Abstract
Purpose
Improved therapies and imaging modalities are needed for the treatment of breast cancer brain metastases (BCBM). ANG1005 is a drug conjugate consisting of paclitaxel covalently linked to Angiopep-2, designed to cross the blood–brain barrier. We conducted a biomarker substudy to evaluate 18F-FLT–PET for response assessment.
Methods
Ten patients with measurable BCBM received ANG1005 at a dose of 550 mg/m2 IV every 21 days. Before and after cycle 1, patients underwent PET imaging with 18F-FLT, a thymidine analog, retention of which reflects cellular proliferation, for comparison with gadolinium-contrast magnetic resonance imaging (Gd-MRI) in brain metastases detection and response assessment. A 20 % change in uptake after one cycle of ANG1005 was deemed significant.
Results
Thirty-two target and twenty non-target metastatic brain lesions were analyzed. The median tumor reduction by MRI after cycle 1 was −17.5 % (n = 10 patients, lower, upper quartiles: −25.5, −4.8 %) in target lesion size compared with baseline. Fifteen of twenty-nine target lesions (52 %) and 12/20 nontarget lesions (60 %) showed a ≥20 % decrease post-therapy in FLT–PET SUV change (odds ratio 0.71, 95 % CI: 0.19, 2.61). The median percentage change in SUVmax was −20.9 % (n = 29 lesions; lower, upper quartiles: −42.4, 2.0 %), and the median percentage change in SUV80 was also −20.9 % (n = 29; lower, upper quartiles: −49.0, 0.0 %). Two patients had confirmed partial responses by PET and MRI lasting 6 and 18 cycles, respectively. Seven patients had stable disease, receiving a median of six cycles.
Conclusions
ANG1005 warrants further study in BCBM. Results demonstrated a moderately strong association between MRI and 18F-FLT–PET imaging.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Approximately 10–30 % of patients with breast cancer develop brain metastases, which are associated with the shortest median survival compared to other sites of metastatic spread [1]. Management of these patients is challenging; the main issues are selective permeability of chemotherapy and targeted therapies across the blood–brain barrier (BBB) and resistance to standard treatments. Additionally, as traditional imaging modalities have limitations in assessing response to treatment [2], both novel treatments and imaging techniques are urgently needed.
ANG1005 (GRN1005) is a Cremophor-free peptide–drug conjugate consisting of three molecules of paclitaxel, covalently linked to a peptide vector, Angiopep-2 [3, 4], which was designed to cross the BBB and enter tumor cells via low-density lipoprotein receptor-related protein-1 (LRP-1)-mediated transcytosis [5–7]. ANG1005 is activated in tumor cells following cleavage by intracellular esterases, releasing conjugated paclitaxel from the Angiopep-2 peptide backbone. Paclitaxel can then bind tubulin and function to stabilize microtubules [3]. The activity of ANG1005 on brain tumors was evaluated using intracerebral human tumor models in nude mice; preclinical studies showed that the brain’s uptake of ANG1005 was approximately 86-fold greater than paclitaxel [3, 8]. Regina et al. demonstrated antineoplastic potency for ANG1005 similar to that of paclitaxel against human cancer cell lines, and a more potent inhibition of intracerebral human tumor xenografts in murine models than paclitaxel [3]. Further, data from multi-center phase I trials showed ANG1005 was associated with manageable toxicity and activity in patients with brain metastases from advanced solid tumors and recurrent malignant gliomas [4, 9], leading the way for phase II studies in patients with breast cancer brain metastases (BCBM).
