Abstract
Abstract
The aim of this study was to evaluate the feasibility of using [18F] 3′-deoxy-3′-fluorothymidine (FLT) positron emission tomography (PET) for the diagnosis and grading of brain tumors.
Methods
The patient population comprised 26 patients (15 males, 11 females) with brain tumors (n=18) or nontumorous lesions (n=8). 2-[18F]fluoro-2-deoxy-d-glucose (FDG) and FLT PET images were obtained using a dedicated PET scanner 1 h after the injection of 370 MBq of FDG or FLT. Uptake of FDG and FLT by the lesions was visually and semiquantitatively assessed in comparison with normal brain tissue.
Results
Of 26 brain lesions, four showed increased FDG uptake compared with normal gray matter (grade 5). These four lesions showed intensely increased FLT uptake and were all high-grade tumors. Twenty-two lesions with similar (grade 4) or decreased (grades 1–3) FDG uptake compared with normal gray matter showed variable pathology. Among the 18 brain tumors, FLT PET showed increased uptake in all 12 high-grade tumors but FDG uptake was variable. In 22 brain lesions with similar or decreased uptake compared with normal gray matter on FDG PET, the sensitivity and specificity of FLT PET for the diagnosis of brain tumor were 79% (11/14) and 63% (5/8), respectively. The uptake ratios of 14 brain tumors on FLT PET were significantly higher than the lesion to gray matter ratios (p=0.012) and lesion to white matter ratios (p=0.036) of FDG uptake and differed significantly between high (5.1±2.6) and low (2.1±1.1) grade tumors (p=0.029). In nine gliomas, FLT uptake was significantly correlated with the Ki-67 proliferation index (rho=0.817, p=0.007).
Conclusion
These findings indicate that FLT PET is useful for evaluating tumor grade and cellular proliferation in brain tumors. It displayed high sensitivity and good contrast in evaluating brain lesions that showed similar or decreased uptake compared with normal gray matter on FDG PET. FLT PET, however, did not appear to be sufficiently useful for differentiating tumors from nontumorous lesions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
2-[18F]fluoro-2-deoxy-D-glucose (FDG) is the most widely used tracer for oncologic positron emission tomography (PET) imaging [1, 2]. This technique, which has significantly enhanced the capabilities of brain imaging, has been applied to tumor detection, noninvasive tumor grading, prediction of prognosis, evaluation of response to treatment, and differentiation of tumor recurrence from radiation necrosis [3–13]. However, the high glucose utilization of normal gray matter limits its utility in the localization of low metabolic brain tumors [1, 3, 10, 11], and the increased FDG uptake of inflammatory lesions may cause false positive results [1, 11].
To overcome these limitations, other tracers such as 11C-methionine, 11C-tyrosine, 18F-tyrosine, and 11C-thymidine, each of which can measure tumor biosynthesis, have been investigated [1, 3, 9, 12, 14–17]. Among these, 11C-thymidine, a pyrimidine nucleoside analog, is rapidly incorporated into DNA and has been used to image cellular proliferation [1, 3, 18]. The clinical applications of 11C-thymidine PET have been limited, however, by the short half-life of 11C and its rapid in vivo degradation, as well as by the difficulties experienced in the radiosynthesis of 11C-thymidine and its high background activity [1, 3, 17, 18].
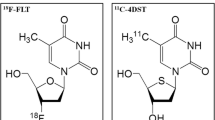
More recently, [18F] 3′-deoxy-3′-fluorothymidine (FLT) was introduced as a PET tracer for tumor imaging [1, 3, 17–19]. FLT has a longer radioactive half-life than 11C-thymidine and its metabolic stability has been confirmed in a pilot study of patients with non-small cell lung cancer [1]. FLT has been found useful for noninvasive assessment of the proliferation rate of colorectal, pancreatic, lung, and esophageal cancer, as well as lymphomas [20–25]. In addition, FLT PET has been utilized in the prognostic assessment and evaluation of response to antiproliferative therapy in colorectal and lung tumors [20, 22, 23]. Its potential clinical use in the evaluation of brain tumors, however, has not been determined. We therefore evaluated the feasibility of FLT PET for the diagnosis and grading of brain tumors.
