N1s XPS spectra of HPA and HPAS. The discrete peaks were assigned to a kind of nitrogen-based functional groups, and their composition ratios (listed in Table 1) were calculated from corresponding peaks areas of nitrogen components. The peaks at 400.4, 398.8, 398.4, and 397.0 eV were ascribed to N-C=O, N(C)3, N-C, and C-N-C, respectively. These peak assignments agree well with previously reported values (Yu et al., 2019). It was reported that the chelation ability of amines with metal ions obeys the following order: primary amine>secondary amine> tertiary amine. In addition, primary, secondary, and tertiary amines were protonated in an acidic solution, and the protonated amines could serve as active binding sites for anionic dyes. All these will determine the adsorption capacity of hybrid hydrogels (Yao et al. 2016). As can be seen in Table 1, HPAS-4 own the highest content of primary amine and secondary amine groups, which would provide enough interaction between HPAS and CR by electrostatic attraction and H-bond interaction.
Due to the introduction of high primary amino content in LA, the hydrogel adsorbent can be easily formed. The SEM images of HPA and HPAS are presented in Fig. 2. As shown in Fig. 2, there is an obvious difference among the surface of hydrogel images. The surface of HPA hydrogel showed plenty of uneven wrinkles and some pores. However, HPAS-2, HPAS-3, and HPAS-4 showed more micropore structures in the images after introducing lignosulfonate amine. The morphology of these two kinds of hydrogel adsorbents is similar with glucan-g-poly(acrylic acid)/sodium lignosulfonate (GL-gPAA/SLS) hydrogels adsorbent (Wang et al., 2017). This 3D porous structure and rough surface may stem from partial overlapping or π-π stacking of aromatic ring structures (Li et al., 2016). Furthermore, with the increasing additive amount of cross-linking agent, the number and size of pores simultaneously increased. Hence, the HPAS hybrid hydrogel may possess an excellent adsorption property compared with those of HPA hydrogels. While there are no obvious pores found in the HPAS-5 hydrogel, which may attribute to the excessive cross-linking and LA chain blocking the formation of pores (Slouf et al., 2020). It can be concluded that the introduction of LA with 3D structure is a benefit for fabricating hierarchical porous structures and improving the adsorption efficiency. The HPAS with a proper amount of cross-linking agent can obtain a perfect structure with high content amino groups and rapid mass transfer 3D hierarchical porous structure, which may extremely enhance the adsorption capacity, and rapid removal of dye in aqueous solution (Akl et al., 2021).
The surface charge of hybrid hydrogels directly affects their adsorption properties. Different adsorption mechanisms for various adsorbents may give rise to different trends according to equilibrium adsorption capacity values with the change in pH. The zeta potential of hybrid hydrogels was determined by pH analysis in the pH range of 2~12, as depicted in Fig. 3. It was clear that the zeta potential decreased with increasing pH on the whole. The isoelectric point (pHip) is the pH value at which a substrate has a zeta potential of zero. The pHip of HPA composed of HP and PEGDGE were 10.6, suggesting that the surface charge of HPA was positive at pH<10.6 and negative at pH >10.6. The exposed abundant amino groups on the surface of HPA can interact with H+ and form cationic groups at pH <10.6, then show strong electrostatic interaction with anionic dyes (Zhu et al., 2016). The additive amount of PEGDGE in HPS affected the according surface charge of HPA. It was found that the pHpzc of HPA-4 was largest at pH 4~5 compared with other HPAs, indicating that HPA-4 exhibited the strongest electrostatic interaction with anionic dyes at pH 4~5. Simultaneously, the pHip of HPAS slightly shifted to low pH after introducing lignosulfonate amine; the according values were about 7.1~7.6 which may be resulting from the anionic sulfonic acid group in LA. Similarly, HPAS-4 showed the largest pHpzc at pH 4~5 compared with other HPAS, indicating that HPAS-4 exhibited the strongest.
3.2 Adsorption Property of HPAS
3.2.1 Effect of PEGDGE Ratio
The performance of HPA and HPAS hybrid hydrogels to remove CR were represented by their adsorption capacities. As a comparison experiment, 20 mg of adsorbent was soaked in 25 mL of 2000 mg/L of CR dye for 24h. As shown in Fig. 4a, HPA adsorbents showed stronger adsorption capacity. At the first initial 1h, the adsorption capacity of all HPA can reach 60% of equilibrium absorption capacity, and the absorption equilibrium is achieved after about 24 h oscillation. It was clear that the additive amount of cross-linking agent seriously affected the adsorption capacity of the final hybrid hydrogel. The adsorption capacity of HPA was in the order of HPA-4 > HPA-3 > HPA-2 > HPA-5. Especially, the adsorption capacity of HPA-4 was 2186 mg/g, which is very competitive compared to reports (Zhu et al., 2016). The excessive cross-linking limited the increasing adsorption ability of adsorbents. By comparison, HPAS hybrid hydrogel adsorbents showed enhanced adsorption efficiency. At the first initial 1 h, the adsorption capacity of all HPAS also can achieve nearly 60% of equilibrium absorption capacity, while the equilibrium was attained after only 3 h stirred. Simultaneously, the additive amount of PEGDGE also affected the adsorption capacity of the resulting hybrid hydrogel. The adsorption capacity of HPAS was in the order of HPAS-4 > HPAS-3 > HPAS-2 > HPAS-5. The adsorption capacity of HPAS-4 was up to 2568 mg/g, which was obviously higher than that of HPA-4. The introduction of LA in hybrid hydrogel clearly improved the resulting adsorption efficiency.
