Abstract
The tellurium oxyanion tellurate is toxic to living organisms even at low concentrations; however, its mechanism of toxicity is poorly understood. Here, we show that exposure of Escherichia coli K-12 to tellurate results in reduction to elemental tellurium (Te[0]) and the formation of intracellular reactive oxygen species (ROS). Toxicity assays performed with E. coli indicated that pre-oxidation of the intracellular thiol pools increases cellular resistance to tellurate—suggesting that intracellular thiols are important in tellurate toxicity. X-ray absorption spectroscopy experiments demonstrated that cysteine reduces tellurate to elemental tellurium. This redox reaction was found to generate superoxide anions. These results indicate that tellurate reduction to Te(0) by cysteine is a source of ROS in the cytoplasm of tellurate-exposed cells.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tellurium (Te) is a group 16 element that naturally occurs in four inorganic oxidation states (Missen et al. 2020). The oxidized forms tellurate (Te[VI], TeO42−) and tellurite (Te[IV], TeO32−) exist as water soluble oxyanions and are known to be toxic to living organisms (Ba et al. 2010; Presentato et al. 2019). Both prokaryotic and eukaryotic microorganisms readily reduce tellurite and tellurate to less toxic elemental tellurium (Te[0]) (Avazéri et al. 1997; Baesman et al. 2009; Gharieb and Gadd 2004; Gharieb et al. 1999; Maltman et al. 2016, 2017; Theisen et al. 2013). Prior toxicological studies of tellurium have primarily focused on the response of microorganisms to tellurite exposure and the mechanisms of tellurite reduction (Chasteen et al. 2009; Pérez et al. 2007; Tremaroli et al. 2007; Zannoni et al. 2007). By comparison, relatively little is known about the cellular response to tellurate and the biochemical pathways that reduce tellurate to Te(0).
Tellurite toxicity studies performed with the model microorganism Escherichia coli K-12 have demonstrated that tellurite exposure results in depletion of the cytoplasmic reduced thiol pool concurrent with a significant loss of cell viability (Turner et al. 1999, 2001). Turner et al. monitored intracellular thiol pools in E. coli during tellurite exposure and found that glutathione is the main target in the observed tellurite-induced thiol depletion. Later work suggested that tellurite exposure triggers the formation of reactive oxygen species (ROS) in the cytoplasm of bacteria (Borsetti et al. 2005; Pérez et al. 2007; Tremaroli et al. 2007). The generation of ROS is accompanied by the development of extensive cellular damage including increased oxidation of cytoplasmic proteins, damage to iron cofactors, and oxidation of membrane lipids (Calderon et al. 2009; Daly et al. 2007; Pérez et al. 2007, 2008; Pradenas et al. 2013). The reduction of tellurite in vitro by the enzymes catalase or NADH-dehydrogenase II was shown to produce superoxide (Díaz-Vásquez et al. 2014; Pérez et al. 2007). This suggests that ROS can be produced directly during the reduction of tellurite. Similarly, superoxide is produced from the abiotic reduction of the selenium oxyanion selenite by glutathione and other thiols (Kessi and Hanselmann 2004; Lin and Spallholz 1993; Staicu and Barton 2017). To date, it remains largely unknown whether similar cellular processes occur in tellurate-exposed cells. Recent work has demonstrated that tellurate toxicity in aerobically-grown E. coli cells was dependent on its transport into the cytoplasm (Goff and Yee 2017). An earlier report had also noted that tellurate exposure resulted in depletion of the cytoplasmic reduced thiol pool of E. coli (Turner et al. 1999). Therefore, interaction with cytoplasmic thiols may contribute to the toxicity of tellurate.
In this study, we investigated the cellular response to tellurate exposure. Experiments were conducted with E. coli to monitor the formation of ROS during tellurate exposure. Because the toxicity of tellurate was found to be dependent on the intracellular thiol pool, in vitro tellurate reduction experiments with thiols were performed to determine if cysteine was involved in tellurate toxicity. The results indicated that cysteine reduces tellurate to elemental tellurium [Te(0)] and that this reaction induces intracellular oxidative stress via the formation of superoxide.
Materials and methods
Strains and growth conditions
Experiments were conducted with the wild-type E. coli K-12 strain. Single gene knock-out strains (ΔfliY::kan and ΔydnJ::kan) were taken from the Keio collection (Baba et al. 2006). Experiments were performed in either LB medium or in M9 medium (0.68 g/L Na2HPO4, 0.3 g/L KH2PO4, 0.05 g/L NaCl, 0.1 g/L NH4Cl, 0.25 g/L MgSO4, 0.012 g/L CaCl2). In the M9 medium, 0.4% (w/v) glucose was added as a carbon source. Cultures were routinely grown at 37 °C in a shaking incubator at 200 rpm.
