Abstract
The catalytic synthesis of 2,5-diformylfuran (DFF) from 5-hydroxymethylfurfural (HMF) attracts extensive attention in the research of biomass conversion. However, it is a challenge to achieve high conversion of HMF under mild conditions with a heterogeneous catalyst, which can be easily separated and recycled. Herein, we report a novel cobalt-based catalyst (CoxOy-N@TiO2) for the oxidation of HMF to DFF with 30 % aqueous tert-butyl hydroperoxide as the oxidant. A series of Co-based catalysts were prepared by a typical impregnation method, and detected by XRD, TEM and FT-IR techniques. Based on the experimental results of catalytic test, it is found that a 91 % conversion of HMF and 40 % selectivity of DFF was obtained with the CoxOy-N@TiO2 catalyst at 80 °C within 5 h. In addition, the CoxOy-N@TiO2 can be recycled up to five times without significant loss of activity.
Graphical Abstract
The efficient oxidation of 5-hydroxymethyl furfural, a biomass-derived platform compound, to produce 2,5-diformylfuran is achieved using CoxOy-N@TiO2 catalyst under mild conditions.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
5-Hydroxymethylfurfural (HMF) has been considered as one of the most common platform chemicals prepared from the biomass, which can be further converted into many high-value-added fundamental chemicals such as 2,5-diformylfuran (DFF), 2,5-furandicarboxylic acid (FDCA), and so on [1–3]. The catalytic oxidation HMF to DFF has provoked much attention in recent years due to the potential application of DFF in the synthesis of drugs [4], fungicides [5], and new functional polymers [6–9]. High yields of DFF are obtained through many routes in the presence of classical oxidants NaOCl, BaMnO4, and pyridinium chlorochromate (PCC) [10–13]. For example, Amarasekara’s research group [26] also found that HMF could be efficiently oxidized to DFF in 63–89 % yield using Mn(III)-salen catalyst and sodium hypochlorite in a phosphate buffer-CH2Cl2 biphasic system at room temperature. However, these stoichiometric oxidants could release serious toxicity wastes after oxidation was finished. Thus, the use of environmentally friendly oxidant such as tert-butyl hydrogen peroxide, hydrogen peroxide or molecular oxygen is desirable [14–16]. We have found that the homogeneous catalytic oxidation of HMF to produce DFF was successfully performed using CuI and 1-hydroxybenzotriazole as the catalysts in which a 93 % conversion and 99 % selectivity of DFF was obtained [17]. The drawback of this reaction system is that the recycle of catalyst is difficult and the temperature is relatively high when compared with the other processes. On the other hand, the supported noble metals (such as Au, Pt, Pd, etc.) were often employed to catalyze the selective oxidation of HMF to DFF using air or hydrogen peroxide as oxidants [18–21]. Moreover, Ru-based catalytic system has also been developed for the efficient and selective oxidation of HMF [22, 23]. Although noble metallic catalyst systems can afford high conversion and good product selectivity, the expensive cost restricts their further industrial applications. Some investigations on the cost–effective non-noble transition metal catalysts to replace the noble metals for the selective conversion of HMF have been explored. Therein, the vanadium based catalysts have been employed for the oxidation of HMF into DFF and a very high yield can be achieved [24]. Furthermore, Moreau et al. [25] reported that a complete transformation of HMF and a 90 % yield of DFF as the main product were obtained with the monolayered V2O5/TiO2 catalyst. Metallic cobalt-based heterogeneous catalysts are well-known to be active in various oxidation processes [26–30]. For instance, the water oxidation and splitting has also been successfully performed using Co-based heterogeneous catalysts [29, 30]. However, there are few examples for the selective oxidation of biomass-derived feedstock. Very recently, our research group reported a highly efficient and heterogeneous CoxOy-N-based catalyst system for the cascade oxidative condensation of furfural with the aliphatic alcohols under mild conditions [31]. Intrigued by the aforementioned work, the selective oxidation of HMF with Co-based catalysts has also been developed with 30 % aqueous tert-butyl hydroperoxide (TBHP) as oxidant. The CoxOy-N@TiO2 material exhibits a superior catalytic activity in the oxidation of HMF to produce DFF, and a 91 % conversion and 40 % selectivity was obtained at 80 °C for 5 h.