3.2.2 Effect of Concentration
The effect of the initial concentration of CR and MB on the adsorption capacity of HPA-4 and HPAS-4 was investigated, and the results are depicted in Fig. 5a. It was clear that HPA-4 and HPAS-4 could remove CR efficiency, while the adsorption capacity for cationic dye MB is much lower than the value for CR, which suggests that the electrostatic interaction is not the key adsorption mechanism (He et al., 2021). A comparison of the adsorption capacity of the different adsorbents for CR, MB, and Cu(II) is given in Fig. 5b. Lignosulfonate amine showed very poor adsorption ability for these pollutants (168 mg/g for CR, 79 mg/g for MB, and 37 mg/g for Cu(II)). HPA hybrid hydrogels composed of hyperbranched polyamide amine and PEGDGE exhibited tremendously enhanced adsorption performance. More shockingly, HPAS hybrid hydrogels showed excellent CR and Cu(II) adsorption performance. The adsorption capacity of HPAS-4 for CR and Cu(II) was 5120 and 249 mg/g, respectively. This result proved that the combination of LA into the hybrid hydrogels was an effective strategy to substantially enhance the adsorption performance of lignosulfonate due to the abundant hierarchical cavity and active groups. The effect of contact time on the adsorption of different dyes by HPAS-4 was also investigated and is shown in Fig. S2. It can be seen that HPAS-4 showed excellent efficiency in the adsorption of CR. At the initial 2 h, the adsorption capacity of CR can reach 70% of Qe, and reaches saturated adsorption capacity at about 3 h. However, HPAS-4 showed lower adsorption performance for MB; the saturated adsorption capacity reached after 10 h.
a Adsorption capacity of HPA-4 and HPAS-4 with different initial concentrations of dyes (m = 20 mg, V = 25 mL, T = 298 K, natural pH); b the ACs of CR, MB, and Cu(II) on LA, HPS-4, and HPAS-4 (C0dye = 6000 mg/L, C0metal = 100 mg/L, m = 20 mg, V = 25 mL, T = 298 K, and t = 24 h); c effect of pH on the adsorption performance of HPAS-4 (C0dye = 1000 mg/L, m = 20 mg, V = 25 mL, T = 298 K, and t = 24 h); d effect of dosage on the adsorption performance and removal efficiency (C0.dye = 3000 mg/L, m = 20 mg, V = 25 mL, T = 298 K, and t = 24 h)
3.2.3 Effect of pH
The adsorption performance and application environment of adsorbents were affected by the pH values of the aqueous solution. That is to say that the electrostatic interactions between adsorbent and adsorbates could be adjusted by changing the surface charge of adsorbents (He et al., 2021). The zeta potential values of adsorbents were measured and are depicted in Fig. 4; furthermore, the adsorption performance of HPAS-4 for CR and MB at different pH values was studied. Twenty milligrams of HPAS-4 was added into 25 mL CR and MB aqueous solutions (1000 mg/L) at different pH values and the mixtures were incubated at 25 °C for 24 h. Figure 5c shows that equilibrium adsorption capacity values of HPAS-4 decreased slightly with increasing pH. When the value of pH was 4~5, the qe of CR was the highest (1248 mg/g) while the pHpzc was also at the most level. That means the electrostatic interaction plays an important role in the adsorption of dyes. Nevertheless, the decrease in qe values is not obvious, which indicated that H-bond interactions dominate the adsorption process due to abundant N and O atoms of adsorbents and adsorbates (He et al., 2021). In addition, π-π interaction between the aromatic ring of lignosulfonate and dyes might be another important adsorption mechanism. The HPAS-4 showed poor adsorption performance for adsorbing MB compared to CR, which suggests the different adsorption mechanisms of CR and MB. H-bond interaction determined the CR adsorption for HPAS and plenty of N and O atoms from LA, PEGDGE, and HP benefit the adsorption of CR. While adsorption of MB mainly depended on π-π stacking from the aromatic ring of lignosulfonate and dyes, HP may shield this interaction to some extent. Furthermore, H-bond interactions were stronger than π-π stacking resulting in adsorbing more CR for HPAS (He et al., 2021).
3.2.4 Effect of Usage
The usage of adsorbent was an important factor in a given adsorption system, which could achieve the adsorption capacity and obtain an efficient method of using minimum adsorbent to adsorb maximum pollutants. Figure 5d shows the relationship of adsorption capacity with adsorbent dosage at a fixed initial concentration and mass of CR. With increasing of HPAS dosage, the adsorption capacity firstly increased and then decreased. The corresponding removal efficiency was increased then up to 100%. It could be explained that the increase in dosage increased the total surface area and adsorption sites, but the mass of the adsorbate is fixed; hence, the amount of pollutants adsorbed on the adsorbents per unit mass decreases. About 25 mg HPAS-4 can remove 100% CR, suggesting that fabricating HP/LA/PEGDGE hybrid hydrogel is an effective and efficient way to remove CR.