Magnetic resonance imaging (MRI) is the gold standard in the assessment and monitoring of patients with brain metastases [10]. While gadolinium (Gd)-enhanced MRI scans demarcate individual tumors and their surrounding anatomy, these studies are limited in that gadolinium enhancement of brain tumors mainly reflects impairment, or leakiness, of the BBB, and interventions that affect BBB permeability alter gadolinium enhancement [11]. Positron emission tomography (PET) is a functional imaging modality that uses radioactive tracers to provide information relevant to different cellular and molecular events [12]. 3′-deoxy-3′-18F fluorothymidine (18F-FLT) is a thymidine analog that acts as a chain terminator in the synthesis of DNA; its retention reflects DNA synthesis [13]. It has been studied as a radiolabeled imaging probe for the assessment of cellular proliferation in malignant tumors [12]. In general, 18F-FLT appears to offer little benefit over standard 18F-FDG for diagnosis and staging of different cancers [14–21], but appears to be more sensitive than 18F-FDG in detecting central nervous system (CNS) tumors [22, 23]. A study in 25 patients with newly diagnosed or recurrent gliomas showed that 18F-FLT was more sensitive than 18F-FDG for detection of high-grade tumors, and this finding was associated with a higher correlation between tumor uptake and Ki-67 index for 18F-FLT than for 18F–FDG [24].
The National Cancer Institute (NCI) participated in a phase II multi-center trial to assess the efficacy of ANG1005 in the treatment of BCBM. As 18F-FLT–PET has shown utility in assessing treatment response in breast cancer patients [25, 26], we conducted an imaging substudy alongside the ongoing multiple-cycle efficacy phase II study.
Methods
Patients
Eligible patients had to have histologically or cytologically confirmed breast cancer with known hormone receptor (HR) and HER2 status (HER2-positive tumors were defined as having an immunohistochemistry score of 3+ or evidence of gene amplification according to FISH). Patients had to have at least one radiologically confirmed and metastatic brain lesion (≥1.0 cm in longest diameter by Gd-MRI of brain) that had not undergone radiosurgery. Prior whole-brain radiotherapy (WBRT) was allowed, if >28 days prior to study enrollment. Corticosteroids and anticonvulsants (not enzyme-inducing antiepileptic drugs), if required, had to be at stable doses for >5 days before baseline Gd-MRI brain and ≥5 days prior to the first dose of ANG1005. Patients with HER2-positive disease already on trastuzumab were recommended to continue the same, provided standard of care criteria were met regarding adequate left ventricular ejection fraction (LVEF) [27]. Exclusion criteria included grade ≥2 neuropathy, CNS disease requiring immediate neurosurgical intervention, and known leptomeningeal disease. The NCI Clinical Cancer Research Institutional Review Board approved the study protocol.
Study design
This was a single institution first-cycle imaging substudy conducted as part of a phase II, multi-center, open-label trial of ANG1005 alone or in combination with trastuzumab in patients with BCBM (NCT01480583). Our primary objective was to determine whether one cycle of ANG1005 therapy is associated with a significant change in 18FLT–PET uptake. Secondary objectives included determining whether percentage change in 18FLT–PET/CT uptake after 1 cycle of ANG1005 is correlated with intracranial tumor response on MRI.
Administration of study treatment
Patients received ANG1005 therapy at a dose of 550 mg/m2 IV every 21 days until intracranial disease progression or unacceptable toxicity. Premedication was not required. Six mg of Neulasta subcutaneously was administered 24 h after each infusion of ANG1005 to all the patients. Adverse events were recorded every 3 weeks and graded according to the NCI Common Toxicity Criteria for Adverse Events (CTCAE), version 4.0.
Efficacy assessments
Patients had to have measurable disease present at baseline, have received at least one cycle of therapy, and have had their disease re-evaluated before they could be considered evaluable for response. This was determined using Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 for peripheral disease [28], and intracranial disease was assessed using modified RECIST criteria (CNS RECIST v1.1) [28]. MRI images were centrally reviewed and lesions measured by a neuroradiologist (coauthor NP). Patients underwent 18F-FLT PET/CT imaging before and after the first cycle of therapy of ANG1005. Dynamic 3D PET emission brain imaging was performed over 30 min, and then a static whole-body PET scan at 1 h post-injection was conducted. With regards to the 18F-FLT PET/CT scan, volumes of interest were drawn in target brain metastases. The maximum pixel value was taken as the maximum standard uptake value, or SUVmax, and the tumor-to-normal (T:N) ratio was calculated. The SUV80 was also determined, the 80 % threshold reflecting the average value of the maximum 20 % pixels. The percentage change before and after cycle 1 was calculated, with a 20 % change considered to be significant. As the historical intracranial overall response rate (ORR) in the target population was assumed to be <10 %, a response rate of ≥20 % would indicate that GRN1005 has clinically meaningful activity in this patient population. Changes in FLT–PET were assessed after the first cycle of therapy. Patients underwent MRI brain scans after every two cycles of therapy until disease progression or withdrawal from study. Results from the Phase II study, which enrolled 61 patients, will be reported elsewhere.
