Abstract
The use of native starch as a thermoplastic polymer is limited by its fragility and high water absorption. Due to the presence of several hydroxyl groups in its structure, water acts as a natural plasticizer of starch, modifying its properties. It is necessary to chemically modify starch molecules by replacing hydroxyl groups with other functional groups to reduce water absorption. Chemical modification of starch granules also alters its swelling and gelatinization behavior. In this contribution we describe the chemical modification of starch and its influence on its hydrophilicity and heat resistance. Acetic acid, maleic anhydride and octanoyl chloride were used as derivatizing reagents. The effectiveness of the treatments was evaluated by means of infrared spectroscopy. Different tests were conducted in order to evaluate the influence of the different chemical modifications on starch structure and properties. Results showed that the treatments effectively reduced starch moisture susceptibility, while substantially altering other properties such as amylose content, swelling power, solubility, and heat resistance. Finally, films were prepared from native and derivatized starch and their surface polarity was evaluated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Natural biodegradable polymers appear as an attractive solution to the problems associated with the production, use and disposal of conventional plastics.
Starch is a very attractive source for the development of biodegradable plastics. Starch is one of the lowest cost biodegradable materials currently available in the global market. It is a versatile biopolymer with great potential for applications in non-food industries [1, 2]. Starch is the most important form of energy storage in plants [3]. It can be found in the form of discrete semi crystalline particles, whose size, shape, morphology and composition depend on the botanical origin. These particles are called granules, and have a diameter ranging from 1 to 100 µm and can present different regular or irregular shapes (spherical, oval, prismatic, lenticular) depending on their botanical origin [4–8].
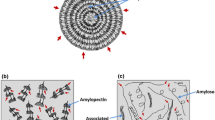
The starch granules are partially crystalline and are composed mainly of two glucopyranose homopolymers: amylose and amylopectin. Amylose is an essentially linear polymer composed of D-glucopyranose units linked by α-1,4 links (Fig. 1) [9, 10]. On the other hand, amylopectin is a highly branched polysaccharide. It consists of thousands of d-glucopyranose units linked by α-1,4 links (Fig. 1).
Starch granules are insoluble in water. The properties of starch depend largely on the content of amylose and amylopectin as well as the shape and size of the granules. The presence of numerous hydroxyl groups determines the hydrophilic nature of starch.
While it is possible to produce plastics from native starch, they are not suitable for use as packaging material due to their poor mechanical properties and high moisture susceptibility. As a result, numerous physical and chemical modification methods have been developed to enhance starch’s positive characteristics, reduce its undesirable qualities and add new attributes [11–18].
Chemical modification of starch is carried out by the reaction or treatment of native starch with chemical reagents to introduce new functional groups, induce the cleavage of molecular chains, promote oxidation of molecules or induce molecular rearrangement [18]. The main methods of chemical modification can be divided into conversion and derivatization. Derivatization refers to the modification of starch by reaction of the hydroxyl groups, replacing them with other groups with the desired functionality. Many types of derivatized starches can be obtained.
The objectives of the present study were to reduce the moisture absorption of potato starch by substituting hydroxyl groups with voluminous hydrophilic groups, to study the overall effect of derivatization on the properties of potato starch, with special emphasis on physical–chemical properties and structure, and to study the effect of the derivatizing agents molecular weight.
Materials and Methods
Materials
Native potato starch was kindly provided by Avebe Argentina SA (Argentina). Acetic acid and acetic anhydride were purchased from Cicarelli (Argentina). Octanoyl chloride, glycerol, potato amylose and amylopectin were purchased from Aldrich (USA). All other reagents used were of analytical grade.
Chemical Treatments
Three different modifications were done in order to derivatize potato starch. The modifiers were acetic acid, maleic anhydride and octanoyl chloride. The molecular weight of acetic acid is 60 g/mol, the maleic anhydride is 98 g/mol, and octanoyl chloride is 162.5 g/mol.
Derivatization treatments were carried out using 0.1 mol of substituent per gram of starch [19]. The treatments were performed under the following conditions (Fig. 2):
-
Acetylation: 50 g of starch were immersed in a solution containing acetic acid (80 ml), acetic anhydride (40 ml), perchloric acid (0.5 ml) and toluene (100 ml) under constant stirring at room temperature for 4 h [19].