Measurement of intracellular oxidative stress
The cell-permeable probe 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) was used as an indicator for the formation of intracellular reactive oxygen species (ROS). Following cleavage of the acetate groups by intracellular esterases and oxidation by intracellular ROS, the nonfluorescent H2DCFDA molecule is converted to the fluorophore 2′,7′-dichlorofluorescein (DCF) (Gomes et al. 2005). Overnight cultures of E. coli were diluted 100-fold into LB medium and grown to mid-log phase (optical density at 600 nm [OD600] ~ 0.6). The mid-log cultures were centrifuged at 5900×g for 5 min. The cell pellet was washed and re-suspended in 0.9% (w/v) NaCl solution. H2DCFDA was then added to a final concentration of 5 µM. The reaction mixture was incubated in the dark for 1 h at room temperature. Following incubation, excess extracellular H2DCFDA was removed by centrifugation at 5900×g for 5 min. The cell pellets were re-suspended in 0.9% NaCl solution, treated with tellurate (0.5–2 mM), and DCF fluorescence signal intensities were monitored at excitation and emission wavelengths of 485 and 528 nm, respectively, on a BioTek Synergy LX plate reader.
Viable cell counts
Experiments were conducted to examine the effect of thiol-blocking on tellurate toxicity in E. coli. Overnight cultures were centrifuged at 5900×g for 5 min, resuspended in phosphate-buffered saline (PBS) (per liter: 8.0 g NaCl, 0.2 g KCl, 1.44 g Na2HPO4, 0.24 g KH2PO4), centrifuged at 5900×g for 5 min, and then resuspended in PBS followed by dilution into fresh M9 medium. Cultures were pre-grown with the thiol-oxidizing agent diamide (1 mM) (Kosower et al. 1969; Pöther et al. 2009).The diamide-treated cultures were grown to OD600 of 0.1 and then spiked with 0.5 mM tellurate. Samples were taken immediately before and after the tellurate spike for viable cell counts. The samples were serially diluted into PBS and then spotted in triplicate (10 µL) onto LB plates. Following overnight incubation at 37 °C, the number of colonies were counted and used to determine the number of viable cells in the cultures.
Cell growth in cystine- and tellurate-treated cultures
We tested if exogenous thiols impact the growth of tellurate-exposed E. coli cells. Overnight cultures of E. coli were centrifuged at 5900×g for 5 min, resuspended in PBS and then diluted into fresh M9 medium. To avoid direct reactions in the cellular medium, we amended the cultures with the oxidized disulfide form of cysteine: cystine. Intracellularly, cystine is reduced to cysteine (Chonoles Imlay et al. 2015). Accordingly, cultures were amended with tellurate (0.5 mM) and/or cystine (0.17 mM), and cell growth was monitored at OD600. The minimum inhibitory concentration (MIC) of tellurate in the presence and absence of cystine (0.17 mM) was also determined for the wild type E. coli, ΔfliY, and ΔydnJ strains. Overnight cultures were diluted into fresh M9 medium in a 96-well plate with an initial cell density of 5 × 105 cells/mL. Increasing tellurate concentrations were added to each well. The MIC was defined as the lowest concentration of tellurate that would inhibit growth.
Abiotic reduction of tellurate by cysteine
In vitro tellurate reduction by cysteine was examined with anoxic solutions in sealed reaction vials purged with and filled with a headspace of N2 gas. Reactions were initiated by mixing 0.5 mM tellurate with varying concentrations of cysteine (0–4 mM). All reactors were shaken gently at room temperature for 24 h to allow the reaction to reach equilibrium. Samples were collected and filtered (0.2 μm) for analysis of the aqueous tellurium and reduced thiol concentrations as described below.
Analytical methods
Samples for aqueous tellurium analysis were acidified with 2% nitric acid and analyzed by Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES) on an iCAP 7400 ICP-OES (Thermo Fisher) at a wavelength of 214.282 nm. Samples for reduced thiol (i.e. glutathione and cysteine) analysis were diluted 100-fold and immediately derivatized using the fluorescent probe monobromobimane (mBBr). mBBr reacts with the sulfhydryl group of thiol molecules to form a stable and highly fluorescent thioether bond (Fahey and Newton 1988). 84 µL of borate/diethylenetriaminepentaacetic acid (DTPA) buffer (100 mM/10 mM, pH 9) and 3 µL of mBBr (50 mM) were added to 800 µL of the sample and allowed to react for 30 min in the dark followed by the addition of 60 µL of 800 mM methanesulfonic acid (MSA). Reduced thiol concentrations were determined using reversed-phase high-pressure liquid chromatography (HPLC) with an Agilent 1100 series fluorescence detector at excitation/emission wavelengths of 390/478 nm. Separations were performed using a gradient method with a ZORBAX Extend-C18 column (Agilent) on an Agilent 1260 Infinity II HPLC system. Solvent A was a 0.25%/1% (v/v) glacial acetic acid/acetonitrile and solvent B was 100% acetonitrile. The following gradient method was used at a flow rate of 0.5 mL per minute: 0–3 min, 3% B; 3–5 min, 12.5% B; 5–18 min 26.4% B; 18–23 min 80% B, 23–33 min, 80% B, 33–35 min, 3% B; 35–40 min 3% B.