Statistical analysis
The primary endpoint on which the sample size for this portion of the study was based on was to determine if there was a change in the FLT–PET uptake as measured by SUV after ANG1005 treatment compared to baseline. We planned to enroll ten patients at our site in order to have 80 % power to detect a one standard deviation change in the level of SUV after treatment compared to before treatment using a 0.05 alpha level two-tailed paired t test. To estimate the degree of correlation between the four FLT–PET variables with the three MRI variables, we performed a Spearman rank correlation analysis on the data; prior to analysis, the median value of each PLT-PET variable was calculated for each of the ten patients (the sample size was 9 or 10 for each estimated correlation coefficient). Strong associations were as follows: |r| > 0.7, moderate association: 0.5 < |r| < 0.7, moderate to weak association: 0.3 < |r| < 0.5, and weak association: |r| < 0.3. Confidence limits (95 %) for the correlation coefficients are reported with each Spearman correlation coefficient (r). Correlation analyses were limited to individual patient medians, not lesions, as lesions from the same individual could not be considered independent of one another. However, plots of individual target lesions were made for presentation purposes. All statistical analyses were performed using SAS (R) version 9.3. All other evaluations were performed with exploratory intent and reported as being hypothetical generating in view of the pilot nature of the study.
Results
Patient characteristics
Patient characteristics and details of prior treatment are listed in Table 1. Median age for all ten patients was 52.5 years. The median duration since the original diagnosis of brain metastases was 12.5 months (minimum, maximum 0.5–25 months). Patients received a median of 3 prior systemic treatments in the metastatic setting prior to study enrollment (minimum, maximum 1–11). All ten patients (100 %) had prior taxane-based treatment.
Two of the ten patients previously had a craniotomy before enrolling in study (2/10; 20 %), and nine of the ten patients had received either whole-brain radiotherapy (WBRT) or stereotactic radiotherapy (SRS) (9/10; 90 %). Five of the ten patients (50 %) had HER2-positive disease.
Toxicity and dose intensity
ANG1005 ± trastuzumab was generally well tolerated. All ten patients (100 %) had hematological toxicity. Six of these patients had grade ≥3 neutropenia. Four patients (40 %) had grade ≥3 lymphopenia, and two patients (20 %) had grade ≥3 thrombocytopenia. Regarding nonhematologic adverse events, the most common grade 1 and 2 adverse events were fatigue (40 %), alopecia (30 %), vomiting (20 %), rash (20 %), and nausea (20 %). Grade ≥3 adverse events included vomiting (20 %), febrile neutropenia (20 %), nausea (10 %), diarrhea (10 %), and fatigue (10 %).
Results by MRI
Tumor reductions as defined by MRI using the modified response evaluation criteria in solid tumors (RECIST) for the central nervous system (CNS) had a median decrease of 17.5 % (n = 10 patients; lower, upper quartiles: −25, −4.8 %) in lesion size compared to baseline (Table 2). Two patients had confirmed partial responses (PR) lasting 6 and 18 cycles, respectively. Seven patients had stable disease (SD), receiving a median of six cycles. One patient had progressive disease (PD) after receiving three cycles.