3.2.5 Adsorption Kinetics
The response rate of adsorption equilibrium between the adsorbent and pollutant is crucial in practical applications, the effect of contact time on HPAS-4 with CR and Cu(II) was investigated, and the results are shown in Fig. 6. It can be seen that HPAS-4 showed excellent efficiency in adsorption of CR and Cu(II). Two adsorption stages could be found for CR and Cu(II). At the initial 20 min, the adsorption capacity of CR can reach 50% of equilibrium adsorption capacity (Qe); then, the adsorption capacity reaches 90% at 80 min, and reaches saturated adsorption capacity at about 180 min. Similarly, the adsorption capacity of HPAS-4 for Cu(II) on contact time exhibited the same tendency. The adsorption capacity of Cu(II) can reach 50% of Qe at 50 min; then, the adsorption capacity reaches 90% at 110 min, and reaches saturated adsorption capacity at about 200 min. HPAS had a large amount of various amine and ether groups for interacting with dye and metal ions. The initial rapid adsorption rate was regarded as being associated with the number of accessible active sites. As the adsorption process goes on, more adsorption sites were occupied by dyes and steric hindrance increased, and the adsorption rate begin to delay. At last, adsorption equilibrium is achieved as the adsorption process continued.
The effect of the contact time (dashed lines: pseudo-first-order model; solid lines: pseudo-second-order model) on the adsorption of CR (a) and Cu(II) (b) with HPAS-4 (C0CR = 2000 mg/L, C0.Cu(II) = 100 mg/L, m = 20 mg, V = 25 mL, T = 298 K); the adsorption isotherms (solid lines: Langmuir model; dashed lines: Freundlich model) of CR (c) and Cu(II) (d) onto HPAS-4
Pseudo-first-order/second-order kinetics was employed to describe the experimental data to further understand the adsorption process as follows:
where k1 (min−1) and k2 (g•mg −1 •min −1)) are the adsorption rate constants for the kinetic pseudo-first-order/second-order model; Qe and Qt are the adsorption capacity (mg/g) of CR or Cu(II) on HPAS-4 at equilibrium and for a given contact time t, respectively. The fitting results were evaluated by the correlation coefficient (R2) and are listed in Table 2. It was obvious that the pseudo-second-order model is more suitable to describe the adsorption process for HPAS-4 according to the values of R2. The better fit of the pseudo-second-order model suggested that the adsorption of CR and Cu(II) on HPAS-4 is dependent on chemisorption or the rate-determining step may involve sharing or exchanging electrons, like H-bond (He et al., 2021). This result is consistent with previous reports on the adsorption of dyes and metal ions on amino or hyperbranched polyamide-functionalized cellulose (Kumari et al., 2016; Maqbool et al., 2021; Zhang et al., 2016). The K2 values listed in Table 2 also indicated that the adsorption is relatively rapid. It is further confirmed that the hierarchical porous structure of HPAS composed of 3D HP and LA with a highly branched cavity structure and a large number of accessible amines and ether groups can enhance the efficient adsorption of pollutants.
3.2.6 Adsorption Isotherms
Figure 6c and d depict the adsorption isotherms of CR and Cu(II) at 288, 298, and 308 K, respectively. As shown in Fig. 6c and d, the adsorption capacity of HPAS towards both CR and Cu(II) ascended with rising initial concentration, which did not reach a saturated state even at high concentration, indicating that high initial concentration supplied the impetus to counterwork the difficult mass transfer at the interface. This result was also consisting of MXene/PEI-modified sodium alginate aerogel adsorption behavior for Congo red from n aqueous solution (Feng et al., 2021). The Langmuir isotherm (Eq. (5)) and Freundlich isotherm (Eq. (6)) were used to unravel the interaction between HPAS and adsorbate molecules. Langmuir adsorption isotherm model assumes that the adsorption of a molecule is a single molecular layer surface adsorption, and the adsorbent surface is uniform with a finite number of identical sites; besides, adsorption will be out of dynamic equilibrium and no further adsorption when the adsorption reaches equilibrium, and the adsorbed particles are completely independent; there is no interaction between them (Wang et al., 2018). Freundlich adsorption isotherm is an empirical equation; it is predicted that the adsorbent surface is not uniform, and the amount of the adsorbate increases with the increasing of concentration; moreover, there is an interaction between the adsorbed molecules (Wang et al., 2018).
where qe (mg/g) is the adsorption capacity at the equilibrium and Ce (mg/L) is the equilibrium concentration of the adsorbate in the solution; qm (mg/g) is the saturated adsorption capacity. KL(L/mg) is the Langmuir adsorption constant related to the maximum adsorption capacity and the energy of adsorption. KF (L/mg) is the Freundlich adsorption constant related to the adsorption capacity and characterization of the interaction strength between the adsorbent and the adsorbate; the higher the KF value, the greater the adsorption capacity of the adsorbent. 1/n is the heterogeneity factor of the Freundlich model, on behalf of the intensity of the adsorption. When 0 < 1/n <1, it is indicated that the adsorption process is advantageous and easy to carry out; if 1/n =1, the adsorption reaction is linear, and there is no interaction between adsorbates; when 1/n >1, the adsorption process is negative and the adsorption reaction is more difficult. Adsorption isotherm parameters are summarized in Table 3. According to the results shown in Fig. 6c, d and Table 3, the R2 of the Langmuir isotherm models for CR and Cu(II) were higher than those of Freundlich isotherm models, which means that the Langmuir isotherm model was a better fit for the adsorption behavior of either CR and Cu(II) using the HPAS hybrid hydrogels. Hence, the CR and Cu(II) were predicted to be adsorbed via the mono-molecular layer on the surface of the HPAS hybrid hydrogels (Table 3).