-
Maleinization: 50 g of starch were immersed in a solution of maleic anhydride (5 g) in acetone (500 ml) under continuous stirring at room temperature for 24 h [19]. During the reaction, the aliphatic ring of the maleic anhydride molecule opens up resulting in a linear chain.
-
Octanoylation: 50 g of starch were immersed in a solution of octanoyl chloride (33 ml) in acetone (500 ml) under continuous stirring at room temperature for 24 h.
Fourier Transformed Infrared Spectroscopy (FTIR)
A Mattson Genesis II FTIR in diffuse reflectance mode (DRIFT) between 400 and 4,000 cm−1 was used to verify the effectiveness of the derivatization treatments. An average of 32 scans with a resolution of 2 cm−1 at room temperature was used. The obtained spectra were normalized considering the intensity of the peak at 2,933 cm−1, which corresponds to the CH2 group. A “Derivatization Index” was calculated as the ratio of the intensity of the normalized ester peaks to the CH2 peaks [20].
Amylose Content Determination
The determination of amylose content was performed according to the colorimetric technique based on the phenomenon of development of a coordination complex between iodine and amylose molecules [21–25]. This method is based on the determination of the amount of complex formed between iodine and amylose by colorimetric analysis. A calibration curve was determined from mixtures of different proportions of commercial amylose and amylopectin, both derived from potato starch. For this purpose, a solution of 100 mg of amylose and 100 mg of amylopectin in 9 ml of 1 N NaOH and 1 ml of C2H5OH were prepared in separate containers. Prepared solutions were allowed to stand for 18 h at room temperature. Following, solutions containing 0, 10, 20, 25 and 30 % amylose were prepared. Iodine reagent was added (0.2 g I2 and 2.0 g of KI in 100 ml of distilled water).
The absorbance at 620 nm was measured using an Agilent 8453 spectrophotometer. Solutions were prepared from native starch and other modified starches. The amylose content of each modified starch was determined by direct comparison with the calibration curve.
X-Ray Diffraction (XRD)
In order to determine the influence of chemical modification in the crystal structure and the crystallinity of potato starch, X-Ray diffractometry was carried out. Powder native and modified starch samples were analyzed using a XRD Philips PW1710 diffractometer (k = 0.179 nm). The samples were analyzed in the range 2θ = 1–40° at a speed of 1°/min. The baseline was taken as a straight line between the intensity of the peak at 2θ = 2.5° and the peak at 2θ = 40°. The crystallinity was determined according to Eq. 1 [26] which takes into account the individual contributions of amorphous and crystalline phases.
Study of Granule Morphology (POM, SEM)
The influence of different derivatization treatments on the morphology of starch granules was studied by means of Polarized Optical Microscopy (POM) and Scanning Electron Microscopy (SEM). An optical Olympus SZH 10 microscope with a magnification of 35X with cross polarizers was used to reveal the existence of crystalline regions (birefringence). Starch samples were dispersed in distilled water at a concentration of 0.01 g/ml to avoid overlapping of granules. Dry starch powder (gold metalized) was studied by scanning electron microscopy using a JEOL JSM-6100 Instrument microscope. Samples were pre-conditioned in a vacuum oven at 35 °C for 48 h.
Differential Scanning Calorimetry (DSC)
A DSC-50 Shimadzu Instrument calorimeter was used to study the gelatinization process of native and modified starch. Scans were conducted from room temperature to 120 °C in sealed aluminum capsules. In each capsule a certain amount of starch and water was placed. Correspondingly, the same amount of water was placed in the reference capsule. The starch powder was preconditioned in a vacuum oven at 35 °C for 48 h.
Thermogravimetric Analysis (TGA)
The effect of chemical modification on the thermal resistance of native and modified potato starch was studied using a DTG-50 Shimadzu Instrument. Dynamic Tests were conducted from 20 to 700 °C in platinum capsules. Nitrogen was used to prevent thermo-oxidative reactions at a rate of 20 ml/min. The samples were preconditioned in a vacuum oven at 35 °C for 48 h.