Materials and methods
Patients
The study cohort consisted of 26 patients (15 men, 11 women, aged 2–67 years; median age 34 years) with suspected brain tumors on brain MRI who underwent FDG PET and FLT PET between December 2002 and January 2004. During the first 2 months of this pilot study, FLT PET was performed in nine consecutive patients regardless of the results of FDG PET. Subsequently, however, FLT PET was performed only on 17 patients whose brain lesions showed decreased or similar FDG uptake compared with normal gray matter. The FLT PET and FDG PET studies were performed within 1 week. Of the 26 patients, 19 underwent PET for initial diagnosis and seven for the evaluation of tumor recurrence after treatment. In 23 patients, histopathological diagnosis was obtained after PET examination. The other three patients were diagnosed on the basis of clinical and radiological follow-up (>6 months).
The institutional review board for clinical investigation approved this study protocol, and informed consent was obtained from all patients studied.
Histopathological diagnosis and grading of brain tumors was performed according to the WHO grading system (malignancy scale) for CNS tumors [26]. The proliferative activity of each tumor was measured by obtaining the Ki-67 proliferation index from immunohistochemical staining of pathologic specimens. After cell staining, a minimum of 500 cells in the region of the tumor with the greatest density of staining was counted per tissue section. The Ki-67 score (%) was estimated by counting the percentage of the counted cells which stained with antibody to Ki-67 nucleus antigen.
[18F]FLT synthesis
A TracerLab MX (GEMS, Belgium) FDG module with a disposable cassette was used for automatic preparation of [18F]FLT [27]. [18F]FLT was prepared by [18F]fluorination of (5′-O-DMTr-2′-deoxy-3′-O-nosyl-β-D-threo-pentofuranosyl)-3-N-BOC-thymine, followed by hydrolysis with 1 N HCl. After HPLC purification, the yield of radiochemical was 48.7±5.6%. The radiochemical purity of the [18F]FLT injectable solution was 98±1.2%, and its specific activity was 3,225–7,692 Ci/mmol.
PET studies
The PET scans were performed with an ECAT EXACT HR+ PET scanner (Siemens-CTI, Knoxville, TN, USA), which has a 50-cm transaxial field of view and a 15.5-cm axial field of view, producing 128 image planes spaced 1.7 mm apart. The transaxial spatial resolution was 4.5 mm full-width at half-maximum (FWHM) at the center of the field of view, and the axial resolution was 4.2 mm FWHM.
All patients fasted for at least 6 h prior to PET scanning. A 5-min transmission scan and a 15-min emission scan were performed 60 min after the intravenous injection of FDG (370 MBq) or FLT (370 MBq) in a quiet and dimly lit room. The transmission scan was performed using an external source of 68Ge. All emission scans were performed in 3D acquisition mode. Attenuation-corrected images were reconstructed with ordered subset expectation maximization (OSEM), with six iterations and 16 subsets. They were post-smoothed with a Shepp filter.
Data analysis
Uptake of FDG and FLT by suspected brain tumors on MRI was assessed visually and quantitatively. Visual analysis was performed by two independent observers (S.J.C., J.S.K.). Anatomical localization of the brain lesions was confirmed by visually comparing the PET scan images with the corresponding MRI scans, and uptake of the brain lesions was assessed by comparison with normal brain tissue. On FDG PET, the degree of FDG uptake by the brain lesion was scored as grade 1 (< normal white matter), grade 2 (= normal white matter), grade 3 (> normal white matter but < normal gray matter), grade 4 (= normal gray matter), or grade 5 (> normal gray matter). On FLT PET, the degree of FLT uptake was scored as grade 1 (< normal brain background), grade 2 (= normal brain background), grade 3 (> normal brain background), or grade 4 (>> normal brain background); brain lesions with grade 3 or 4 uptake were considered tumors.
For quantitative analysis, two regions of interest (ROIs) of 3×3×1 (5.1×5.1×2.4 mm) and 6×6×1 (10.2×10.2×2.4 mm)voxels were manually placed on the highest uptake area of the lesion and the normal brain tissue of the contralateral side, respectively. In cases of cerebellum or brain stem lesions, the ipsilateral cerebral hemisphere was selected as the reference. When the margin and uptake of brain lesions were difficult to define, the location of ROIs for brain lesions was facilitated by use of coregistration of PET and MR images. Using the mean standardized uptake value (SUV) of each ROI, the uptake ratio of the lesion to normal brain tissue was calculated.
Statistical analysis
The sensitivity and specificity of FLT PET for the differential diagnosis of tumors and nontumorous lesions were calculated. The Wilcoxon signed rank test was used to evaluate the differences between FDG and FLT uptake in brain tumors. The Kruskal-Wallis test and the Mann-Whitney test were used to compare FLT and FDG uptake among low-grade tumors, high-grade tumors, and nontumorous lesions. Spearman’s correlation was used to assess the relationship between the uptake of FDG and FLT in brain tumors, and between the proliferation index and tumor uptake in gliomas. A p value of <0.05 was considered significantly significant.