The thermodynamic parameters were used to investigate the effect of temperature on the adsorption of CR and Cu(II), which can be calculated by the following Eqs. 7–9:
where Kd is the thermodynamic equilibrium constant, which can be calculated from Fig. S3. C0 is the initial concentration of the dye or metal (mg/L), Ce is the adsorption balance concentration (mg/L), V is the volume of dye or metal solution (mL), and m is the amount of adsorbent (mg). ΔG (kJ•mol−1) is the Gibbs free energy change, R (8.314 J•mol−1•K−1) is the gas constant, T (K) is the temperature, ΔH (kJ•mol−1) is the enthalpy change, and ΔS (J•mol−1•K−1) is the entropy change. The specific thermodynamic parameters (ΔG, ΔH, and ΔS) are listed in Table 4. The value of ΔG was negative at all the temperatures, indicating the adsorption of CR and Cu(II) was spontaneous, and the ΔG absolute values of CR and Cu(II) increased as the temperature rises, which demonstrated that the higher the temperature, the greater the amount of adsorption. The positive ΔH and ΔS respectively indicated the endothermic adsorption process and the random increases at the hybrid hydrogel-solution interface during the process of adsorption (Hu et al., 2020; Zhang et al., 2021c), which was consistent with the previous reporter (Bai et al., 2022; Yu et al., 2019). More importantly, HPAS-4 had higher CR ACs compared with most adsorbents summarized in Table 5. These results suggested that HPAS could be a promising adsorbent for removing organic dye wastewater.
3.3 Adsorption Mechanism of HPAS
The HPAS adsorbent with a hierarchical porous 3D structure and a large number of active amino and ether groups showed shocking removable ability for organic dyes especially CR from wastewater. In fact, several factors may strongly affect the adsorption properties of adsorbents. Herein, FTIR and XPS measurements were used for studying the adsorption mechanisms of HPAS hybrid hydrogels. As shown in Fig. 7a and Fig. S4 (the magnified FTIR spectra), the characteristic peaks between 647–900 and 2911 cm−1 of CR were observed on the adsorbed HPAS-4 + CR (Chatterjee et al., 2018). Furthermore, a new peak at 1508 cm−1 assigning N = N stretching vibration to CR was also found. The peak at 1067 cm−1 attributed to the SO3− group of CR after CR adsorption was shifted to 1037 cm−1 (HPAS-4 + CR), while the corresponding peak of SO3− in LA was at 1037 cm−1, which indicated the stronger interaction between CR and HPAS. This also indicated the electrostatic interaction between SO3− and protonated amine groups (≡N+) (Yao et al., 2017). In addition, the O–H and N–H stretching vibration at 3467 cm−1 was also shifted to 3330 cm−1 and became broader which clarifies the strong interaction between HPAS and CR via hydrogen bond formation. The slight shift of band positions was found at near 1650 cm−1 (O = C-NH) probably due to CR on HPAS-4 via hydrogen bonds (Mokhtar et al., 2020). These results indicated that hydrogen bond H-bond interactions may be the key mechanism of the CR adsorption process. After Cu(II) adsorption on HPAS, the peaks at 3500–3000 cm−1 attributed to the overlapping of O–H and N–H vibration became broader and obviously shifted, and the intensity of peak at 1650 cm−1 (O = C-NH) obviously decreased, indicating that oxygen- and nitrogen-containing groups on HPAS could serve as electron donors to harvest Cu(II) by complexation or chelation (Yu et al., 2019).
Figure 7b shows the new Cu2p and enhanced S2p peaks in XPS spectra of HPAS + CR and HPAS + Cu(II), suggesting the pollutant adsorption onto the HPAS. Figure 7c, d, and e depict the N1s, O1s, and S2p XPS spectra of HPAS before and after adsorption, respectively. The N1s and O1s peaks of pristine HPAS located at 399.46 and 532.46 eV obviously shifted to 399.38 and 531.8 eV after adsorbing CR, respectively. Furthermore, the S2p peak shape of pristine HPAS was different from that of HPAS after adsorbing CR. Similarly, after adsorbing Cu(II), the observed N1s and O1s peaks of HPAS were also shifted. The shift in the binding energy of the N1s and O1s peaks strongly indicated that O- and N-containing groups were involved in the adsorption interactions, which was also in good agreement with the FTIR analysis results (Yu et al., 2019). Figure 7f shows the high-resolution Cu2p XPS spectra; characteristic peaks located at 953.7, 936.2, 934.9, and 932.9 eV were individually assigned to zero-valent Cu(0), Cu(OH)2, Cu(I), and Cu(II), which indicated that the adsorbing Cu(II) was partially reduced for the native reducibility of terminated-amino groups in HPAS. It was further revealed that the removal of Cu(II) was accompanied by the generation of copper derivatives (Cu(0) and hydroxides) on HPAS except complexation. These results were consistent with the previous report (Yu et al., 2019). According to the comprehensive results of experimental, FTIR, and XPS analysis, the CR and Cu(II) adsorption mechanism of HPAS was proposed and is shown in Scheme 2. H-bond dominated the CR adsorption process of HPAS accompanied with electrostatic attraction and π-π stacking interaction occurred between aromatic rings of CR molecules and lignosulfonate, which is similar with ANF/PAMAM aerogels. However, the removal of Cu(II) by HPAS was primarily determined by the complexation/chelation of amine- and/or acylamino-groups accompanied with precipitation of copper derivatives on HPAS.