Solubility and Swelling Capacity
The influence of chemical modification on the solubility and swelling power of starch was determined according to the technique described by Schoch [27]. The method involves placing a sample of 2 g of starch in a test tube with 35 ml of distilled water. The suspension is stirred at 300 rpm for one hour and then placed in a water bath at 90 °C for another hour. Finally the sample is centrifuged at 12,500 rpm for 5 min. The supernatant contains the molecules of amylose and amylopectin that have been solubilized. In contrast, the precipitated phase corresponds to the swollen starch grains, which have absorbed a large amount of water. The latter has the characteristics of a gel.
To determine the solubility, the amount of solid present in the supernatant is measured (mS). The solvent is removed by placing the sample in a vacuum oven at 35 °C for 48 h. Then, the solubility is calculated as the ratio between the dissolved mass to the initial mass (Eq. 2):
To determine the degree of swelling (Swelling Power—SP), the mass of precipitate is measured by direct weighting (mP) and it is related to the initial mass (m0) of starch (Eq. 3), taking into account the soluble fraction (mS):
Moisture Absorption
Moisture absorption tests were performed on samples of native and modified starch powder. The samples were dried in a vacuum oven at 40 °C for 24 h until constant weight is reached. They were subsequently placed in a chamber with controlled humidity (75 % HR—sodium chloride saturated solution). The samples were weighed at regular intervals until equilibrium moisture content was reached (constant weight). Tests were performed in triplicate. Water absorption was calculated according to Eq. 4:
Results and Discussion
Fourier Transformed Infrared Spectroscopy (FTIR)
The infrared spectroscopy technique has proved effective for evaluating the functional groups present in the chemical structure of various materials and has been used successfully in the study of different types of chemically modified starch [19, 28, 29].
Native starch presents several characteristic absorbance bands and peaks (Fig. 3). The region at 2,880–2,900 and 3,200 cm−1 corresponds to hydroxyl groups. The presence of CH2 groups determines the existence of the peaks at 2,920–2,836, 2,880, 1,405–1,465 and 1,245 cm−1. The vibration of OH and CH groups in the glucopyranose rings is evidenced by the peaks at 1,415 and 1,320 cm−1. In addition, the peak at 1,255 cm−1 corresponds to the vibration of the CO group, while the peaks at 1,150 and 1,040 cm−1 is related to the vibration of the COC group of the glycosidic bond.
Several changes were observed when analyzing the spectra of chemically modified starches. The spectra of modified starches show a peak at 1,703 cm−1 corresponding to carboxylic groups (CO2) and another peak at 1,162 cm−1, which corresponds to the vibration of CO groups (Fig. 3). An increase in the intensity of the peak at 1,240 cm−1 is also observed and is attributed to the presence of more CO groups. The presence of these peaks demonstrates the existence of ester groups and C = O bonds, which verifies the esterification of starch molecules [19]. In the case of acetylated and octanoylated starch, another peak appears at a wavelength of 1,375 cm−1 corresponding to the deformation of C-CH3 groups [29].
A derivatization index was calculated by dividing the intensity of the peak at 1,703 cm−1 to the intensity of that at 2,933 cm−1. This index is related to the amount of C = O esterification bonds created during the derivatization treatments. Results are shown in Table 1.
Amylose Content
During derivatization treatments, migration of amylose molecules to the outside of the starch granules takes place [30]. This process is favored by the incorporation of voluminous substituent groups within the structure of the starch granule. When the starch is washed after each treatment, dissolved amylose molecules are lost.
The amylose content of chemically modified potato starch was found to be lower than that of native starch (Table 1). Acetylated starch showed a slightly lower amount of amylose than native starch. Maleated starch lost about 50 % of the original amylose content, while in octanoylated starch no amylose remained within the granule. In the latter case, it is possible that other factors besides the incorporation of large substituent groups are affecting the loss of amylose as discussed later on. These results agree with those reported by Liu and colleagues [31].
X-Ray Diffraction (DRX)
The diffraction pattern of starch granules was not affected by the chemical treatments. Figure 4 shows the spectra of native and modified potato starch, where diffraction peaks corresponding to the ‘A’ pattern [5, 32] can be observed. The characteristic peaks can be seen at the following angles 2θ = 5°, 15°, 17°, 20°, 22° and 23° [26, 33].
The crystallinity of acetylated and maleated potato starch remained constant for practical purposes. The crystallinity of octanoyl starch was greater than that of native starch (Table 1). This was attributed to lower amylose content of octanoyl strach. The elimination of a highly amorphous phase leads to a higher apparent crystallinity. Cheetham and Tao [33] showed that the largest proportion of crystallinity in starch granules of corn starch is due to the organization of amylopectin molecules. They also showed that this effect is not linear.