Experimental Section
Reagents
HMF, Co(OAc)2·4H2O, Al2O3, ZnO, SiO2 and Kaolin were provided by Aladdin Reagent Co. Ltd (Shanghai, China) and used as received. 30 % aqueous TBHP and H2O2 are of analytical grade and obtained from commercial source. DFF as the standard sample is purchased from Alfa Aesar. Dimethylsulfoxide (DMSO) and other solvents are purified by distillation. All other reagents are analytical grade and obtained from commercial. Oxygen supplied in a high-pressure cylinder was used through a reducing valve without further treatment.
Characterization of Catalysts and Product Analysis
X-ray diffraction (XRD) measurement was performed by using a diffractometer with Cu Ka radiation (0.02° resolution) in the range from 10° to 80° [2θ]. Moreover, the surface morphology and particle size of the platinum catalysts were obtained by using a transmission electron microscope (TEM: JEM-2100, JEOL). The BET surface areas, pore volumes, and average pore diameters of the prepared samples were obtained from N2 (77 K) adsorption measurement using a Micro-meritics ASAP2020M system. Therein, the samples were pretreated under vacuum at 250 °C for 4 h before measurement. The average pore diameter data were calculated according to the Barrett–Joyner–Halenda (BJH) model in absorption and desorption periods.
The quantitative analysis of the product was performed on a GC with a hydrogen ion flame detector; the qualitative analysis of the product was carried out on an Agilent 6890/5973 gas chromatograph–mass spectrometer (GC–MS) instrument.
The Preparation of TiO2 Support
The support TiO2 is prepared by the homogenous precipitation similar to literature method [32]. In general, 20 g TiCl4 (17 wt%) and 15 g urea were added into de-ionized water, and the mixture was heated to 100 °C under stirring and kept for 12 h. After the reaction, the obtained white precipitate was filtered and washed to the neutral pH with water. The solid was dried at 110 °C overnight. Then, the crystal product was grinded to the power and further calcined at 300 °C for 5 h. Finally, the obtained TiO2 material was light yellow power (yield is about 5.2 g).
The Preparation of Co-Based Catalysts
In a typical procedure, the mixture of Co(OAc)2.4H2O (1 mmol, 0.25 g) and 1,10-phenanthroline (2 mmol, 0.4 g) in ethanol was stirred for 20–30 h at room temperature. Then, the TiO2 support (2 g) was added and the whole reaction mixture was stirred at 60 °C for 4–5 h. After the reaction mixture was cooled down to room temperature, ethanol was removed slowly under vacuum. The remaining solid was dried at 60 °C over night. Then the dried solid was ground to powder and calcined at 800 °C for 2 h in nitrogen atmosphere and cooled down to room temperature. The final powder was designated as CoxOy-N@TiO2. The theoretical Co content is 3 wt%, which is based on TiO2. Similarly, the other Co-based catalyst (CoxOy-N@SiO2, CoxOy-N@Kaolin, et al.) were also synthesized by the above process.
General Procedure for the Oxidation of 5-Hydroxymethylfurfural (HMF)
All oxidation experiments are performed in a 120 mL autoclave equipped with magnetic stirring and automatic temperature control. A typical procedure for the oxidation of HMF is as follows. CH3CN (15 mL) solution of HMF (1.26 g, 0.01 mol), Co/TiO2 (0.55 g) and 30 % aqueous TBHP (5 eq.) was placed into the reactor and the reactor was sealed. Under being stirred, the autoclave was preheated to 80 °C, and kept for 5 h. After reaction, the autoclave was cooled down to room temperature and the as-obtained mixture was analyzed by GC and GC–MS.
Analysis and Separation of Oxidized Products
The products were analyzed with the internal standard technique by gas chromatography using a flame ionization detector (all products are determined on GC–MS). Moreover, for the oxidation of HMF, the product is separated from the product solutions as follows.