3.4 Reusability Test of HPAS
Given the importance of the reusability of adsorbent before practical application, the recyclability of HPAS hybrid hydrogels was investigated after adsorbing CR and Cu(II). Ethylene diamine (EDA) and ethanol were used as desorption solvents for desorbing CR, and the desorption of Cu(II) was conducted by 0.1 M HCl (He et al., 2021; Yu et al., 2019). Figure 8 shows the recyclability of HPAS-4 adsorbing CR and Cu(II). After 5 cycles of adsorption–desorption, HPAS-4 still exhibited excellent reusability and show high adsorption capacity and removal efficiency for CR and Cu(II), and more than 96% CR and 84% Cu(II) could be removed. In addition, the morphology of HPAS-4 before and after CR adsorption was further investigated by SEM measurement. As shown in Fig. 9, it was found that there still has no change before and after adsorption for HPAS. That is to say, the adsorbent has good recovery performance and great application prospects in the field of CR removal of wastewater.
4 Conclusions
In summary, we developed ultra-high-capacity lignosulfonate-based hybrid hydrogel adsorbents for Congo red removal by one-pot synthesized with HP, LA, and PEGDGE. A hierarchical network could be formed by cross-link reaction from HP with the branched 3D network and three-dimensional LA chain, and abundant N and O atoms were introduced into the hybrid hydrogels. The special 3D network and increased active sites improved the mass transfer of dye and affinity between dye and adsorbents; this hybrid hydrogel showed a super high capacity for CR compared with most other reports. The adsorption of dye fitted well with pseudo-second-order kinetics and the Langmuir isotherm, and the process was controlled by intraparticle diffusion. Investigation of the pH effect and adsorption mechanism indicated that the adsorption interaction mainly depends on H-bonds accompanied with electrostatic attraction and π-π stacking interaction. The adsorption of Cu(II) is mainly determined by the complexation/chelation accompanied with the precipitation of copper derivatives on HPAS. The results of this study provide a new method for the preparation of high-performance biomass-based adsorbents in the field of water purification by adsorption.
Data Availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Akl, Z. F., Zaki, E. G., & Elsaeed, S. M. (2021). Green hydrogel-biochar composite for enhanced adsorption of uranium. ACS Omega, 6(50), 34193–34205. https://doi.org/10.1021/acsomega.1c01559
Aliabadi, R. S., & Mahmoodi, N. O. (2018). Synthesis and characterization of polypyrrole, polyaniline nanoparticles and their nanocomposite for removal of azo dyes; sunset yellow and Congo red. Journal of Cleaner Production, 179, 235–245. https://doi.org/10.1016/j.jclepro.2018.01.035
Bai, L., Zhou, Y., Zhang, P., & Li, S. (2022). Construction of a carbon/lignosulfonate adsorbent to remove Pb2+ and Cu2+. ACS Omega, 7(1), 351–361. https://doi.org/10.1021/acsomega.1c04746
Beyki, M. H., Alijani, H., & Fazli, Y. (2016). Solvent free synthesized MnFe2O4@polyamid resin as a novel green nanohybrid for fast removing Congo red. Journal of Molecular Liquids, 216, 6–11. https://doi.org/10.1016/j.molliq.2016.01.017
Bhat, S. A., Zafar, F., Mondal, A. H., Mirza, A. Z., Haq, Q. M. R., & Nishat, N. (2020). Efficient removal of Congo red dye from aqueous solution by adsorbent films of polyvinyl alcohol/melamine-formaldehyde composite and bactericidal effects. Journal of Cleaner Production, 255, 122–262. https://doi.org/10.1016/j.jclepro.2020.120062
Chatterjee, S., Tran, H. N., Godfred, O.-B., & Woo, S. H. (2018). Supersorption capacity of anionic dye by newer chitosan hydrogel capsules via green surfactant exchange method. ACS Sustainable Chemistry Engineering, 6(3), 3604–3614. https://doi.org/10.1021/acssuschemeng.7b03929
Chen, L., Zhua, Y., Cui, Y., Dai, R., Shan, Z., & Chen, H. (2021). Fabrication of starch-based high-performance adsorptive hydrogels using a novel effective pretreatment and adsorption for cationic methylene blue dye: Behavior and mechanism. Chemistry Engineering Journal, 405, 126953.