There are other factors that contribute to the overall crystallinity. Chemical modification alters the regularity of the amylose and amylopectin chains, thereof reducing the crystallinity. However, in this contribution, this effect is shielded by the loss of amylose. Without this latter effect, it would be expected that the crystallinity of the modified starches resulted lower [34].
Starch Granule Morphology (POM, SEM)
Chemical modification of starch involves a series of physical and chemical phenomena that occur at the interface between the environment and the granules. Polarized Light Optical Microscopy (POM) had previously been used to detect structural and morphological changes that occur during the chemical modification of starch granule regions [35, 36]. The optical microscopy evaluation showed differences in the morphology of the native and the different derivatized starch granules. In the case of acetylated starch some defects on the surface of the granules were observed, such as cracks and depressions. The same types of defects were observed on the surface of maleated and octanoylated starch granules. The most pronounced effect was observed in the case of octanoylated starch. Chemical modification did not cause changes in birefringence as indicated by the presence of Maltese crosses (Fig. 5).
Results obtained by scanning electron microscopy (SEM) permitted to extend the information obtained by optical microscopy (POM). Different types of defects on the surface of starch granules could be appreciated. Native starch presented a smooth and flawless surface while derivatized starches presented surface defects caused by the chemical treatment.
The chemical modification induced different types of morphological damage in the granular structure, being the most common ones the sinking, blistering and cracking of the granules.
The sinking and collapse phenomenon is attributed to the pressure exerted by the bulky groups incorporated. Due to the complex structure of starch granules, the hilum is the most susceptible zone to the effect of chemical modification, because it is the center from which all amylopectin chains originate [6, 14, 37]. The bulky substituent groups react with the amorphous region near the hilum. These groups exert a separation of chains or “pushing apart” effect [14, 37]. The hilum (mainly amorphous) is less resistant to this effect that the rest of the granule (more crystalline). A gradual collapse of the grain structure takes place (Fig. 6a). In a first stage, the central granule is “disrupted” and the surface sinks to the interior. If the failure of the inner area is greater, the granule acquires a toroidal structure. Finally, a complete fragmentation of the granules can take place.
On the other hand, the chemical modification can also lead to the formation of blisters (Fig. 6b) and cracks (Fig. 6c) on the surface of the granules. The potato starch granules, unlike other species, have no channels in their structure [38, 39]. For this reason, the penetration of reagents occurs by diffusion through the walls of the granules, leading to blistering and cracking.
Differential Scanning Calorimetry (DSC)
The different treatments caused different structural changes in the starch granules. These changes also affected the transition temperatures and associated heat of the gelatinization process [38, 39]. Table 1 presents the results of thermal analysis performed on samples of native and modified starch. There was a significant reduction in enthalpy of gelatinization (ΔHGel) as well as a reduction in the characteristic temperatures of the gelatinization process (TOnset, TPeak, TEndset). This effect was more significant for the octanoylated starch.
The observed effects have been explained in terms of physical and chemical changes in starch. The reactive groups incorporated into potato starch chains reduce the number of intra and intermolecular hydrogen bonding, facilitating the access of water and thus reducing the gelatinization temperature [40].
The substitution occurs preferentially in the amorphous regions, which promotes a greater degree of swelling and weakens the adjacent crystalline sections [14, 34]. The increase in the temperature range at which gelatinization occurs can be attributed to an increase in the homogeneity between the amorphous and crystalline phases of starch granules [35] or a plasticizing effect acting over the amorphous regions of the starch granules [41–43].
Thermogravimetric Analysis (TGA)
The results of thermogravimetric analysis of native and modified starch are shown in Fig. 7. It clearly shows the effect of the different treatments on the thermal resistance of the starch granules. The degradation of native starch begins at 227 ± 1 °C, with a maximum rate of degradation to 310 ± 1 °C. The substitution of hydroxyl groups caused an increase in the thermal resistance of starch. The acetylation showed the more pronounced effect, increasing the onset of the decomposition to temperatures above 260 °C and shifting the peak temperature to 325 °C, despite being the treatment that causes less morphological changes.