X-ray absorption spectroscopy
Precipitates that were formed following the reduction of tellurate by cysteine abiotically and by E. coli were analyzed using X-ray absorption spectroscopy. Te K-edge (31,814 eV) x-ray absorption spectroscopy measurements (Boyanov and Kemner 2019) were carried out at the MR-CAT/EnviroCAT bending magnet beamline (Sector 10, Advanced Photon Source) (Kropf et al. 2010). The abiotic sample was prepared by reacting 1 mM of tellurate with 6 mM of cysteine. The precipitate was collected by centrifugation at 8100×g for 20 min and then further air dried, resulting in a hydrated paste. The E. coli sample was prepared from a mid-log phase culture transferred into fresh LB medium containing 0.1 mM tellurate, incubated for 24 h, and harvested by centrifugation at 8000×g for 15 min. The cell pellet and the Te-cysteine sample were transferred to sample holders and sealed between two layers of Kapton film in a 1.5 mm thick plexiglass well. Standards included sodium tellurate dihydrate powder (Strem Chemicals), sodium tellurite powder (Alfa Aeser), and Te(0) powder (Sigma-Aldrich). The Te powders were ground and mounted on the adhesive side of Kapton tape. X-ray absorption near edge spectra (XANES) and extended x-ray absorption fine structure (EXAFS) spectra were collected at room temperature inside a N2-purged sample cell. The abiotic sample and Te standards were measured in transmission mode using gas-filled ionization chambers. The E. coli sample was measured in fluorescence mode using a four-element energy dispersive detector (Vortex). Energy calibration was established by setting the inflection point in the spectrum from Te powder metal to 31,814 eV and then maintained continuously by collecting data from the reference simultaneously with the data from the samples. Radiation-induced changes in the spectra were not detected. No differences were observed between spectra from fresh areas on the samples so all scans from each sample were averaged to produce the final spectrum. Normalization, background removal, and Fourier transforms of the data were done using the programs AUTOBK and FEFFIT (Newville et al. 1993, 1995).
Superoxide production measurements
Superoxide formation during the reduction of tellurate by cysteine was monitored using the tetrazolium salt WST-1. Superoxide radicals reduce WST-1 to a yellow-colored formazan product detectable at 450 nm by UV–Vis spectroscopy (Tan and Berridge 2000). The reaction was initiated by mixing 3 mM cysteine with 0.5 mM tellurate in 50 mM phosphate buffer (pH 7) containing WST-1 (0.3 mg/mL) and monitored at 450 nm. Superoxide dismutase (SOD) (2 mg/mL) was added to a subset of reactors to quench the reduction of WST-1 by the superoxide anion. Anoxic experiments were performed in deoxygenated phosphate buffer prepared by bubbling with N2 in stoppered test tubes that were then filled with a headspace of N2.
Results
Tellurate toxicity and the role of thiols
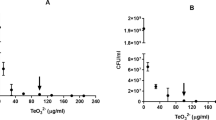
Cytoplasmic ROS were detected in E. coli cultures upon tellurate exposure. Cells exposed to tellurate showed higher DCF fluorescence signals compared to the unexposed control cultures (Fig. 1a). Exposure to increasing tellurate concentrations resulted in increasing DCF fluorescence. We observed 3.5, 5.6, and 7.2-fold increases in fluorescence signal following exposure to 0.5, 1, and 2 mM tellurate, respectively, at the end of 6 h of exposure. These results indicate dose dependent formation of cytoplasmic ROS.
Tellurate toxicity in E. coli cells. a Generation of intracellular ROS in tellurate-exposed E. coli cells was monitored using the fluorescent probe H2DCFDA. b Cell viability following tellurate (0.5 mM) exposure. Cells were pre-treated with (white bars) or without (grey bars) the thiol-oxidizing agent diamide (1 mM). Viable cell counts were performed and the counts were normalized against t = 0 values for the same cultures. c Growth of cultures treated with tellurate (0.5 mM) and/or cystine (0.17 mM). For all datasets, each data point is the average of three replicates and error bars represent ± SD
Growth experiments showed that pre-treatment of cultures with the thiol-oxidizing agent diamide was protective against tellurate toxicity. At 30 min following tellurate (0.5 mM) exposure, 63 ± 23% of the diamide-treated cells remained viable compared to 1.4 ± 0.45% of the untreated cells (Fig. 1b). A similar protective effect was observed at 120 min post-exposure.
The addition of cystine to the culture medium significantly enhanced tellurate toxicity (Fig. 1c). Simultaneous addition of tellurate and cystine to E. coli cultures entirely abolished growth. By comparison, the addition of tellurate without cystine had a moderate toxicity effect, with a prolonged lag phase and cultures reaching an OD600 that was 79% of the untreated control. The addition of cystine alone had a minor impact on cell growth, with a slightly increased lag phase relative to the untreated cells; however, both cultures reached the same maximum OD600 after 25 h. To determine if the enhanced tellurate toxicity is dependent on translocation of the cystine into the cytoplasm of the cells, we measured the MIC of tellurate in knock-out strains of the two characterized cystine transporters of E. coli: ΔydjN and ΔfliY. FliY-YecSC is a high-affinity cystine transporter with a Km in the nanomolar range (Butler et al. 1993; Chonoles Imlay et al. 2015) YdjN has a lower affinity for cystine with a Km in the micromolar range and is the primary cystine transporter in E. coli under cystine-rich growth conditions relevant to our experiments here (Chonoles Imlay et al. 2015). In the presence of cystine, ΔydjN showed greater resistance to tellurate compared to the wild-type strain (Table 1). In the absence of cystine, no difference was observed between the two strains. ΔfliY had similar tellurate sensitivity as the wild-type strain.