Results
Of the 26 lesions, 18 were brain tumors and 8 were nontumorous lesions (Table 1). Of the 18 tumors, 12 were high grade (III or IV), while six were low grade (I or II). Among the benign lesions, two cases with postoperative change showed fluid collection or hemorrhage with gadolinium enhancement at the operation site on MRI. These two cases were confirmed as nontumorous lesions by clinical and MR follow-up. Another two cases with inflammation were nonspecific inflammatory granulomas of unknown etiology. One case with subacute infarction was 17-day-old ischemic infarct at the time of the FDG PET study. All but one brain tumor showed various degrees of enhancement on gadolinium-enhanced MR.
The results of pathology and ROI-based uptake ratio according to the visual grade of FDG and FLT uptake are summarized in Tables 2 and 3. All six lesions showing increased (grade 5) or similar uptake (grade 4) compared with normal gray matter on FDG PET were malignant tumors. Twenty lesions with decreased uptake (grades 1–3) compared with normal gray matter showed variable pathology. On FLT PET, 12 of 18 lesions with increased uptake (grades 3 and 4) were high-grade tumors. However, none of eight lesions without increased uptake (grades 1 and 2) were high-grade tumors. Of 18 brain tumors, FLT PET showed increased uptake in all 12 high-grade tumors, but FDG uptake was variable.
FLT uptake ratios of 18 brain tumors were correlated with lesion to normal gray matter (rho=0.54, p=0.02) and also lesion to white matter ratios (rho=0.55, p=0.019) of FDG uptake, but FLT uptake ratios were significantly higher than lesion to normal gray matter (p<0.001) and lesion to white matter ratios (p=0.035) of FDG uptake.
Except in four patients who underwent FLT PET during the initial period of the study, we did not perform FLT PET in patients with brain lesions showing increased uptake compared with normal gray matter on FDG PET. We therefore assessed the diagnostic ability of FLT PET in 22 patients whose brain lesions showed decreased or similar uptake (grades 1–3, or 4) compared with normal gray matter on FDG PET. FLT PET was successful in detecting 11 of the 14 brain tumors (Figs. 1, 2), with all three false negatives being grade II astrocytomas (Fig. 3). Of the eight nontumorous lesions, five showed no uptake of FLT (Table 1). The three false positive cases consisted of one case each of subacute infarction (Fig. 4), multiple sclerosis, and radiation necrosis. Thus, the diagnostic sensitivity and specificity of FLT PET were 78.6% and 62.5%, respectively. In this population, the FLT uptake ratios of the 14 brain tumors were significantly higher than lesion to white matter ratios (p=0.036) as well as the lesion to gray matter ratios (p=0.012) of FDG uptake. When we compared the FLT uptake by high-grade and low-grade tumors and nontumorous lesions showing isometabolism or hypometabolism in relation to normal gray matter on FDG PET, we found that FLT uptake by high-grade tumors was significantly higher than that by low-grade tumors (p=0.029), whereas the difference between low-grade tumors and nontumorous lesions was not significant (p=0.414). The differences in FDG uptake among these three groups were not significant in this population (Table 4).
An 11-year-old male with a germ cell tumor of the basal ganglia. MRI shows subtle changes (arrow) in the right basal ganglia. On FDG PET, the right basal ganglia lesion shows slightly decreased uptake compared with contralateral basal ganglia but increased uptake compared with normal white matter. FLT PET, however, reveals intensely increased uptake, suggesting the presence of a malignant tumor (arrow). Based on the FLT PET results, a stereotactic biopsy could be performed in the right basal ganglia
In the nine gliomas, we evaluated the relationship between tumor uptake and proliferative activity, as measured by the Ki-67 index, and as well as tumor grade. We found that the FLT uptake by gliomas was significantly correlated with tumor grade (rho=0.871, p=0.001) and also with cellular proliferation (rho=0.817, p=0.007) (Fig. 5). However, lesion to gray matter ratios and lesion to white matter ratios of FDG uptake were not significantly correlated with either tumor grade or cellular proliferation.