Swelling Power, Solubility and Moisture Absorption
The effects of chemical modification on swelling power, solubility and moisture absorption of starch were studied [11, 44–48]. The complex process of swelling of starch granules is closely linked to the structure of the amylose and amylopectin chains and is clearly altered by the effect of the chemical treatments.
It has been shown that chemical treatments have a marked effect on different structural characteristics of the starch granules, and these features also affect the swelling capacity. The main factors are summarized in Table 2. It is not possible to predict the overall effect of a particular type of treatment in the structure of the starch granule. There are cases where the chemical modification resulted in a higher swelling capacity [35, 49] and others where the opposite took place [37, 50, 51].
Table 1 presents swelling capacity for the different derivatized potato starches. Acetylated starch showed a higher degree of swelling that native starch. Modification with maleic anhydride and octanoyl chloride showed a reduced swelling power. This effect is explained by assuming that the resulting swelling capacity is the sum of the contributions of the various factors mentioned above. In all cases the incorporation of bulky groups favors swelling. However, treatments damage the granular structure of starch, reducing its integrity and lowering its swelling capacity. Furthermore, a reduction in amylose content was also found, which causes a further decrease in the swelling capacity.
Like the swelling power, solubility of starch is mainly related to the intensity of interactions between amylose and amylopectin chains in the amorphous and crystalline phases [7, 14, 27, 37, 52]. The results showed that the solubility of starch increases with chemical modification. It is also highly dependent on factors that facilitate the penetration of water, such as an increase in the distance between chains of amylopectin, or the loss of integrity of the granular structure.
Finally, it was noticed that all treatments were very effective in reducing moisture absorption. During a first stage, the absorption rate was the same for all materials. However, afterwards the different types of modified starch showed lower absorption rates (Fig. 8). The octanoylated starch was the most effective treatment for reducing the moisture sensitivity of starch granules, achieving a reduction of about 25 % in the final value. The moisture absorption tests were performed at room temperature, preserving the structure of starch granules. The reduction in moisture absorption relative to native starch is also presented in Table 1.
The lower value of equilibrium moisture content is related to different factors. The most important one is the polarity of the octanoyl group compared to the other substituents. The larger and less hydrophilic nature of the substituent group results in a lower moisture absorption. Moreover, the reduction of amylose content in the chemical modification reduces the ability to absorb moisture. This is because amylose is the main component of the amorphous phase, where the absorption mainly takes place.
Conclusions
During this work the important effect of three different derivatization treatments on the characteristics of potato starch was demonstrated. Chemically modified potato starch treatments were verified by infrared spectroscopy. The selected chemical modification treatments leaded to the incorporation of bulky and hydrophobic groups to the starch chains, changing the nature and strength of the interactions between the chains of amylose and amylopectin. Starches with reduced moisture sensitivity and increased thermal resistance were obtained. The reduction in the moisture absorption of starch granules was attributed to a reduction in the number of hydroxyl groups that are available to form hydrogen bonds with water. Derivatization with octanoyl chloride produces a greater reduction in water absorption value and in the morphological structure of granule.
In this contribution, we showed that the treatments also produced other changes that completely alter the behavior of the modified starches. Treatments caused a decrease in amylose content. All these simultaneous changes in both chemical composition and structure modify the physical behavior of starch such as swelling behavior, moisture absorption, solubility, thermal transitions, heat resistance, and crystallinity, among others.