After being distilled to remove the solvent (CH3CN), the reaction mixture was transferred into a flask and saturated aqueous NaHSO3 solution was added, then the mixture was stirred for 2 h under N2 atmosphere. The reaction mixture was placed in the refrigerator for 3 h, and then the resulting solid was collected by filtration. The as-obtained solid was washed with aqueous solution of sodium chloride to remove HMF and by-products. After that, the washed solid was transferred into a two-neck flask, the solution of HCl was added under N2 atmosphere and the mixture was stirred at room temperature for 1 h, then at 50 °C for 2 h. Finally, the product was regenerated and extracted with diethyl ether. As a result, the purity is more than 99 % from GC analysis. The difference between the GC yield and separated yield is less than 10 %.
Result and Discussions
Characterization of Co-Based Catalysts
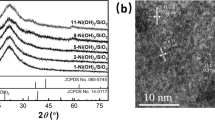
The XRD patterns of the supported CoxOy-N catalyst were shown in Fig. 1. All of samples show the characteristic peaks of Co, Co2O3 and Co3O4, which is consistent with those reported by the Ref. [33]. For CoxOy-N@Kaolin catalysts, no peaks of the supports were observed. However, the XRD patterns of CoxOy-N@TiO2, CoxOy-N@SiO2, CoxOy-N@ZnO and CoxOy-N@Al2O3 displayed the characteristic diffraction peaks of TiO2, SiO2, ZnO and Al2O3.
The Co content of CoxOy-N@TiO2 as determined in the XPS and ICP studies were 1.87 and 1.90 wt% (see Figure S2 and Table S1 in supporting information). According to the best fit of Co2p3/2 peaks, peaks with binding energy of 784.86, 781.95, 781.23, 780.52 and 777.57 eV can be attributed to a satellite peak of cobalt, bonding of cobalt and nitrogen, Co2O3, CoO and Co0, respectively (see Fig. 2). The atomic conc. % of them are approximately 2:3:3:2:1.
Moreover, the fresh and the used CoxOy-N@TiO2 catalysts were further characterized by TEM technique. As shown in Fig. 3, the size of most cobalt oxide-N particles in the samples is about 50 nm. On the other hand, the size of particle keeps almost unchanged after being employed in the catalytic oxidation of HMF with 30 % aqueous TBHP as oxidant.
At last, textural properties of CoxOy-N@TiO2 derived from the nitrogen physisorption are shown in Table 1. The BET surface area and pore volume of the fresh CoxOy-N@TiO2 catalyst is 110 m2 g−1 and 0.0019 cm3 g−1. After being reused for four times, the BET surface area of catalyst and pore volume become a little smaller, which can be contributed to precipitation of some by-products in the catalytic processes (shown in Table 1).
The Catalytic Conversion of HMF into DFF Using Co-Based Catalysts
The selective oxidation of HMF to DFF was performed in CH3CN using different Co-based catalysts where the reaction equation is given in the Scheme 1. The experimental results are summarized in Table 2. It is found that a 91 % conversion of HMF and 40 % selectivity of DFF was obtained when CoxOy-N@TiO2 was used as the catalyst. Then, the catalytic activities of CoxOy-N@Kaolin, CoxOy-N@ZnO, CoxOy-N@Al2O3 and CoxOy-N@SiO2 were also investigated in which the conversion of HMF was respectively 80, 66, 65, or 51 % and the selectivity of DFF was 31, 23, 20 or 33 %, respectively (entries 2, 3, 4 and 5). Obviously, the CoxOy-N@TiO2 catalyst exhibited better catalytic performance in the selective oxidation of HMF into DFF. Furthermore, several oxidants including O2 and 30 % aqueous H2O2 were also used to replace 30 % aqueous TBHP for the oxidation of HMF to DFF in the presence of the CoxOy-N@TiO2 catalyst (shown in entries 6 and 7 of Table 2). As a result, it was found that the conversion of HMF is only 5 % using molecular oxygen as oxidant. Otherwise, the conversion is 36 % when the 30 % aqueous H2O2 was employed as oxidant. These data indicated that 30 % aqueous TBHP is preferable oxidant for the oxidation of HMF with the CoxOy-N@TiO2 catalyst. That is probably because that TBHP is a stronger oxidant agent than H2O2 and O2, which can activate C–H bond more easily. In this work, the catalytic activity of the metallic cobalt is weaker than precious metal, so TBHP is preferable for this reaction [22].