Analysis of intracranial response by FLT–PET
Target lesions chosen by MRI evaluation were then reviewed for FLT–PET quantitation; additional clearly visible lesions were considered nontarget lesions, and SUV for those lesions were interpreted separately. Of the 32 target (T) and 20 nontarget (NT) metastatic brain lesions measured by MRI, 29 T and 20 NT were measured by FLT–PET. At 30 min, the SUVMAX ranged from 0.8 to 6.3 at baseline, mean 3.0. In total, 15/29 target lesions and 12/20 nontarget lesions showed a >20 % decrease post-therapy (odds ratio 0.71; 95 % CI 0.19, 2.61). Figure 1 depicts a waterfall plot for the best response for each patient and for percent reduction of the individual target (n = 29) lesions. The median percentage change in SUVmax was −20.9 % (n = 29 lesions; lower, upper quartiles: −42.4 , 2.0 %), and for SUV80 was also −20.9 % (n = 29; lower, upper quartiles: −49.0, 0.0 %). The tumor-to-normal (T:N) ratios ranged from 3.3 to 45.1, (mean 15.0) at baseline and after one cycle; the median percent change in T:N was −21.3 % (n = 29; lower, upper quartiles: −23.8, −9.9 %).
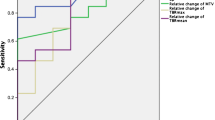
Comparison of MRI and FLT–PET imaging. a Percentage change SUVmax versus target lesions only. The 20 % decrease threshold is demarked by a dashed line. b Median SUVMAX percent change in patients with target lesions (n = 9) versus maximum response (same data as in Table 3); in each pane, the solid black line is the linear regression fit, with the dashed lines representing the 95 % confidence interval. c Percentage change median SUVMAX (Target) shown as a waterfall plot (lesions are color-coded by patient to match Fig. 1a). A LOESS (locally weighted scatterplot smoothed) regression (solid blue line) was also applied to the data
Association between MRI and FLT–PET imaging
There was a moderately strong association (Spearman r > 0.7) between MRI and FLT–PET imaging based on percent change in SUVMAX between baseline and post cycle 1 (Table 3). The additional FLT–PET parameters were at least moderately associated with each other, as indicated by the similar Spearman r values (r = 0.63 or r = 0.80) for each association. Figure 1a depicts the best MRI response versus percentage change in SUVMAX (same data as in Table 3). The percentage change in SUVMAX in measurable individual target lesions appeared to be related to the median of individual lesion maximum response (Fig. 1b); however, a Spearman r could not be assigned due to lack of independent samples (29 lesions from 9 patients). The data shown in Fig. 1 a–b appeared to follow a nonlinear pattern; thus, a locally weighted scatterplot smoothing (LOESS) regression was used to visualize the relationship (Fig. 1c), and a linear regression line is shown for comparison purposes. This pattern may be due to a small sample size, particularly as FLT–PET percent changes approach positive territory. There appears to be a linear pattern where both MRI and FLT–PET measured negative percent changes in tumor uptake (indicating tumor shrinkage from one cycle of ANG1005). Figure 2 depicts baseline MRI scans and 18FLT PET scans pre- and post one cycle of ANG1005 for patients 5–7. Caution should be used when interpreting these results, as sample size is small.
Responses of individual patients. Baseline MRIs and 18FLT–PET scans pre and post cycle 1 of ANG1005 are presented for each patient. Patients 5 and 7 had a partial response to treatment, and patient 7 remained on study for 18 cycles. Patient 6 had a mixed response, in that some lesions responded (decreased in size by 40–50 %) and some progressed (increased in size by 40–50 %). This illustrates how tumor heterogeneity can impact response assessment and treatment benefit. R right, inf inferior, NT nontarget, temp temporal, L left
Discussion
This report shows the impact of ANG1005, an agent with activity in BCBM, on the uptake of radiolabeled fluorothymidine. 18F-FLT–PET was performed at baseline and after one cycle of ANG1005, whereas MRI Brain was performed at baseline and after two cycles of ANG1005. Based on the results, 18F-FLT–PET may be a useful tool to predict response in this setting, as it appears to correlate well (Spearman r > 0.7) with best MRI response and the median percentage change in FLT–PET by patient. These findings are encouraging, given the lack of effective treatments and accurate imaging modalities for patients with BCBM.