References
Mathew AP, Dufresne A (2002) Morphological investigation of nanocomposites from sorbitol plasticized starch and tunicin whiskers. Biomacromolecules 3(3):609–617
Shen L, Haufe J, Patel MK (2009) PRO-BIP2009: product overview and market projection of emerging bio-based plastics, 2009, Group Science, Technology and Society (STS); Copernicus Institute for Sustainable Development and innovation. Utrecht University, Utrecht, Netherlands
Stevens ES (2002) Green plastics, an introduction to the new science of biodegradable plastics. Princeton University Press, New Jersey, USA
Zobel HF (1988) Molecules to granules: a comprehensive starch review. Starch Stärke 40(2):44–50
Hoover R (2001) Composition, molecular structure, and physicochemical properties of tuber and root starches: a review. Carbohydr Polym 45(3):253–267
Hoover R, Hughes T, Chung HJ, Liu Q (2010) Composition, molecular structure, properties, and modification of pulse starches: a review. Food Res Int 43(2):399–413
Bertoft E, Blennow A (2009) Structure of potato starch. In: Singh J, Kaur L (eds) Advances in potato starch chemistry and technology. Academic Press, San Diego, pp 83–98
García NL, Famá L, Dufresne A, Aranguren M, Goyanes S (2009) A comparison between the physico-chemical properties of tuber and cereal starches. Food Res Int 42(8):976–982
Gérard C, Barron C, Colonna P, Planchot V (2001) Amylose determination in genetically modified starches. Carbohydr Polym 44(1):19–27
Vandeputte GE, Delcour JA (2004) From sucrose to starch granule to starch physical behaviour: a focus on rice starch. Carbohydr Polym 58(3):245–266
BeMiller JN, Whistler RL (2009) Starch: chemistry and technology. In: Taylor Steve L (ed) Food science and technology international series. Academic Press, New York, NY, p 879
Bertolini AC (ed) (2010) Starches: characterization, properties, and applications. CRC Press, Boca Raton, FL, p 288
Huber KC, BeMiller JN (2010) Modified starch: chemistry and properties. In: Bertolini AC (ed) Starches: characterization, properties, and applications. CRC Press, Boca Raton, FL, pp 145–203
Singh J, Kaur L, McCarthy OJ (2009) Potato starch and its modification. In: Singh J, Kaur L (eds) Advances in potato chemistry and technology. Academic Press, San Diego, pp 273–318
Tester RF, Debon SJJ (2000) Annealing of starch—a review. Int J Biol Macromol 27(1):1–12
Van Beynum GMA, Roels JA (1985) Starch conversion technology. Medium: X; Size: p 363
Whistler RL, BeMiller JN (eds) (1993) Industrial gums: polysaccharides and their derivatives, 3rd edn. Academic Press, San Diego, CA, p 642
Wurzburg OB (ed) (1986) Modified starches: properties and uses. CRC Press, Boca Raton, FA
Cyras V, Tolosa Zenklusen M, Vazquez A (2006) Relationship between structure and properties of modified potato starch biodegradable films. J Appl Polym Sci 101(6):4313–4319
Mbougueng PD, Tenin D, Scher J, Tchiégang C (2012) Influence of acetylation on physicochemical, functional and thermal properties of potato and cassava starches. J Food Eng 108(2):320–326
McCready RM, Hassid WZ (1943) The separation and quantitative estimation of amylose and amylopectin in potato starch. J Am Chem Soc 65(6):1154–1157
Bates FL, French D, Rundle RE (1943) Amylose and amylopectin content of starches determined by their iodine complex formation1. J Am Chem Soc 65(2):142–148
Saenger W (1984) The structure of the blue starch-iodine complex. Naturwissenschaften 71(1):31–36
Hollo J, Szeitli J (1968) The reaction of starch with iodine. In: Radley JA (ed) Starch and its derivatives. Champan and Hall Ltd, London, pp 203–246
Rundle RE, Foster JF, Baldwin RR (1944) On the nature of the starch—iodine complex1. J Am Chem Soc 66(12):2116–2120
Liu Q, Donner E, Tarn R, Singh J, Chung H-J (2009) Advanced analytical techniques to evaluate the quality of potato and potato starch. In: Singh J, Kaur L (eds) Advances in potato chemistry and technology. Academic Press, San Diego, pp 221–248
Schoch TJ (1964) Swelling power and solubility of granular starches. In: Whistler RI, Smith RJ, BeMiller JN (eds) Methods in carbohydrate chemistry, vol IV. Academic Press, New York, pp 106–108
Chi H, Xu K, Wu X, Chen Q, Xue D, Song C, Zhang W, Wang P (2008) Effect of acetylation on the properties of corn starch. Food Chem 106(3):923–928
Fringant C, Desbrières J, Rinaudo M (1996) Physical properties of acetylated starch-based materials: relation with their molecular characteristics. Polymer 37(13):2663–2673