Discussion
For the diagnosis and treatment of brain tumors, it is essential to evaluate the grade of the malignancy and the extent of the tumor [16]. The results of this study revealed that FLT PET is useful for evaluating the histologic grade and cellular proliferation of brain tumors, as well as for the detection and delineation of brain tumors that show decreased or similar uptake compared with normal gray matter on FDG PET (Figs. 1, 2). FLT PET, however, did not appear sufficiently useful for differentiating tumors from nontumorous lesions, inasmuch as we observed several false negative and false positive cases (Figs. 3, 4).
FLT uptake is related to proliferation and tumor grade. FLT permeates the cell membrane by facilitated diffusion [19, 25] and is phosphorylated by the S-phase-specific enzyme thymidine kinase 1 to 3′-fluorothymidine monophosphate (FLT-MP), which leads to intracellular trapping due to the lack a hydroxyl group on the monophosphate [18, 19, 21]. During DNA synthesis, the concentration of thymidine kinase 1 increases almost tenfold and it is thus an accurate marker of cellular proliferation [1, 18]. With respect to the mechanism of FLT uptake by brain tumors, the permeability of the blood–brain barrier (BBB) should be considered, insofar as under normal conditions FLT is poorly transported across the BBB. We have shown here that FLT uptake can be correlated with cellular proliferation and grading of brain tumors. However, we found that three nontumorous lesions with gadolinium enhancement on MRI showed increased FLT uptake, despite showing no significant uptake on FDG PET.
FDG uptake by brain tumors is not correlated with abnormality in BBB and is relatively independent of blood flow [28–30]. In contrast, the uptake of 201Tl, which is poorly transported across the BBB, like FLT, has been found to reflect the degree of proliferative activity in brain tumor cells when combined with disruption of the BBB [31]. Our results suggest that both thymidine kinase 1 activity and BBB permeability are major factors in the FLT uptake by brain lesions, although we did not compare FLT uptake with the severity of BBB disruption. This factor may limit the clinical usefulness of FLT PET in the differential diagnosis of brain tumors and nontumorous lesions.
In this study, FLT PET showed high sensitivity and good contrast in evaluating brain lesions showing decreased or similar uptake compared with normal gray matter on FDG PET. However, we observed three false negative cases, all of which were grade II astrocytomas (Fig. 3). These false negative results may have been due to the partial volume effect, as well as to the low cellular density and proliferative activity of these tumor cells. In addition, our finding that the other low-grade tumors (two gangliogliomas and one DNET) showed increased FLT uptake suggests that decreased uptake of FLT by grade II astrocytomas may reflect some metabolic property of astrocytoma cells.
During the first 2 months of this study, we performed FLT PET on nine consecutive patients regardless of their FDG PET results. In these patients, four tumors showing increased uptake compared with normal gray matter on FDG PET showed intensely increased FLT uptake and were high-grade tumors. Thus, in these cases, FLT PET seemed to have no advantage over FDG PET in evaluating the lesions. Although increased FDG uptake by various types of inflammatory lesion can cause false positive results, the detection of brain tumors and their differentiation from benign lesions are frequently complicated when tumors show decreased or similar uptake compared with normal gray matter on FDG PET [12], and the tumor grade is least predictable when tumor FDG uptake is less than gray matter uptake but higher than white matter uptake [8, 9]. Subsequently, therefore, we performed FLT PET only in 17 patients whose brain lesions showed decreased or similar uptake compared with normal gray matter on FDG PET. As a result, we could not evaluate the diagnostic ability of FDG PET and directly compare it with that of FLT PET in all brain lesions of the study period. Although FDG uptake has been reported to correlate positively with the histological malignancy of gliomas [29, 32], contradictory results have also been reported [11, 30, 31]. In this study, brain lesions showing decreased uptake compared with normal gray matter (grades 1–3) on FDG PET were of various histological types and malignancies. In contrast, FLT uptake showed significant correlations with the histological grade, even in brain tumors showing decreased or similar uptake compared with normal gray matter on FDG PET, and the uptake ratio of tumor to normal brain background on FLT PET was significantly higher than those of tumor to normal gray matter or white matter on FDG PET. These results suggest that FLT PET is superior to FDG PET in the delineation of brain tumors and in evaluating their histological grade and proliferative activity. So, it may be possible that the role of FLT in assessing response would be more relevant.
In conclusion, we have shown here that FLT PET is useful for the evaluation of tumor grade and cellular proliferation in brain tumors. In addition, FLT PET shows high sensitivity and good contrast in evaluating brain lesions displaying decreased or similar uptake compared with normal gray matter on FDG PET. This method, however, does not appear to be capable of differentiating tumors from nontumorous lesions. Further studies including a larger number of patients with nontumorous lesions are needed. These studies should evaluate the impact of FLT PET on the prognosis and therapeutic response of brain tumors in order to clarify further the clinical usefulness of FLT PET.