Contrast-enhanced MRI detection of brain metastases represents gadolinium leakage through the BBB, as opposed to actual tumor volume [29, 30]. Pseudoresponse and pseudoprogression are terms describing inaccurate CNS response assessment, and both have been described with conventional MRI imaging. Pseudoresponse is a phenomenon whereby contrast uptake is reduced due to a reduction in vascular permeability, which is seen in patients on steroids or in patients with glioblastoma multiforme (GBM) treated with antiangiogenic agents [31]. On the other hand, cytotoxic therapy or radiation therapy can cause cell damage and local inflammation, which may increase vascular permeability, resulting in early increases in contrast enhancement, i.e., pseudoprogression [32–34]. Therefore, improved strategies are needed to more accurately determine treatment response in this setting [25].
There is considerable interest for development of 18F-FLT–PET as a cancer imaging biomarker, especially as it is becoming more readily available. Therefore, studies focusing on its mechanism of action and potential clinical applications are important. An advantage of 18F-FLT–PET is that uptake in normal brain parenchyma is low, which allows visualization of brain tumors with high contrast. However, a limitation is that benign lesions disrupting the BBB cannot be distinguished from malignant tumors. Additionally, the extent to which 18F-FLT–PET correlates with proliferative index in different tumor types is variable, as 18F-FLT–PET cannot discriminate between moderately proliferative tumors driven by thymidine salvage from those dependent on de novo thymidine synthesis. However, 18F-FLT–PET accurately quantified the proliferation activity of malignant brain tumors in a study of 25 patients. Research is ongoing; NCT02328300 is assessing 18F-FLT–PET and MRI for the evaluation of pseudoprogression in patients with brain metastases, and NCT01621906 is comparing MRI with 18F-FLT–PET in patients with BCBM receiving whole-brain radiotherapy (WBRT)± the multikinase inhibitor sorafenib. The available evidence suggests that FLT–PET imaging in this setting may improve response assessment [35, 36]. Our preliminary evaluation of FLT–PET imaging for CNS disease in BCBM suggests that it is a promising tool that could serve as a complementary assessment method, supporting or clarifying MRI findings. We noted a correlation between FLT–PET change after one cycle and ultimate best response (r = 0.75) after ANG1005. While these results have to be considered preliminary due to the small size (and the very wide confidence interval), they certainly support an expanded look at the imaging technique. Improving our ability to determine who may be benefiting from therapy is a critical piece in improving the study of new agents in this setting, several of which are in clinical development [37].
Phase I and II clinical studies have demonstrated signs of both CNS and peripheral antitumor activity of ANG1005 in patients with brain metastases from lung and breast cancer [4, 9, 38]. Additionally, ANG1005 received orphan drug designation from the FDA for treatment of GBM in 2014, and for BCBM in March 2015 [39]. In the phase I trial, 5/27 (18.5 %) patients were noted to have a PR, and 11/27 (41 %) had SD at doses ≥420 mg/m2. Our imaging trial was a substudy of a phase II trial conducted by Lin et al., to evaluate the CNS and peripheral antitumor activity of ANG1005 in patients with BCBM [38]. Safety and tolerability of ANG1005 resembled a taxane profile. In the phase II study, 61 patients were treated. For patients who were treated at the 550 mg/m2 dose, best responses in the CNS were as follows: ten patients had a PR (20 %), 31 patients had SD (61 %), and ten patients had disease progression (20 %). The best observed responses for peripheral disease in this patient group were as follows: one patient had a CR (4 %), seven patients had a PR (25 %), 14 patients had SD (50 %), and six patients had disease progression (21 %). At the 650 mg/m2 dose, CNS responses were as follows: four patients had a PR (40 %), four patients had SD (40 %), and two patients had PD (20 %). Peripheral response rates for patients treated at this dose were as follows: PR = one patient (25 %), SD = two patients (50 %), and one patient had PD (25 %). Of note, five of the ten patients in our imaging substudy (50 %) had HER2-positive disease, which may be due to selection bias of patients being enrolled.