Reduction of tellurate by cysteine in vitro generates superoxide
As exogenous and intracellular thiols both had a significant impact on tellurate toxicity, we further investigated the reactions between tellurate with the major intracellular thiols of E. coli: cysteine and glutathione (Wheldrake 1967). In vitro experiments showed that glutathione was unreactive towards tellurate (Fig. S1). Measurements of soluble tellurium and reduced glutathione in reactors showed no reaction after 24 h at all tested glutathione:tellurate ratios. In contrast, cysteine was highly reactive towards tellurate. The formation of a dark precipitate was visually observed within minutes of tellurate reaction with cysteine. XANES and EXAFS analyses of the precipitate indicated that the soluble tellurate oxyanion was reduced and precipitated as its elemental form [Te(0)] without a detectable reaction intermediate (Fig. 2a). The XANES spectra of oxidized Te(IV) and Te(VI) in the tellurite and tellurate standards show a well pronounced white line, whereas the lower valent Te(0) standard has a significantly suppressed white line. Similar spectral dependence on Te valence has been observed previously (Harada and Takahashi 2008; Qin et al. 2017). The edge position and the white line of the Te-cysteine spectrum closely match those of elemental tellurium, indicating that the reaction of cysteine with tellurate resulted in its complete reduction to Te(0). Similarly, the spectrum of tellurium associated with E. coli biomass in cultures grown with tellurate matches the XANES spectrum of Te(0) (Fig. 2a). The EXAFS data also indicate complete reduction of tellurate to Te(0) in both the cysteine experiments and in the E. coli sample (Fig. 2b).
In vitro reaction between cysteine and tellurate. a Te K-edge XANES data and b corresponding EXAFS data from the abiotic cysteine reactions and E. coli samples. The experimental spectra match the Te(0) standard, indicated by the suppressed white line (XANES) and the peak from Te0–Te0 coordination in the Te(0) standard (EXAFS). c Tellurium and cysteine concentrations remaining in solution as a function of the initial concentration of cysteine added. The initial tellurate concentration in each reactor was approximately 0.5 mM. Final tellurium (black circles) and cysteine (open squares) concentrations were measured after 24 h of reaction
Reaction of tellurate with cysteine resulted in the loss of soluble tellurium from the dissolved phase (Fig. 2c). Soluble tellurium concentrations decreased linearly with increasing cysteine up to cysteine:tellurate ratios of 6:1 which indicated that 6 mol of cysteine were needed to reduce 1 mol of tellurate. Complete consumption of cysteine was observed in reactors containing 2:1, 4:1, and 6:1 ratios of cysteine to tellurate, suggesting its complete oxidation to cystine (Fig. 2c). When cysteine and tellurate were reacted at an 8:1 ratio, a portion of the added cysteine remained in excess. Control experiments without added tellurate showed no loss of cysteine (data not shown).
Finally, we found that the reaction between cysteine and tellurate directly yielded ROS. Reaction of cysteine (3 mM) and tellurate (0.5 mM) in the presence of the superoxide-reactive molecule WST-1 formed a yellow-colored formazan product that was detectable spectrophotometrically at 450 nm (A450). At 60 min, reactors containing both cysteine and tellurate had approximately 30-fold increased A450 compared to the control reactors containing tellurate or cysteine alone (Fig. 3a). The maximal rate (0.088 min−1) of formazan formation was reached after 20 min of reaction. To verify that the superoxide anion is the reductant of WST-1 under these conditions, we repeated the reaction with the addition of superoxide dismutase (SOD) (2 mg/mL) (Fig. 3a). SOD inhibited formazan product formation which resulted in A450 at 60 min that was 86% lower than in the reactors without added SOD. To determine if the generation of superoxide anion is dependent on molecular oxygen, these experiments were repeated in deoxygenated solution. Similar to the experiments performed under oxygenated conditions, we observed approximately 30-fold greater A450 relative to the control following 60 min of reaction (Fig. 3b). Together, these results indicate that the reduction of tellurate by cysteine generates superoxide via a reaction mechanism that does not involve molecular oxygen.
Generation of superoxide anion during reduction of tellurate by cysteine. a Formation of the superoxide anion during tellurate (0.5 mM) reduction by cysteine (3 mM) under normal atmospheric conditions. Each data point is the average of three replicates and error bars represent ± SD. Error bars are present but are often smaller than the plotted point. b Formation of the superoxide anion during tellurate (0.5 mM) reduction by cysteine (3 mM) under anoxic conditions
Discussion
The results showed that cystine supplementation to the culture medium of E. coli significantly increased tellurate toxicity (Fig. 1c). This toxicity effect is similar to previous observations that have been made with tellurite-exposed cells. The addition of either cysteine or cystine to culture media enhances the toxicity of tellurite in E. coli (Scala and Williams 1963) and in the purple non-sulfur bacterium Rhodobacter sphaeroides (Moore and Kaplan 1992). Furthermore, cysteine, glutathione, 2-mercaptoethanol, and dithiotreitol have been shown to enhance tellurite toxicity in Staphylococcus aureus (Pugin et al. 2014; Turner et al. 2007). Once transported inside the cell, cystine is reduced to cysteine (Chonoles Imlay et al. 2015). We observed that an E. coli knock-out strain deficient in the cystine transporter YdjN showed greater resistance to tellurate in the presence of cystine compared to the wild-type strain grown under the same conditions (Table 1). This suggests that cystine-mediated tellurate toxicity is dependent upon transport of the extracellular cystine into the cell where it would be reduced to cysteine. We propose that intracellular cysteine is the thiol species enhancing the toxicity of tellurate.