References
Shields AF, Grierson JR, Dohmen BM, Machulla HJ, Stayanoff JC, Lawhorn-Crews JM, et al. Imaging proliferation in vivo with [F-18]FLT and positron emission tomography. Nat Med 1998;4(11):1334–6.
Wagner M, Seitz U, Buck A, Neumaier B, Schultheiss S, Bangerter M, et al. 3′-[18F]fluoro-3′-deoxythymidine([18F]-FLT) as positron emission tomography tracer for imaging proliferation in a murine B-cell lymphoma model and in the human disease. Cancer Res 2003;63:2681–7.
Eary JF, Mankoff DA, Spence AM, Berger MS, Olshen A, Link JM, et al. 2-[C-11]thymidine imaging of malignant brain tumors. Cancer Res 1999;59:615–21.
Schifter T, Hoffman JM, Hanson MW, Boyko OB, Beam C, Paine S, et al. Serial FDG-PET studies in the prediction of survival in patients with primary tumors. J Comput Assist Tomogr 1993;17(4):509–16.
Kim CK, Gupta NC, Chandramouli B, Alavi A. Standardized uptake values of FDG: body surface area correction is preferable to body weight correction. J Nucl Med 1994;35:164–7.
Herholz K, Pietrzyk U, Voges J, Schroder R, Halber M, Treuer H, et al. Correlation of glucose consumption and tumor cell density in astrocytomas. A stereotactic PET study. J Neurosurg 1993;79:853–8.
Zasadny KR, Wahl RL. Standardized uptake values of normal fissues at PET with 2-[fluorine-18]-fluoro-2-deoxy-D-glucose: variations with body weight and a method for correction. Radiology 1993;189:847–50.
Delbeke D, Meyerowitz C, Lapidus RL, Maciunas RJ, Jennings MT, Moots PL, et al. Optimal cutoff levels of F-18 fluorodeoxyglucose uptake in the differentiation of low-grade from high-grade brain tumors with PET. Radiology 1995;195:47–52.
Kaschten B, Stevenaert A, Sadzot B, Deprez M, Degueldre C, Del Fiore G, et al. Preoperative evaluation of 54 gliomas by PET with fluorine-18-fluorodeoxyglucose and/or carbon-11-methionine. J Nucl Med 1998;39:778–85.
Weber W, Bartenstein P, Gross MW, Kinzel D, Daschner H, Feldmann HJ, et al. Fluorine-18-FDG PET and iodine-123-IMT SPECT in the evaluation of brain tumors. J Nucl Med 1997;38:802–8.
Oriuchi N, Tomiyoshi K, Inoue T, Ahmad K, Sarwar M, Tokunaga M, et al. Independent thallium-201 accumulation and fluorine-18-fluorodeoxyglucose metabolism in glioma. J Nucl Med 1996;37:457–62.
Chung JK, Kim YK, Kim SK, Lee YJ, Paek S, Yeo JS, et al. Usefulness of 11C-methionine PET in the evaluation of brain lesions that are hypo- or isometabolic on 18F-FDG PET. Eur J Nucl Med Mol Imaging 2002;29:176–82.
Alavi JB, Alavi A, Chawluk J, Kushner M, Powe J, Hickey W, et al. Positron emission tomography in patients with glioma. A predictor of prognosis. Cancer 1988;62:1074–78.
Inoue T, Shibasaki T, Oriuchi N, Aoyagi K, Tomiyoshi K, Amano S, et al. 18Fα-methyl tyrosine PET studies in patients with brain tumors. J Nucl Med 1999;40:399–405.
Tomiyoshi K, Amed K, Muhammad S, Higuchi T, Inoue T, Endo K, et al. Synthesis of isomers of 18F labelled amino acid radiopharmaceutical: 2- and 3-L−18F-alpha-methyltyrosine using a separation and purification system. Nucl Med Commun 1997;18:169–75.
Ogawa T, Kanno I, Shishido F, Inugami A, Higano S, Fujita H, et al. Clinical value of PET with 18F-fluorodeoxyglucose and L-methyl-11C-methionine for diagnosis of recurrent brain tumor and radiation injury. Acta Radiol 1991;32:197–202.