ANG1005 is an interesting compound in that it delivers a well-understood and effective anticancer agent both to the CNS and systemically. Paclitaxel also combines well with other anticancer agents, in particular DNA-damaging agents. Studies of ANG1005 in combination with other agents will be an important future direction. Tests have shown that patients do not develop antibodies to ANG1005, even after numerous cycles of treatment in some cases, and patients do not require premedication. Neurocognitive toxicities have not been observed, and systemic toxicities are the well-known effects of paclitaxel [40, 41]. A phase II, open-label, multi-center study of ANG1005 in breast cancer patients with recurrent brain metastases is currently ongoing, but closed to accrual (NCT02048059); the primary outcome measure is intracranial objective response rate (ORR). The trial planned to enroll 56 evaluable patients, and the expected completion date is October 2016. While further studies with ANG1005 should be conducted, the role of Angiopep-2 should also be further explored, as it is possible that conjugation of anticancer agents with this vector could increase their efficacy in the treatment of brain metastases. In summary, therapy for BCBM is an important unmet need, as is assessment of therapeutic outcome. ANG1005 has activity in BCBM, with a manageable toxicity profile. FLT–PET imaging could potentially represent a complementary assessment method, which could improve MRI evaluation of CNS response. Further studies of ANG1005 are warranted, and combination studies should be developed.
References
Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH et al (2010) Metastatic behavior of breast cancer subtypes. J Clin Oncol 28(20):3271–3277
Gempt J, Bette S, Buchmann N, Ryang YM, Forschler A, Pyka T et al (2015) Volumetric analysis of F-18-FET-PET imaging for brain metastases. World Neurosurg 84(6):1790–1797
Regina A, Demeule M, Che C, Lavallee I, Poirier J, Gabathuler R et al (2008) Antitumour activity of ANG1005, a conjugate between paclitaxel and the new brain delivery vector Angiopep-2. Br J Pharmacol 155(2):185–197
Kurzrock R, Gabrail N, Chandhasin C, Moulder S, Smith C, Brenner A et al (2012) Safety, pharmacokinetics, and activity of GRN1005, a novel conjugate of angiopep-2, a peptide facilitating brain penetration, and paclitaxel, in patients with advanced solid tumors. Mol Cancer Ther 11(2):308–316
Demeule M, Currie JC, Bertrand Y, Che C, Nguyen T, Regina A et al (2008) Involvement of the low-density lipoprotein receptor-related protein in the transcytosis of the brain delivery vector angiopep-2. J Neurochem 106(4):1534–1544
Demeule M, Regina A, Che C, Poirier J, Nguyen T, Gabathuler R et al (2008) Identification and design of peptides as a new drug delivery system for the brain. J Pharmacol Exp Ther 324(3):1064–1072
Bertrand Y, Currie JC, Poirier J, Demeule M, Abulrob A, Fatehi D et al (2011) Influence of glioma tumour microenvironment on the transport of ANG1005 via low-density lipoprotein receptor-related protein 1. Br J Cancer 105(11):1697–1707
Thomas FC, Taskar K, Rudraraju V, Goda S, Thorsheim HR, Gaasch JA et al (2009) Uptake of ANG1005, a novel paclitaxel derivative, through the blood-brain barrier into brain and experimental brain metastases of breast cancer. Pharm Res 26(11):2486–2494
Drappatz J, Brenner A, Wong ET, Eichler A, Schiff D, Groves MD et al (2013) Phase I study of GRN1005 in recurrent malignant glioma. Clin Cancer Res 19(6):1567–1576