Ferrini LMK, Rocha TS, Demiate IM, Franco CML (2008) Effect of acid-methanol treatment on the physicochemical and structural characteristics of cassava and maize starches. Starch Stärke 60(8):417–425
Liu H, Ramsden L, Corke H (1999) Physical properties of cross-linked and acetylated normal and waxy rice starch. Starch Stärke 51(7):249–252
Imberty A, Buléon A, Tran V, Péerez S (1991) Recent advances in knowledge of starch structure. Starch Stärke 43(10):375–384
Cheetham NWH, Tao L (1998) Variation in crystalline type with amylose content in maize starch granules: an X-ray powder diffraction study. Carbohydr Polym 36(4):277–284
Adebowale KO, Lawal OS (2003) Microstructure, physicochemical properties and retrogradation behaviour of Mucuna bean (Mucuna pruriens) starch on heat moisture treatments. Food Hydrocoll 17(3):265–272
Kaur L, Singh N, Singh J (2004) Factors influencing the properties of hydroxypropylated potato starches. Carbohydr Polym 55(2):211–223
Kim HR, Hermansson A-M, Eriksson CE (1992) Structural characteristics of hydroxypropyl potato starch granules depending on their molar substitution. Starch Stärke 44(3):111–116
Singh J, Kaur L, McCarthy OJ (2007) Factors influencing the physico-chemical, morphological, thermal and rheological properties of some chemically modified starches for food applications–a review. Food Hydrocoll 21(1):1–22
Huber KC, BeMiller JN (2001) Location of sites of reaction within starch granules. Cereal Chem 78(2):173–180
Huber KC, BeMiller JN (2010) Modified starch: chemistry and properties. In: Bertolini AC (ed) Starches: characterization, properties, and applications. CRC Press, Boca Raton, FL
Perera C, Hoover R, Martin AM (1997) The effect of hydroxypropylation on the structure and physicochemical properties of native, defatted and heat-moisture treated potato starches. Food Res Int 30(3–4):235–247
Chang YP, Abd Karim A, Seow CC (2006) Interactive plasticizing-antiplasticizing effects of water and glycerol on the tensile properties of tapioca starch films. Food Hydrocoll 20(1):1–8
Han JA, Lee BH, Lim WJ, Lim ST (2005) Utilization of hydroxypropylated waxy rice and corn starches in Korean waxy rice cake to retard retrogradation. Cereal Chem 82(1):88–92
Singh J, Kaur L, Singh N (2004) Effect of acetylation on some properties of corn and potato starches. Starch Stärke 56(12):586–601
Jayakody L, Hoover R (2008) Effect of annealing on the molecular structure and physicochemical properties of starches from different botanical origins—a review. Carbohydr Polym 74(3):691–703
Varatharajan V, Hoover R, Liu Q, Seetharaman K (2010) The impact of heat-moisture treatment on the molecular structure and physicochemical properties of normal and waxy potato starches. Carbohydr Polym 81(2):466–475
Hoover R, Manuel H (1996) The effect of heat-moisture treatment on the structure and physicochemical properties of normal maize, waxy maize, dull waxy maize and amylomaize V starches. J Cereal Sci 23(2):153–162
Hoover R, Manuel H (1996) Effect of heat–moisture treatment on the structure and physicochemical properties of legume starches. Food Res Int 29(8):731–750
Thomas DJ, Atwell WA (eds) (1999) Starches. Eagan Press, St. Paul, MN, p 94
Rutenberg MW, Solarek D (1984) Starch derivatives: production and uses. In: Whistler RL, BeMiller JN, Paschall EF (eds) Starch: chemistry and technology. Academic Press, London, p 77
Adebowale KO, Adeniyl Afotabib T, Lawal OS (2002) Isolation, chemical modification and physicochemical characterisation of Bambarra groundnut (Voandzeia subterranean) starch and flour. Food Chem 78(3):6
Hirsch JB, Kokini JL (2002) Understanding the mechanism of cross-linking agents (POCl3, STMP, and EPI) through swelling behavior and pasting properties of cross-linked waxy maize starches. Cereal Chem 79(1):102–107
Tester RF, Morrison WR (1990) Swelling and gelatinization of cereal starches. I. Effects of amylopectin, amylose, and lipids. Cereal Chem 67:551–557
Acknowledgments
The authors thank the National Research Council (Conicet-Argentina) for the funding provided (PIP0014).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morán, J.I., Cyras, V.P. & Vázquez, A. Preparation and Characterization of Three Different Derivatized Potato Starches. J Polym Environ 21, 395–404 (2013). https://doi.org/10.1007/s10924-012-0539-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-012-0539-x