Shields AF, Grierson JR, Kozawa SM, Zheng M. Development of labeled thymidine analogs for imaging tumor proliferation. Nucl Med Biol 1996;23:17–22.
Grierson JR, Shields AF. Radiosynthesis of 3′-deoxy-3′-[18F]fluorothymidine: [18F]FLT for imaging of cellular proliferation in vivo. Nucl Med Biol 2000;27:143–56.
Mier W, Haberkorn U, Eisenhut M. [18F]FLT; portrait of a proliferation marker. Eur J Nucl Med Mol Imaging 2002;29:165–9.
Francis DL, Visvikis D, Costa DC, Arulampalam TH, Townsend C, Luthra SK, et al. Potential impact of [18F]3′-deoxy-3′-fluorothymidine versus [18F]fluoro-2-deoxy-D-glucose in positron emission tomography for colorectal cancer. Eur J Nucl Med Mol Imaging 2003;30:988–94.
Seitz U, Wagner M, Neumaier B, Wawra E, Glatting G, Leder G, et al. Evaluation of pyrimidine metabolising enzymes and in vitro uptake of 3′-[18F]fluoro-3′-deoxythymidine ([18F]FLT) in pancreatic cancer cell lines. Eur J Nucl Med Mol Imaging 2002;29:1174–81.
Vesselle H, Grierson J, Muzi M, Pugsley JM, Schmidt RA, Rabinowitz P, et al. In vivo validation of 3′deoxy-3′-[18F]fluorothymidine ([18F]FLT) as a proliferation imaging tracer in humans: correlation of [18F]FLT uptake by positron emission tomography with Ki-67 immunohistochemistry and flow cytometry in human lung tumors. Clin Cancer Res 2002;8:3315–23.
Buck AK, Schirrmeister H, Hetzel M, Von Der Heide M, Halter G, Glatting G, et al. 3-deoxy-3-[18F]fluorothymidine-positron emission tomography for noninvasive assessment of proliferation in pulmonary nodules. Cancer Res 2002;62:3331–4.
Dittmann H, Dohmen BM, Kehlbach R, Bartusek G, Pritzkow M, Sarbia M, et al. Early changes in [18F]FLT uptake after chemotherapy: an experimental study. Eur J Nucl Med Mol Imaging 2002;29:1462–9.
Vijayalakshmi D, Belt JA. Sodium-dependent nucleoside transport in mouse intestinal epithelial cells. Two transport systems with differing substrate specificities. J Biol Chem 1988;263:19419–23.
Kleihues P, Louis DN, Scheithauer BW, Rorke LB, Reifenberger G, Burger PC, et al. The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol 2002;61(3):215–25.
Oh SJ, Mosdzianowski C, Chi DY, Kim JY, Kang SH, Ryu JS. Fully automated synthesis system of 3′-deoxy-3′-[18F]fluorothymidine. Nucl Med Biol 2004;31:803–9.
Di Chiro G. Positron emission tomography using [18F]fluorodeoxyglucose in brain tumors. A powerful diagnostic and prognostic tool. Invest Radiol 1987;22:360–71.
Di Chiro G, DeLaPaz RL, Brooks RA, Sokoloff L, Kornblith PL, Smith BH, et al. Glucose utilization of cerebral gliomas measured by [18F]fluorodeoxyglucose and positron emission tomography. Neurology 1982;32:1323–9.
Tyler JL, Diksic M, Villemure JG, Evans AC, Meyer E, Yamamoto YL, et al. Metabolic and hemodynamic evaluation of gliomas using positron emission tomography. J Nucl Med 1987;28:1123–33.
Sasaki M, Kuwabara Y, Yoshida T, Nakagawa M, Fukumura T, Mihara F, et al. A comparative study of thallium-201 SPET, carbon-11 methionine PET and fluorine-18 fluorodeoxyglucose PET for the differentiation of astrocytic tumours. Eur J Nucl Med 1998;25:1261–9.
Patronas P, Brooks RA, DeLaPaz RL, Smith BH, Kornblith PL, Di Chiro G. Glycolytic rate (PET) and contrast enhancement (CT) in human cerebral gliomas. Am J Neuroradiol 1983;4:533–5.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choi, S.J., Kim, J.S., Kim, J.H. et al. [18F]3′-deoxy-3′-fluorothymidine PET for the diagnosis and grading of brain tumors. Eur J Nucl Med Mol Imaging 32, 653–659 (2005). https://doi.org/10.1007/s00259-004-1742-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-004-1742-3