In addition, the turnover frequency (TOF) and turnover number (TON) studies indicate that the CoxOy-N@TiO2 catalyst can give higher TON and TOF than the other structurally similar catalysts (see Table 2). In addition, Vuyyuru and Strasser [34] reported heterogeneous chemical and electrochemical catalysis the transformation of HMF to DFF. The conversion of HMF and selectivity of DFF are 85.55 and 9.63 % in the former catalytic system, and they are respectively 29.03 and 26.51 % in the latter. The study from Eyjólfsdóttir‘s group showed the supported Ru(OH)x makes the conversion of HMF up to over 90 %, but the selectivity of DFF is less than 10 % [35]. Obviously, the CoxOy-N@TiO2 catalyst system exhibites a higher effiency in the transformation of HMF to DFF.
In the following, different solvents including NMP, CH3CN, DMSO, t-butanol, toluene and THF have been employed in the selective oxidation of HMF to DFF in the presence of the CoxOy-N@TiO2 catalyst (Table 3). Herein, a 38 or 45 % conversion of HMF was obtained, when Ether or THF was employed as the solvent. In addition, the conversion of HMF in t-butanol, toluene, NMP, and DMSO solvent are 55, 60, 72 and 75 %, respectively. So CH3CN showed the best performance.
Besides, the effect of reaction time on the oxidation of HMF into DFF was also investigated. As shown in Fig. 4, the conversion of HMF and the selectivity of DFF kept almost constant after reacting for 5 h. So the investigations on this reaction would be operated for 5 h in our further studies.
In order to study the stability of catalyst, the recycling experiment of the CoxOy-N@TiO2 has been performed in the coxidation of HMF into DFF. In each run, the catalyst was separated, and washed with distilled water and anhydrous ethanol in turn, then dried at 60 °C for 12 h before being reused in the next run. The experimental results are shown in Fig. 5, the conversion of HMF and selectivity of DFF has no significant change, which shows that the CoxOy-N@TiO2 catalyst possesses good stability, great reusability and recyclability.
Conclusions
In summary, a novel oxidation process of HMF to DFF using heterogeneous CoxOy-N@TiO2 catalyst has been developed. This method allows the oxidation to be performed with the simple t-BOOH as the oxidant, in which a 91 % conversion of HMF and 40 % selectivity of DFF was obtained at 80 °C for 5 h. It will provide a promising route for the application of biomass-derived platform compounds.
References
Van Putten, R.-J., van der Waal, J.C., de Jong, E., Rasrendra, C.B., Heeres, H.J., de Vries, J.G.: Hydroxymethylfurfural, a versatile platform chemical made from renewable resources. Chem. Rev. 113, 1499–1597 (2013)
Caes, B.R., Teixeira, R.E., Knapp, K.G., Raines, R.T.: Biomass to furanics: renewable routes to chemicals and fuels. ACS Sustain. Chem. Eng. 3, 2591–2605 (2015)
Gallezot, P.: Conversion of biomass to selected chemical products. Chem. Soc. Rev. 41, 1538–1558 (2012)
Hopkins, K.T., Wilson, W.D., Bender, B.C., McCurdy, D.R., Hall, J.E., Tidwell, R.R., Kumar, A., Bajic, M., Boykin, D.W.: Extended aromatic furan amidino derivatives as anti-pneumocystis carinii agents. J. Med. Chem. 41, 3872–3878 (1998)
Corma, A., Iborra, S., Velty, A.: Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 107, 2411–2502 (2007)
Amarasekara, A.S., Green, D., Williams, L.D.: Renewable resources based polymers: synthesis and characterization of 2,5-diformylfuran-urea resin. Eur. Polym. J. 45, 595–598 (2009)