The thiol-oxidizing agent diamide was used to test the impact of intracellular disulfide stress on tellurate toxicity. Diamide oxidizes sulfhydryl moieties within the cytoplasm (Kosower et al. 1969). Cultures that were pre-grown with diamide prior to tellurate exposure showed greater resistance to tellurate compared to cultures without pre-oxidation of the thiol pool (Fig. 1b). We noted also that the diamide pre-treatment resulted in less visible precipitation of dark Te(0) solids in the culture medium, leading us to hypothesize that tellurate reduction and toxicity are both linked to the intracellular thiol pool of E. coli.
Cysteine reduced tellurate to Te(0) in vitro (Fig. 2), and the reaction generated superoxide anion (Fig. 3). We note that the concentrations of cysteine used for our experiments are relevant to the cytoplasmic concentrations of cysteine reported previously for E. coli (Park and Imlay 2003). Interestingly, tellurate did not react with glutathione even when glutathione was provided in excess. Notably, the standard reduction potentials of the cystine/cysteine and glutathione disulfide/glutathione redox couples are very similar (− 250 and − 264 mV, respectively) and the standard reduction potentials for the tellurate/tellurite and tellurite/elemental tellurium redox couples are relatively high (+ 892 mV, and + 827 mV, respectively) (Bouroushian 2010; Park and Imlay 2003). Thus, tellurate reduction by either cysteine or glutathione is thermodynamically favorable. The lack of glutathione reactivity suggests that other factors control the differences in their observed reactivities with tellurate. Interestingly, cysteine and glutathione also differ greatly in their reactivities with free intracellular iron. The contrasting reactivity of the two thiols towards iron is thought to be related to the differences in coordination chemistry and the rates of electron transfer (McAuliffe and Murray 1972; Park and Imlay 2003). A similar phenomenon may be occurring here with tellurate.
Tellurate exposure resulted in cytoplasmic ROS formation in E. coli (Fig. 1a). Induction of intracellular oxidative stress by tellurate is similar to the toxicity mechanism of many other metals and metalloids, including selenite (Bébien et al. 2002), selenate (Bébien et al. 2002), tellurite (Pérez et al. 2007), gold (Muñoz-Villagrán et al. 2020), silver (Tian et al. 2018), copper (Dupont et al. 2011), and cadmium (Pacheco et al. 2008). For example, tellurite reduction in vitro by the E. coli catalase or NADH-dehydrogenase II enzymes have been shown to generate superoxide anion (Díaz-Vásquez et al. 2014; Pérez et al. 2007). Reduction of selenite with glutathione or cysteine also results in the formation of superoxide (Kessi and Hanselmann 2004; Kramer and Ames 1988; Spallholz 1994). Likewise, we demonstrate here that reaction between tellurate and cysteine yields superoxide. Formation of superoxide within the cytoplasm of cells can directly damage the [Fe-S] centers of enzymes, cause membrane lipid peroxidation, as well as protein oxidation (Chasteen et al. 2009). Subsequent Fenton or Haber–Weiss reactions can also generate hydroxyl radicals which cause DNA damage (Imlay and Linn 1988).
In contrast to the common paradigm of reduction of toxic metals and metalloids to their less toxic forms as a cellular defense mechanism (Bruins et al. 2000), this work and prior studies (Díaz-Vásquez et al. 2014; Pérez et al. 2007) indicate that the reduction of tellurium oxyanions generates harmful ROS. While Te(0) is indeed less toxic than either tellurite or tellurate, arriving at that point appears to come at a significant and often deadly cost (Borsetti et al. 2005; Pérez et al. 2007; Tremaroli et al. 2007). As cysteine is ubiquitous across all domains of life, the mode of tellurate toxicity described here likely extends beyond E. coli to other prokaryotic and eukaryotic organisms.