Nowosielski M, Radbruch A (2015) The emerging role of advanced neuroimaging techniques for brain metastases. Chin Clin Oncol 4(2):23
Ellingson BM, Wen PY, van den Bent MJ, Cloughesy TF (2014) Pros and cons of current brain tumor imaging. Neuro Oncol 16(Suppl 7):vii2–vii11
Beadsmoore C, Newman D, MacIver D, Pawaroo D (2015) Positron emission tomography computed tomography: a guide for the general radiologist. Can Assoc Radiol J 66(4):332–347
Peck M, Pollack HA, Friesen A, Muzi M, Shoner SC, Shankland EG et al (2015) Applications of PET imaging with the proliferation marker [18F]-FLT. Q J Nucl Med Mol Imaging 59(1):95–104
Yamamoto Y, Nishiyama Y, Kimura N, Ishikawa S, Okuda M, Bandoh S et al (2008) Comparison of (18)F-FLT PET and (18)F-FDG PET for preoperative staging in non-small cell lung cancer. Eur J Nucl Med Mol Imaging 35(2):236–245
Halter G, Buck AK, Schirrmeister H, Aksoy E, Liewald F, Glatting G et al (2004) Lymph node staging in lung cancer using [18F]FDG-PET. Thorac Cardiovasc Surg 52(2):96–101
Cobben DC, Elsinga PH, Hoekstra HJ, Suurmeijer AJ, Vaalburg W, Maas B et al (2004) Is 18F-3′-fluoro-3′-deoxy-l-thymidine useful for the staging and restaging of non-small cell lung cancer? J Nucl Med 45(10):1677–1682
Buck AK, Halter G, Schirrmeister H, Kotzerke J, Wurziger I, Glatting G et al (2003) Imaging proliferation in lung tumors with PET: 18F-FLT versus 18F-FDG. J Nucl Med 44(9):1426–1431
Francis DL, Visvikis D, Costa DC, Arulampalam TH, Townsend C, Luthra SK et al (2003) Potential impact of [18F]3′-deoxy-3′-fluorothymidine versus [18F]fluoro-2-deoxy-d-glucose in positron emission tomography for colorectal cancer. Eur J Nucl Med Mol Imaging 30(7):988–994
Herrmann K, Ott K, Buck AK, Lordick F, Wilhelm D, Souvatzoglou M et al (2007) Imaging gastric cancer with PET and the radiotracers 18F-FLT and 18F-FDG: a comparative analysis. J Nucl Med 48(12):1945–1950
Cobben DC, van der Laan BF, Maas B, Vaalburg W, Suurmeijer AJ, Hoekstra HJ et al (2004) 18F-FLT PET for visualization of laryngeal cancer: comparison with 18F-FDG PET. J Nucl Med 45(2):226–231
Troost EG, Vogel WV, Merkx MA, Slootweg PJ, Marres HA, Peeters WJ et al (2007) 18F-FLT PET does not discriminate between reactive and metastatic lymph nodes in primary head and neck cancer patients. J Nucl Med 48(5):726–735
Choi SJ, Kim JS, Kim JH, Oh SJ, Lee JG, Kim CJ et al (2005) [18F]3′-deoxy-3′-fluorothymidine PET for the diagnosis and grading of brain tumors. Eur J Nucl Med Mol Imaging 32(6):653–659
Muzi M, Spence AM, O’Sullivan F, Mankoff DA, Wells JM, Grierson JR et al (2006) Kinetic analysis of 3′-deoxy-3′-18F-fluorothymidine in patients with gliomas. J Nucl Med 47(10):1612–1621
Chen K, Bandy D, Reiman E, Huang SC, Lawson M, Feng D et al (1998) Noninvasive quantification of the cerebral metabolic rate for glucose using positron emission tomography, 18F-fluoro-2-deoxyglucose, the Patlak method, and an image-derived input function. J Cereb Blood Flow Metab 18(7):716–723
Pio BS, Park CK, Pietras R, Hsueh WA, Satyamurthy N, Pegram MD et al (2006) Usefulness of 3′-[F-18]fluoro-3′-deoxythymidine with positron emission tomography in predicting breast cancer response to therapy. Mol Imaging Biol 8(1):36–42
Kenny LM, Vigushin DM, Al-Nahhas A, Osman S, Luthra SK, Shousha S et al (2005) Quantification of cellular proliferation in tumor and normal tissues of patients with breast cancer by [18F]fluorothymidine-positron emission tomography imaging: evaluation of analytical methods. Cancer Res 65(21):10104–10112