Delidovich, I., Hausoul, P.J.C., Deng, L., Pfützenreuter, R., Rose, M., Palkovits, R.: Alternative monomers based on lignocellulose and their use for polymer production. Chem. Rev. 116, 1540–1599 (2016)
Xiang, T., Liu, X., Yi, P., Guo, M., Chen, Y., Wesdemiotis, C., Xu, J., Pang, Y.: Schiff base polymers derived from 2,5-diformylfuran. Polym. Int. 62, 1517–1523 (2013)
Ma, J., Yu, X., Xu, J., Pang, Y.: Synthesis and crystallinity of poly(butylene 2,5-furandicarboxylate). Polymer 53, 4145–4151 (2012)
El-Hajj, T., Martin, J.C., Descotes, G.: Dérivés de l’hydroxyméthyl-5 furfural. I. Synthése de dérivés du di-et terfuranne. J. Heterocycl. Chem. 20, 233–235 (1983)
Cottier, L., Descotes, G., Lewkowski, J., Skowronski, R.: Oxidation of 5-hydroxymethyl furfural under sonochemical conditions. Pol. J. Chem. 68, 693–698 (1994)
Cottier, L., Descotes, G., Lewkowski, J., Skowronski, R.: Ultrasonically accelerated syntheses of furan-2,4-dicarbaldehyde from 5-hydroxymethyl-2-furfural. Org. Prep. Proced. Int. 27, 564–566 (1995)
Reijendam, J.W., Heeres, G.J., Janssen, M.J.: Polyenyl-substituted furans and thiophenes. A study of the electronic spectra. Tetrahedron 26, 1291–1301 (1970)
Lv, G., Wang, H., Yang, Y., Deng, T., Chen, C., Zhu, Y., Hou, X.: Graphene oxide: a convenient metal-free carbocatalyst for facilitating aerobic oxidation of 5-hydroxymethylfurfural into 2,5-diformylfuran. ACS Catal. 5, 5636–5646 (2015)
Nie, J., Xie, J., Liu, H.: Efficient aerobic oxidation of 5-hydroxymethylfurfural to 2,5-diformylfuran on supported Ru catalysts. J. Catal. 301, 83–91 (2013)
Partenheimer, W., Grushin, V.V.: Synthesis of 2,5-diformylfuran and furan-2,5-dicarboxylic acid by catalytic air-oxidation of 5-hydroxymethylfurfural: unexpectedly selective aerobic oxidation of benzyl alcohol to benzaldehyde with metal = bromide catalysts. Adv. Synth. Catal. 343, 102–111 (2001)
Tong, X., Sun, Y., Bai, X., Li, Y.: Highly efficient aerobic oxidation of biomass derived 5-hydroxymethyl furfural to produce 2,5-diformylfuran in the presence of copper salts. RSC Adv. 4, 44307–44311 (2014)
Zhu, Y., Shen, M., Xia, Y.: Au/MnO2 nanostructured catalysts and their catalytic performance for the oxidation of 5-(hydroxymethyl)furfural. Catal. Commun. 64, 37–43 (2015)
Zhou, C., Deng, W., Wan, X., Zhang, Q., Yang, Y., Wang, Y.: Functionalized carbon nanotubes for biomass conversion: the base-free aerobic oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid over platinum supported on a carbon nanotubecatalyst. ChemCatChem 7, 2853–2863 (2015)
Casanova, O., Iborra, S., Corma, A.: Biomass into chemicals: aerobic oxidation of 5-hydroxymethyl-2-furfural into 2,5-furandicarboxylic acid with gold nanoparticle catalysts. ChemSusChem 2, 1138–1144 (2009)
Wan, X., Zhou, C., Chen, J., Deng, W., Zhang, Q., Yang, Y., Wang, Y.: Base-free aerobic oxidation of 5-hydroxymethyl-furfural to 2,5-furandicarboxylic acid in water catalyzed by functionalized carbon nanotube-supported Au–Pd alloy nanoparticles. ACS Catal. 4, 2175–2185 (2014)
Hansen, T.S., Sádaba, I., García-Suárez, E.J., Riisager, A.: Cu catalyzed oxidation of 5-hydroxymethylfurfural to 2,5-diformylfuran and 2,5-furandicarboxylic acid under benign reaction conditions. Appl. Catal. A Gen. 456, 44–50 (2013)
Takagaki, A., Takahashi, M., Nishimura, S., Ebitani, K.: One-pot synthesis of 2,5-diformylfuran from carbohydrate derivatives by sulfonated resin and hydrotalcite-supported ruthenium catalysts. ACS Catal. 1, 1562–1565 (2011)