Dogan, D., & Türkdemir, H. (2005). Electrochemical oxidation of textile dye indigo. Journal of Chemical Technology & Biotechnology, 80(8), 916–923. https://doi.org/10.1002/jctb.1262
Du, J., Dong, Z., Yang, X., & Zhao, L. (2021). Facile fabrication of polymeric quaternary ammonium salt hydrogel by radiation for dyes removal from aqueous solution. Radiation Physics and Chemistry, 188, 109670. https://doi.org/10.1016/j.radphyschem.2021.109670
Fan, X., Peng, L., Wang, X., Han, S., Yang, L., Wang, H., & Hao, C. (2022). Efficient capture of lead ion and methylene blue by functionalized biomass carbon-based adsorbent for wastewater treatment. Industrial Crop Production, 183, 114966. https://doi.org/10.1016/j.indcrop.2022.114966
Feng, Y., Wang, H., Xu, J., Du, X., Cheng, X., Du, Z., & Wang, H. (2021). Fabrication of MXene/PEI functionalized sodium alginate aerogel and its excellent adsorption behavior for Cr(VI) and Congo Red from aqueous solution. Journal Hazardous Materials, 416, 125777. https://doi.org/10.1016/j.jhazmat.2021.125777
Gao, S., Wei, G., Liu, Q., Liu, Q., Gao, T., & Yao, J. (2019). Efficient removal of congo red from pH-unregulated aqueous solutions by lignosulfonate-based polycatecholamine. Journal of Applied Polymer Science, 137, 48640. https://doi.org/10.1002/app.48640
Geng, J., Gu, F., & Chang, J. M. (2019). Fabrication of magnetic lignosulfonate using ultrasonic-assisted in situ synthesis for efficient removal of Cr(VI) and Rhodamine B from wastewater. Journal of Hazardous Materials, 375, 174–181. https://doi.org/10.1016/j.jhazmat.2019.04.086
Gu, F., Geng, J., Li, M., Chang, J., & Cui, Y. (2019). Synthesis of chitosan-lignosulfonate composites as an adsorbent for dyes and metal ions removal from wastewater. ACS Omega, 4, 21421–21430. https://doi.org/10.1021/acsomega.9b03128
Hao, J., Zhou, M., Zhou, S., Zhang, A., Pang, X., Sun, J., & Qiao, Y. (2021). Facile separation of aromatic pollutant /water by lignosulfonate based super paramagnetic composites. Colloids Surface A., 616, 126312. https://doi.org/10.1016/j.colsurfa.2021.126312
He, H., Zhuang, L., Chen, S., Liu, H., & Li, Q. (2016). Structure design of a hyperbranched polyamine adsorbent for CO2 adsorption. Green Chemistry, 18(21), 5859–5869. https://doi.org/10.1039/C6GC01416J
He, Z., Wu, F., Guan, S., Liu, L., Li, J., & Huang, Y. (2021). Polyamide amine/aramid nanofiber composite aerogels as an ultra-high capacity adsorbent for Congo red removal. Journal of Material Chemistry A, 9, 13320–13331. https://doi.org/10.1039/D1TA02801D
Hu, L., Guang, C., Liu, Y., Su, Z., Gong, S., Yao, Y., & Wang, Y. (2020). Adsorption behavior of dyes from an aqueous solution onto composite magnetic lignin adsorbent. Chemosphere, 246, 125757. https://doi.org/10.1016/j.chemosphere.2019.125757
Jana, S., Pradhan, S. S., & Tripathy, T. (2017). Poly(N, N-dimethylacrylamide-co-acrylamide) grafted hydroxyethyl cellulose hydrogel: A useful congo red dye remover. Journal of Polymers and the Environment, 26(7), 2730–2747. https://doi.org/10.1007/s10924-017-1168-1
Ji, K., Xu, H., Ma, X., Yin, J., & Jiang, X. S. (2017). Hyperbranched poly(ether amine)@poly(vinylidene fluoride) (hPEA@PVDF) porous membranes for selective adsorption and molecular filtration of hydrophilic dyes. Journal Material Chemistry A, 5(21), 10470–10479. https://doi.org/10.1039/C7TA02176C
Jiang, C. L., Wang, X. H., Qin, D. M., Da, W. X., Hou, B. X., & Hao, C. (2019). Construction of magnetic lignin-based adsorbent and its adsorption properties for dyes. Journal of Hazardous Materials, 369, 50–61. https://doi.org/10.1016/j.jhazmat.2019.02.021
Jiang, M., Niu, N., & Chen, L. (2022). A template synthesized strategy on bentonite-doped lignin hydrogel spheres for organic dyes removal. Separation Purification Technology, 285, 120376. https://doi.org/10.1016/j.seppur.2021.120376
Kumari, S., Mankotia, D., & Chauhan, G. S. (2016). Crosslinked cellulose dialdehyde for Congo red removal from its aqueous solutions. Journal of Environmental Chemical Engineering, 4(1), 1126–1136. https://doi.org/10.1016/j.jece.2016.01.008
Li, F., Wang, X., Yuan, T., & Sun, R. (2016). A lignosulfonate-modified graphene hydrogel with ultrahigh adsorption capacity for Pb(II) removal. Journal Material Chemistry a, 4(30), 11888–11896. https://doi.org/10.1039/C6TA03779H
Li, L., Zhang, J., & Wang, A. (2018). Removal of organic pollutants from water using superwetting materials. Chemical Record, 18(2), 118–136. https://doi.org/10.1002/tcr.201700029
Li, M. (2005). Studies on photo-electro-chemical catalytic degradation of acid scarlet 3R dye. Science in China, Series b: Chemistry, Life Sciences, & Earth Sciences, 48(4), 297–304. https://doi.org/10.1360/042004-69