References
Avazéri C, Turner RJ, Pommier J, Weiner JH, Giordano G, Verméglio A (1997) Tellurite reductase activity of nitrate reductase is responsible for the basal resistance of Escherichia coli to tellurite. Microbiology 143:1181–1189. https://doi.org/10.1099/00221287-143-4-1181
Ba LA, Döring M, Jamier V, Jacob C (2010) Tellurium: an element with great biological potency and potential. Org Biomol Chem 8:4203–4216. https://doi.org/10.1039/C0OB00086H
Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H (2006) Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol. https://doi.org/10.1038/msb4100050
Baesman SM, Stolz JF, Kulp TR, Oremland RS (2009) Enrichment and isolation of Bacillus beveridgei sp. nov., a facultative anaerobic haloalkaliphile from Mono Lake, California, that respires oxyanions of tellurium, selenium, and arsenic. Extremophiles 13:695–705. https://doi.org/10.1007/s00792-009-0257-z
Bébien M, Lagniel G, Garin J, Touati D, Verméglio A, Labarre J (2002) Involvement of superoxide dismutases in the response of Escherichia coli to selenium oxides. J Bacteriol 184:1556. https://doi.org/10.1128/JB.184.6.1556-1564.2002
Borsetti F, Tremaroli V, Michelacci F, Borghese R, Winterstein C, Daldal F, Zannoni D (2005) Tellurite effects on Rhodobacter capsulatus cell viability and superoxide dismutase activity under oxidative stress conditions. Res Microbiol 156:807–813. https://doi.org/10.1016/j.resmic.2005.03.011
Bouroushian M (2010) Electrochemistry of the chalcogens. In: Bouroushian M (ed) Electrochemistry of metal chalcogenides. Springer, Berlin, pp 57–75. https://doi.org/10.1007/978-3-642-03967-6_2
Boyanov MI, Kemner KM (2019) Application of synchrotron X-ray absorption spectroscopy and microscopy techniques to the study of biogeochemical processes. In: Alessi DS, Veeramani H, Kenney JPL (eds) Analytical geomicrobiology: a handbook of instrumental techniques. Cambridge University Press, Cambridge, pp 238–261. https://doi.org/10.1017/9781107707399.010
Bruins MR, Kapil S, Oehme FW (2000) Microbial resistance to metals in the environment. Ecotoxicol Environ Saf 45:198–207. https://doi.org/10.1006/eesa.1999.1860
Butler JD, Levin SW, Facchiano A, Miele L, Mukherjee AB (1993) Amino acid composition and N-terminal sequence of purified Cystine Binding Protein of Escherichia coli. Life Sci 52:1209–1215. https://doi.org/10.1016/0024-3205(93)90103-A
Calderon IL, Elías AO, Fuentes EL, Pradenas GA, Castro ME, Arenas FA, Perez JM, Vasquez CC (2009) Tellurite-mediated disabling of [4Fe–4S] clusters of Escherichia coli dehydratases. Microbiology 155:1840–1846
Chasteen TG, Fuentes DE, Tantaleán JC, Vásquez CC (2009) Tellurite: history, oxidative stress, and molecular mechanisms of resistance. FEMS Microbiol Rev 33:820–832. https://doi.org/10.1111/j.1574-6976.2009.00177.x
Chonoles Imlay KR, Korshunov S, Imlay JA (2015) Physiological roles and adverse effects of the two cystine importers of Escherichia coli. J Bacteriol 197:3629. https://doi.org/10.1128/JB.00277-15
Daly MJ, Gaidamakova EK, Matrosova VY, Vasilenko A, Zhai M, Leapman RD, Lai B, Ravel B, Li S-MW, Kemner KM, Fredrickson JK (2007) Protein oxidation implicated as the primary determinant of bacterial radioresistance. PLoS Biol 5:e92. https://doi.org/10.1371/journal.pbio.0050092
Díaz-Vásquez WA, Abarca-Lagunas MJ, Arenas FA, Pinto CA, Cornejo FA, Wansapura PT, Appuhamillage GA, Chasteen TG, Vásquez CC (2014) Tellurite reduction by Escherichia coli NDH-II dehydrogenase results in superoxide production in membranes of toxicant-exposed cells. Biometals 27:237–246. https://doi.org/10.1007/s10534-013-9701-8
Dupont CL, Grass G, Rensing C (2011) Copper toxicity and the origin of bacterial resistance—new insights and applications. Metallomics 3:1109–1118
Fahey RC, Newton GL (1988) Determination of low molecular weight thiols using monobromobimane fluorescent labeling and high-performance liquid chromatography—NASA-CR-182932
Gharieb MM, Gadd GM (2004) Role of glutathione in detoxification of metal(loid)s by Saccharomyces cerevisiae. Biometals 17:183–188. https://doi.org/10.1023/B:BIOM.0000018402.22057.62
Gharieb MM, Kierans M, Gadd GM (1999) Transformation and tolerance of tellurite by filamentous fungi: accumulation, reduction, and volatilization. Mycol Res 103:299–305
Goff J, Yee N (2017) Tellurate enters Escherichia coli K-12 cells via the SulT-type sulfate transporter CysPUWA. FEMS Microbiol Lett. https://doi.org/10.1093/femsle/fnx241
Gomes A, Fernandes E, Lima JLFC (2005) Fluorescence probes used for detection of reactive oxygen species. J Biochem Biophys Methods 65:45–80. https://doi.org/10.1016/j.jbbm.2005.10.003