Sádaba, I., Gorbanev, Y.Y., Riisager, A., Putluru, S.S.R., Berg, R.W., Riisager, A.: Catalytic performance of zeolite-supported vanadia in the aerobic oxidation of 5-hydroxymethylfurfural to 2,5-diformylfuran. ChemCatChem 5, 284–293 (2013)
Moreau, C., Durand, R., Pourcheron, C., Tichit, D.: Selective oxidation of 5-hydroxymethylfurfural to 2,5-furan-dicarboxaldehyde in the presence of titania supported vanadia catalysts. Surf. Sci. Catal. 108, 399–406 (1997)
Amarasekara, A.S., Green, D., McMillan, E.: Efficient oxidation of 5-hydroxymethylfurfural to 2,5-diformylfuran using Mn(III)-salen catalysts. Catal. Commun. 9, 286–288 (2008)
Jia, C., Schwickardi, M., Weidenthaler, C., Schmidt, W., Korhonen, S., Weckhuysen, B.M., Schüth, F.: Co3O4–SiO2 nanocomposite: a very active catalyst for CO oxidation with unusual catalytic behavior. J. Am. Chem. Soc. 133, 11279–11288 (2011)
Banerjee, D., Jagadeesh, R.V., Junge, K., Pohl, M.-M., Radnik, J., Brückner, A., Beller, M.: Convenient and mild epoxidation of alkenes using heterogeneous cobalt oxide catalysts. Angew. Chem. 126, 4448–4452 (2014)
Kanan, M.W., Nocera, D.G.: In situ formation of an oxygen-evolving catalyst in neutral water containing phosphate and Co2+. Science 321(47), 1072–1075 (2008)
Wang, J.H., Cui, W., Liu, Q., Xing, Z.C., Asiri, A.M., Sun, X.P.: Recent progress in cobalt-based heterogeneous catalysts for electrochemical water splitting. Adv. Mater. 28(2), 215–230 (2016)
Yu, L., Liao, S., Ning, L., Xue, S., Liu, Z., Tong, X.: Sustainable and cost–effective protocol for cascade oxidative condensation of furfural with aliphatic alcohols. ACS Sustain. Chem. Eng. 4, 1894–1898 (2016)
Chen, Y., Lin, A., Gan, F.: Preparation of nano-TiO2 from TiCl4 by dialysis hydrolysis. Powder Technol. 167, 109–116 (2006)
Jagadeesh, R.V., Junge, H., Pohl, M.-M., Radnik, J., Brückner, A., Beller, M.: Selective oxidation of alcohols to esters using heterogeneous Co3O4-N@C catalysts under mild conditions. J. Am. Chem. Soc. 135, 10776–10782 (2013)
Vuyyuru, K.R., Strasser, P.: Oxidation of biomass derived 5-hydroxymethylfurfural using heterogeneous and electrochemical catalysis. Catal. Today 195, 144–154 (2012)
Ståhlberg, T., Eyjólfsdóttir, E., Gorbanev, Y.Y., Sádaba, I., Riisager, A.: Aerobic oxidation of 5-(hydroxymethyl)furfural in ionic liquids with solid ruthenium hydroxide catalysts. Catal. Lett. 142, 1089–1097 (2012)
Acknowledgments
We are grateful for the support of the National Natural Science Foundation of China (No. 21336008) and Innovation and Entrepreneurship Training Program for College Students (No. 201410060054).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
12649_2016_9724_MOESM1_ESM.docx
Electronic Supplementary Information (ESI) available: IR spectra of the supported CoxOy-N catalyst, full XPS spectrum of CoxOy-N@TiO2 catalyst, XPS analysis for the composition of CoxOy-N@TiO2 catalyst, the results of both GC–MS and GC analysis of the catalytic products can be found online version. See doi: 10.1039/x0xx00000x (DOCX 180 kb)
Rights and permissions
About this article
Cite this article
Ning, L., Liao, S., Sun, Y. et al. The Efficient Oxidation of Biomass-Derived 5-Hydroxymethyl Furfural to Produce 2,5-Diformylfuran Over Supported Cobalt Catalysts. Waste Biomass Valor 9, 95–101 (2018). https://doi.org/10.1007/s12649-016-9724-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-016-9724-9