Maqbool, M., Sadaf, S., Bhatti, H. N., Rehmat, S., Kausar, A., Alissa, S. A., & Iqbal, M. (2021). Sodium alginate and polypyrrole composites with algal dead biomass for the adsorption of Congo red dye: Kinetics, thermodynamics and desorption studies. Surface Interfaces, 25, 101183. https://doi.org/10.1016/j.surfin.2021.101183
Meng, X., Scheidemantle, B., Li, M., Wang, Y., Zhao, X., González, M. T., Singh, P., Pu, Y., Wyman, C. E., Ozcan, S., Cai, C. M., & Ragauskas, A. J. (2020). Synthesis, characterization, and utilization of a lignin-based adsorbent for effective removal of Azo Dye from aqueous solution. ACS Omega, 5(6), 2865–2877. https://doi.org/10.1021/acsomega.9b03717
Moghaddam, S. S., Alavi Moghaddam, M. R., & Arami, M. (2010). Coagulation/flocculation process for dye removal using sludge from water treatment plant: Optimization through response surface methodology. Journal of Hazardous Materials, 175(1–3), 651–657. https://doi.org/10.1016/j.jhazmat.2009.10.058
Mokhtar, A., Abdelkrim, S., Djelad, A., Sardi, A., Boukoussa, B., Sassi, M., & Bengueddach, A. (2020). Adsorption behavior of cationic and anionic dyes on magadiite-chitosan composite beads. Carbohydrate Polymer, 229, 115399. https://doi.org/10.1016/j.carbpol.2019.115399
Mondal, A. K., Wu, S., Xu, D., Zou, Q., Chen, L., Huang, L., Huang, F., & Ni, Y. (2021). Preparation of lignosulfonate ionic hydrogels for supercapacitors, sensors and dye adsorbent applications. International Journal of Biological Macromolecules, 187, 189–199. https://doi.org/10.1016/j.ijbiomac.2021.07.021
Panzarasa, G., Osypova, A., Ribera, J., Schwarze, F. W. M. R., Quasso, F., & Consolati, G. (2018). Hybrid adsorbent materials obtained by the combination of poly(ethylene-alt-maleic anhydride) with lignin and lignosulfonate. Journal Polymers and Environment, 26, 4293–4302. https://doi.org/10.1007/s10924-018-1299-z
Pu, Y., Xie, Z., Ye, H., & ShiW,. (2021). Amidation modified waste polystyrene foam as an efficient recyclable adsorbent for organic dyes removal. Water Science Technology, 83(9), 2192–2206. https://doi.org/10.2166/wst.2021.129
Sajjadi, M., Ahmadpoor, F., Nasrollahzadeh, M., & Ghafuri, H. (2021). Lignin-derived (nano)materials for environmental pollution remediation: Current challenges and future perspectives. International Journal of Biological Macromolecules, 178, 394–423. https://doi.org/10.1016/j.ijbiomac.2021.02.165
Slouf, M., Strachota, B., Strachota, A., Gajdosova, V., Bertschova, V., & Nohava, J. (2020). Macro-, micro- and nanomechanical characterization of crosslinked polymers with very broad range of mechanical properties. Polymers, 12, 2951. https://doi.org/10.3390/polym12122951
Sun, Y., Bai, L., Han, C., Lv, X., Sun, X., & Wang, T. (2022). Hybrid amino-functionalized TiO2/sodium lignosulfonate surface moleculary imprinted polymer for effective scavenging of methylene blue from wastewater. Journal Cleaner Production, 337, 130457. https://doi.org/10.1016/j.jclepro.2022.130457
Tang, Y. F., Ai, S. J., Lin, T. P., Li, Y. Q., & Zhou, R. (2021). Quaternary ammonium functionalized lignosulfonate for simultaneous adsorption of anionic/cationic dyes and desinfection. Chemistry Select, 6(33), 8537–8545. https://doi.org/10.1002/slct.202100475
Teng, X., Xu, H., Song, W., Shi, J., Xin, J., Hiscox, W. C., & Zhang, J. (2017). Preparation and properties of hydrogels based on PEGylated lignosulfonate Amine. ACS Omega, 2(1), 251–259. https://doi.org/10.1021/acsomega.6b00296
Wang, H., Yan, K., & Chen, J. (2021). Preparation of hydroxyapatite microspheres by hydrothermal self-assembly of marine shell for effective adsorption of Congo Red. Materials Letter, 304, 130573. https://doi.org/10.1016/j.matlet.2021.130573
Wang, X., Wang, Y., Hou, H., Wang, J., & Hao, C. (2017). Ultrasonic method to synthesize glucan-g-poly(acrylic acid)/sodium lignosulfonate hydrogels and studies of their adsorption of Cu2+ from aqueous solution. ACS Sustainable Chemistry Engineering, 5, 6438–6446. https://doi.org/10.1021/acssuschemeng.7b00332
Wang, Y. Y., Zhu, L. L., Wang, X. H., Zheng, W. R., Hao, C., Jiang, C. L., & Wu, J. B. (2018). Synthesis of aminated calcium lignosulfonate and its adsorption properties for azo dyes. Journal of Industrial and Engineering Chemistry, 6, 321–330. https://doi.org/10.1016/j.jiec.2017.12.030
Wei, S., Chen, W., Tong, Z., Jiang, N., & Zhu, M. (2021). Synthesis of a functional biomass lignin-based hydrogel with highswelling and adsorption capability towards Acid Red 73. Environment Science Pollution Research, 28, 51306–51320. https://doi.org/10.1007/s11356-021-14324-4
Xia, N. N., Zhang, H. Y., Hu, Z. H., Kong, F., & He, F. (2021). A functionalized bio-based materials with abundant mesopores and catechol groups for efficient removal of boron. Chemosphere, 263, 128202. https://doi.org/10.1016/j.chemosphere.2020.128202