Harada T, Takahashi Y (2008) Origin of the difference in the distribution behavior of tellurium and selenium in a soil–water system. Geochim Cosmochim Acta 72:1281–1294. https://doi.org/10.1016/j.gca.2007.12.008
Imlay JA, Linn S (1988) DNA damage and oxygen radical toxicity. Science 240:1302–1309. https://doi.org/10.1126/science.3287616
Kessi J, Hanselmann KW (2004) Similarities between the abiotic reduction of selenite with glutathione and the dissimilatory reaction mediated by Rhodospirillum rubrum and Escherichia coli. J Biol Chem 279:50662–50669. https://doi.org/10.1074/jbc.M405887200
Kosower NS, Kosower EM, Wertheim B, Correa WS (1969) Diamide, a new reagent for the intracellular oxidation of glutathione to the disulfide. Biochem Biophys Res Commun 37:593–596. https://doi.org/10.1016/0006-291X(69)90850-X
Kramer GF, Ames BN (1988) Mechanims of mutagenicity and toxicity of sodium selenite (Na2SeO3) in in Salmonella typhimurium. Mutat Res 201:169–180. https://doi.org/10.1016/0027-5107(88)90123-6
Kropf A, Katsoudas J, Chattopadhyay S, Shibata T, Lang E, Zyryanov V, Ravel B, McIvor K, Kemner K, Scheckel K (2010) The new MRCAT (Sector 10) bending magnet beamline at the advanced photon source. In: AIP Conference Proceedings, vol 1. American Institute of Physics, p 299–302
Lin Y, Spallholz JE (1993) Generation of reactive oxygen species from the reaction of selenium compounds with thiols and mammary tumor cells. Biochem Pharmacol 45:429–437
Maltman C, Walter G, Yurkov V (2016) A diverse community of metal(loid) oxide respiring bacteria is associated with tube worms in the vicinity of the Juan de Fuca Ridge Black Smoker Field. PLoS ONE 11:e0149812. https://doi.org/10.1371/journal.pone.0149812
Maltman C, Donald LJ, Yurkov V (2017) Tellurite and tellurate reduction by the aerobic anoxygenic phototroph Erythromonas ursincola, strain KR99 is carried out by a novel membrane associated enzyme. Microorganisms 5:20. https://doi.org/10.3390/microorganisms5020020
McAuliffe CA, Murray SG (1972) Metal complexes of sulphur-containing amino acids. Inorg Chim Acta Rev 6:103–121. https://doi.org/10.1016/0073-8085(72)80013-5
Missen OP, Ram R, Mills SJ, Etschmann B, Reith F, Shuster J, Smith DJ, Brugger J (2020) Love is in the earth: a review of tellurium (bio)geochemistry in surface environments. Earth Sci Rev 204:103150. https://doi.org/10.1016/j.earscirev.2020.103150
Moore MD, Kaplan S (1992) Identification of intrinsic high-level resistance to rare-earth oxides and oxyanions in members of the class Proteobacteria: characterization of tellurite, selenite, and rhodium sesquioxide reduction in Rhodobacter sphaeroides. J Bacteriol 174:1505–1514. https://doi.org/10.1128/jb.174.5.1505-1514.1992
Muñoz-Villagrán C, Contreras F, Cornejo F, Figueroa M, Valenzuela-Bezanilla D, Luraschi R, Reinoso C, Rivas-Pardo J, Vásquez C, Castro M (2020) Understanding gold toxicity in aerobically-grown Escherichia coli. Biol Res 53:1–9
Newville M, Līviņš P, Yacoby Y, Rehr JJ, Stern EA (1993) Near-edge x-ray-absorption fine structure of Pb: a comparison of theory and experiment. Phys Rev B 47:14126–14131. https://doi.org/10.1103/PhysRevB.47.14126
Newville M, Ravel B, Haskel D, Rehr JJ, Stern EA, Yacoby Y (1995) Analysis of multiple-scattering XAFS data using theoretical standards. Phys B 208–209:154–156. https://doi.org/10.1016/0921-4526(94)00655-F
Pacheco CC, Passos JF, Castro AR, Moradas-Ferreira P, De Marco P (2008) Role of respiration and glutathione in cadmium-induced oxidative stress in Escherichia coli K-12. Arch Microbiol 189:271–278. https://doi.org/10.1007/s00203-007-0316-8
Park S, Imlay JA (2003) High levels of intracellular cysteine promote oxidative DNA damage by driving the Fenton reaction. J Bacteriol 185:1942–1950. https://doi.org/10.1128/jb.185.6.1942-1950.2003
Pérez JM, Calderón IL, Arenas FA, Fuentes DE, Pradenas GA, Fuentes EL, Sandoval JM, Castro ME, Elías AO, Vásquez CC (2007) Bacterial toxicity of potassium tellurite: unveiling an ancient enigma. PLoS ONE 2:e211. https://doi.org/10.1371/journal.pone.0000211
Pérez JM, Arenas FA, Pradenas GA, Sandoval JM, Vásquez CC (2008) Escherichia coli YqhD exhibits aldehyde reductase activity and protects from the harmful effect of lipid peroxidation-derived aldehydes. J Biol Chem 283:7346–7353
Pöther D-C, Liebeke M, Hochgräfe F, Antelmann H, Becher D, Lalk M, Lindequist U, Borovok I, Cohen G, Aharonowitz Y, Hecker M (2009) Diamide triggers mainly S thiolations in the cytoplasmic proteomes of Bacillus subtilis and Staphylococcus aureus. J Bacteriol 191:7520–7530. https://doi.org/10.1128/jb.00937-09