Trastuzumab package insert. http://www.accessdata.fda.gov/drugsatfda_docs/label/2000/trasgen020900lb.htm. Accessed 9 Oct 2015
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247
Young RJ, Sills AK, Brem S, Knopp EA (2005) Neuroimaging of metastatic brain disease Neurosurgery 57(5 Suppl):S10–S23 discusssion S1–4
Cha S (2009) Neuroimaging in neuro-oncology. Neurotherapeutics 6(3):465–477
da Cruz LCH Jr, Rodriguez I, Domingues RC, Gasparetto EL, Sorensen AG (2011) Pseudoprogression and pseudoresponse: imaging challenges in the assessment of posttreatment glioma. AJNR Am J Neuroradiol 32(11):1978–1985
Li YQ, Chen P, Haimovitz-Friedman A, Reilly RM, Wong CS (2003) Endothelial apoptosis initiates acute blood-brain barrier disruption after ionizing radiation. Cancer Res 63(18):5950–5956
Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ (2008) Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol 9(5):453–461
Fiegler W, Langer M, Scheer M, Kazner E (1986) Reversible computed tomographic changes following brain tumor irradiation induced by the “early-delayed reaction” after radiation. Der Radiol 26(4):206–209
Thorsen F, Fite B, Mahakian LM, Seo JW, Qin S, Harrison V et al (2013) Multimodal imaging enables early detection and characterization of changes in tumor permeability of brain metastases. J Control Release 172(3):812–822
Schiepers C, Chen W, Dahlbom M, Cloughesy T, Hoh CK, Huang SC (2007) 18F-fluorothymidine kinetics of malignant brain tumors. Eur J Nucl Med Mol Imaging 34(7):1003–1011
Bates SE (2015) Central nervous system metastasis from breast cancer. Oncologist 20(1):3–4
Lin NU, Gabrail NY, Sarantopoulos J, Schwartzberg LS, Kesari S, Bates SE et al (2014) Evaluation of CNS and peripheral antitumor activity of ANG1005 in patients with brain metastases from breast tumors and other advanced solid tumors. J Clin Oncol 32:5s (suppl; abstr 2523)
FDA approves ANG1005 for the treatment of glioblastoma multiforme. http://angiochem.com/angiochem%E2%80%99s-ang1005-received-orphan-drug-designation-fda-treatment-glioblastoma-multiform. Accessed 6 Feb 2016
Lim E, Lin NU (2014) Updates on the management of breast cancer brain metastases. Oncology 28(7):572–578
Paclitaxel (Taxol) package insert data. https://www.medicines.org.uk/emc/PIL.25823.latest.pdf. Accessed 19 Aug 2015
Acknowledgments
We would like to thank all the patients, their families, the research, and allied health professional staff in the National Cancer Institute for their valuable contributions to this study. ANG1005 was provided by Angiochem, and representatives from the company reviewed the manuscript. None of the authors have a financial relationship with Angiochem or have other relevant conflicts of interest to disclose.
Author contributions
Both Susan E. Bates and Antonio T. Fojo had full access to all of the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
None of the authors have any relevant disclosures.
Rights and permissions
About this article
Cite this article
O’Sullivan, C.C., Lindenberg, M., Bryla, C. et al. ANG1005 for breast cancer brain metastases: correlation between 18F-FLT–PET after first cycle and MRI in response assessment. Breast Cancer Res Treat 160, 51–59 (2016). https://doi.org/10.1007/s10549-016-3972-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-016-3972-z