Xu, L., Sun, P., Jiang, X., Chen, J., Wang, J., Zhang, H., & Zhu, W. (2020). Hierarchical quasi waxberry-like Ba5Si8O21 microspheres: Facile green rotating hydrothermal synthesis, formation mechanism and high adsorption performance for Congo red. Chemical Engineering Journal, 384(15), 123387. https://doi.org/10.1016/j.cej.2019.123387
Xu, W., Chen, Y., Kang, J., & Li, B. (2019). Synthesis of polyaniline/lignosulfonate for highly efficient removal of acid red 94 from aqueous solution. Polymer Bulletin, 76, 4103–4116. https://doi.org/10.1007/s00289-018-2586-5
Yadav, A., Bagotia, N., Sharma, A. K., & Kumar, S. (2021). Advances in decontamination of wastewater using biomass-based composites: A critical review. Science Total Environment, 2021(784), 147108. https://doi.org/10.1016/j.scitotenv.2021.147108
Yao, Q., Xie, J., Liu, J., Kang, H., & Liu, Y. (2014). Adsorption of lead ions using a modified lignin hydrogel. Journal of Polymer Research, 21, 465. https://doi.org/10.1007/s10965-014-0465-9
Yao, W., Yu, S., Wang, J., Zou, Y., Lu, S., Ai, Y., Alharbi, N. S., Alsaedi, A., Hayat, T., & Wang, X. (2017). Enhanced removal of methyl orange on calcined glycerol-modified nanocrystallined Mg/Al layered double hydroxides. Chemical Engineering Journal, 307, 476–486. https://doi.org/10.1016/j.cej.2016.08.117
Yiamsawas, D., Kangwansupamonkon, W., & Kiatkamjornwong, S. (2021). Lignin-based microgels by inverse suspension polymerization: Syntheses and dye removal. Macromolecular Chemistry and Physics, 222, 2100285. https://doi.org/10.1002/macp.202100285
Yu, D., Wang, Y., Wu, M., Zhang, L., Wang, L., & Ni, H. (2019). Surface functionalization of cellulose with hyperbranched polyamide for efficient adsorption of organic dyes and heavy metals. Journal of Cleaner Production, 232, 774–783. https://doi.org/10.1016/j.jclepro.2019.06.024
Zhang, F., Wang, B., Jie, P., Zhu, J., & Cheng, F. (2021a). Preparation of chitosan/lignosulfonate for effectively removing Pb(II) in water. Polymer, 228, 123878. https://doi.org/10.1016/j.polymer.2021.123878
Zhang, J., Lu, W., Li, H., Zhan, S., & Qiu, Z. (2021b). Polyethyleneimine-impregnated alkali treated waste bamboo powder for effective dye removal. Water Science and Technology, 83(5), 1183–1197. https://doi.org/10.2166/wst.2021.041
Zhang, J., Ma, C., Li, H., Wang, X., Ning, F., Kang, M., & Qiu, Z. (2021c). Polyethyleneimine modified magnetic microcrystalline cellulose for effective removal of congo red: Adsorption properties and mechanisms. Fiber Polymer, 22(6), 1580–1593. https://doi.org/10.1007/s12221-021-0543-7
Zhang, N., Zang, G.-L., Shi, C., Yu, H., & Sheng, G. (2016). A novel adsorbent TEMPO-mediated oxidized cellulose nanofibrils modified with PEI: Preparation, characterization, and application for Cu(II) removal. Journal of Hazardous Materials, 316, 11–18. https://doi.org/10.1016/j.jhazmat.2016.05.018
Zhang, Y., Liang, L., Chen, Y., Chen, X.-M., & Liu, Y. (2019). Construction and efficient dye adsorption of supramolecular hydrogels by cyclodextrin pseudorotaxane and clay. Soft Matter, 15(1), 73–77. https://doi.org/10.1039/C8SM02203H
Zhou, J., Lv, Q., & Luo, J. (2017). Efficient removal of organic dyes from aqueous solution by rapid adsorption onto polypyrrole-based composites. Journal of Cleaner Production, 167, 739–748. https://doi.org/10.1016/j.jclepro.2017.08.196
Zhu, W., Liu, L., Liao, Q., Chen, X., Qian, Z., Shen, J., Liang, J., & Yao, J. (2016). Functionalization of cellulose with hyperbranched polyethylenimine for selective dye adsorption and separation. Cellulose, 23, 3785–3797. https://doi.org/10.1007/s10570-016-1045-4
Funding
This work was supported by the Key Research and Development Program of Science and Technology, Department of Zhejiang Province [No. 2018C03004], the Public Technology Research Program of Zhejiang Province [No. LGF18B070005], Major (key) scientific and technological research projects of Jinhua (No. 2021–3-168), and Applied Basic Research Project of Longgang Institute of Zhejiang Sci-Tech University (No.LGYJY202109).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Ruichao Li and Nini Tian. The first draft of the manuscript was written by Ruichao Li and Nini Tian. Jiantang Jiang, Doufeng Wu, Min Xia, Huagang Ni, Peng Ye, Xingtong Zong, Liang Zong, and Yumei Wang commented on previous versions of the manuscript and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, R., Tian, N., Jiang, J. et al. Lignosulfonate-Based Hybrid Hydrogels Functionalized with Hyperbranched Polyamide Amine as Ultra-High-Capacity Adsorbent for Congo Red Removal from Aqueous Solutions. Water Air Soil Pollut 234, 140 (2023). https://doi.org/10.1007/s11270-023-06162-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-023-06162-6