Pradenas GA, Díaz-Vásquez WA, Pérez-Donoso JM, Vásquez CC (2013) Monounsaturated fatty acids are substrates for aldehyde generation in tellurite-exposed Escherichia coli. Biomed Res Int 2013:563756. https://doi.org/10.1155/2013/563756
Presentato A, Turner RJ, Vasquez CC, Yurkov V, Zannoni D (2019) Tellurite-dependent blackening of bacteria emerges from the dark ages. Environ Chem 16:266–288. https://doi.org/10.1071/EN18238
Pugin B, Cornejo F, García J, Díaz Vásquez W, Arenas Salinas F, Vásquez C (2014) Thiol-mediated reduction of Staphylococcus aureus tellurite resistance. Adv Microbiol 4:183–190. https://doi.org/10.4236/aim.2014.44024
Qin H-B, Takeichi Y, Nitani H, Terada Y, Takahashi Y (2017) Tellurium distribution and speciation in contaminated soils from abandoned mine tailings: comparison with selenium. Environ Sci Technol 51:6027–6035. https://doi.org/10.1021/acs.est.7b00955
Scala J, Williams HH (1963) A comparison of selenite and tellurite toxicity in Escherichia coli. Arch Biochem Biophys 101:319–324. https://doi.org/10.1016/s0003-9861(63)80019-3
Spallholz JE (1994) On the nature of selenium toxicity and carcinostatic activity. Free Radical Biol Med 17:45–64. https://doi.org/10.1016/0891-5849(94)90007-8
Staicu LC, Barton LL (2017) Bacterial metabolism of selenium—for survival or profit. In: van Hullebusch ED (ed) Bioremediation of selenium contaminated wastewater. Springer International Publishing, Cham, pp 1–31. https://doi.org/10.1007/978-3-319-57831-6_1
Tan AS, Berridge MV (2000) Superoxide produced by activated neutrophils efficiently reduces the tetrazolium salt, WST-1 to produce a soluble formazan: a simple colorimetric assay for measuring respiratory burst activation and for screening anti-inflammatory agents. J Immunol Methods 238:59–68. https://doi.org/10.1016/s0022-1759(00)00156-3
Theisen J, Zylstra GJ, Yee N (2013) Genetic evidence for a molybdopterin-containing tellurate reductase. Appl Environ Microbiol 79:3171–3175. https://doi.org/10.1128/AEM.03996-12
Tian X, Jiang X, Welch C, Croley TR, Wong T-Y, Chen C, Fan S, Chong Y, Li R, Ge C, Chen C, Yin J-J (2018) Bactericidal effects of silver nanoparticles on lactobacilli and the underlying mechanism. ACS Appl Mater Interfaces 10:8443–8450. https://doi.org/10.1021/acsami.7b17274
Tremaroli V, Fedi S, Zannoni D (2007) Evidence for a tellurite-dependent generation of reactive oxygen species and absence of a tellurite-mediated adaptive response to oxidative stress in cells of Pseudomonas pseudoalcaligenes KF707. Arch Microbiol 187:127–135. https://doi.org/10.1007/s00203-006-0179-4
Turner RJ, Weiner JH, Taylor DE (1999) Tellurite-mediated thiol oxidation in Escherichia coli. Microbiology 145:2549–2557. https://doi.org/10.1099/00221287-145-9-2549
Turner RJ, Aharonowitz Y, Weiner JH, Taylor DE (2001) Glutathione is a target in tellurite toxicity and is protected by tellurite resistance determinants in Escherichia coli. Can J Microbiol 47:33–40
Turner MS, Lo R, Giffard PM (2007) Inhibition of Staphylococcus aureus growth on tellurite-containing media by Lactobacillus reuteri is dependent on CyuC and thiol production. Appl Environ Microbiol 73:1005–1009. https://doi.org/10.1128/aem.02100-06
Wheldrake JF (1967) Intracellular concentration of cysteine in Escherichia coli and its relation to repression of the sulphate-activating enzymes. Biochem J 105:697–699. https://doi.org/10.1042/bj1050697
Zannoni D, Borsetti F, Harrison JJ, Turner RJ (2007) The bacterial response to the chalcogen metalloids Se and Te. In: Poole RK (ed) Advances in microbial physiology, vol 53. Academic Press, New York, pp 1–312. https://doi.org/10.1016/S0065-2911(07)53001-8
Acknowledgements
This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility, operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357. We thank the MRCAT beamline staff for assistance during data collection at the synchrotron. MIB and KMK were supported by the Argonne Wetlands Hydrobiogeochemistry Scientific Focus Area funded by the Environmental System Science Program, Office of Biological and Environmental Research, Office of Science, U.S. Department of Energy (DOE), under contract DE-AC02-06CH11357. MRCAT/EnviroCAT operations are supported by DOE and the MRCAT/EnviroCAT member institutions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Goff, J.L., Boyanov, M.I., Kemner, K.M. et al. The role of cysteine in tellurate reduction and toxicity. Biometals 34, 937–946 (2021). https://doi.org/10.1007/s10534-021-00319-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-021-00319-8