Abstract
Safe disposal of petroleum oil sludge generated from crude oil storage tank bottom is a major challenge for petroleum refineries across the globe. The presence of long chain hydrocarbons in petroleum oil sludge are known to have effects on the environment through bioaccumulation or biosorption. The present study was focused to develop a modified bioremediation process using hydrocarbonoclastic microbial-assisted biocarrier matrix (MABC) mediated through biosurfactants and biocatalysts for the efficient treatment of petroleum industrial oily sludge. The development of hydrocarbonoclastic microbial-assisted biocarrier matrix was confirmed by scanning electron microscopy analysis. The biocatalysts such as lipase, laccase, esterase and biosurfactant produced by MABC system were found to be 40 U/mg, 18 U/mg, 36 U/mg and 220 mg/g of oil sludge respectively using one variable at a time approach. Further, the response surface methodology was used to determine the optimum treatment conditions (Time, pH, Mass of biocarrier matrix and Amount of oil sludge) for the enhanced removal of total petroleum hydrocarbons (TPH) present in the oil sludge and TPH was degraded by 88.78% at Hydraulic Retention Time of 7 days. The biodegradation of oil sludge was confirmed using Gas Chromatography–Mass Spectrometry analysis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Petroleum oil refinery is one of the major industrial sector around the world contributing to world economy (Suganthi et al. 2018). The rapid increase in the human population has created a significant demand in the consumption of petroleum products to meet the transportation, energy, human health care and cosmetics requirements, which has led to a remarkable growth and development of petroleum oil refining industrial sector across the globe (Kuznetsova and Kravchenko 2020). More particularly, during the storage and transportation of crude oil, under the influence of gravity difference between the lighter and heavier molecules present in the crude oil; heavy hydrocarbons, metallic salts, condensed resins and asphaltene settle at the bottom of the tank by forming sticky and black colour aggregates, termed as “oil sludge” (Ramirez and Collins 2018). The expansion of the petroleum refining industries has been accompanied with the sharp increase in the release of petroleum oil sludge during the process of oil storage, shipment and refining operations (Zhang et al. 2011; Hassanzadeh et al. 2018; Chen et al. 2019a). The petroleum refining industry having a production capability of 105,000 barrels per day gives roughly 50 tons of crude oil sludge (COS) per year with the specific gravity (@15 °C) around 0.905 (Mansur et al. 2015; Al Zubaidi 2018; Johnson and Affam 2019). It has been estimated that, nearly 28,000 tons of COS per year in India and more than one billion tons of COS per year in worldwide is generated by petroleum industries (Hu et al. 2013; Suganthi et al. 2018). Petroleum oil sludge mainly exists in the form of stable water-in-oil (W/O) emulsion, having composition oil, 15–50%; water, 30–50% and inorganic solids, 10–12% (w/w) (Xiaogang et al. 2009; He et al. 2019). The constituents of oil COS waste predominantly includes recalcitrant poly-aromatic hydrocarbons (PAHs) (naphthalene, anthracene, benzopyrene, etc.), aliphatic hydrocarbons (propane, pentane, hexane, octane, propene, cyclopentene, etc.), unsaturated hydrocarbons, alicyclic hydrocarbons, considerable amount of heavy metals (lead, zinc, chromium, nickel, manganese, cadmium, copper, iron) and NSO (nitrogen–sulfur–oxygen compounds) (Janbandhu and Fulekar 2011; Hu et al. 2013; Wang et al. 2018). Hence, this is classified under hazardous waste materials in petroleum refining industries. PAHs and long chain aliphatic hydrocarbons are highly persistent in the environment and acts as carcinogens and mutagens (DNA damage and chromosomal aberration), thereby it causes lethal effects on the biotic as well as abiotic factors. These constitute a big challenge to microbial degradation of petroleum oil sludge/COS (Dhote et al. 2018). Moreover, the percolation of the untreated hydrocarbons/partially treated hydrocarbons into the ground water system as well as the soil nearby treatment units becomes unavoidable (Suganthi et al. 2018). Hence, the petroleum industries must ensure that the toxic hydrocarbons present in the COS must undergo several stages of treatment for the removal of recalcitrant compounds to satisfy the discharging standards prescribed by Environmental Protection Agency (EPA). Currently, various physico-chemical methods such as incineration (Scala and Chirone 2004), stabilization/solidification (Karamalidis and Voudrias 2007), solvent extraction (Zubaidy and Abouelnasr 2010), freeze/thawing (Lin et al. 2008), photo-catalysis (da Rocha et al. 2010), combustion (Zhou et al. 2009), pyrolysis (Liu et al. 2009), chemical oxidation (Ferrarese et al. 2008), ultrasonic treatment (Xu et al. 2009), supercritical water oxidation (SCWO) technology (Cui et al. 2009) and microwave radiation technology (Kuo and Lee 2010) are in practice due to the unavailability of efficient eco-friendly treatment processes (Suganthi et al. 2018). However, these methods have several disadvantages like non-eco-friendly, in nature, low treatment efficiency, hotspot of secondary toxicants, high operational and maintenance cost and requires skilled labour to operate (He et al. 2019; Li et al. 2020).
Hence, the cost-effective bioremediation process such as landfilling (Aguelmous et al. 2020), land farming (Besalatpour et al. 2011), phytoremediation (Nanekar et al. 2015), biodegradation (Ward et al. 2003; Suganthi et al. 2018) and surfactant flushing (Karthick et al. 2019) have gained importance in field level applications. However, these methods were reported to be time-consuming process, contributes to GHGs emissions, and also their effectiveness is limited by environmental factors (Caliman et al. 2011; Saimmai et al. 2012; Ismail et al. 2013; Sarika Saxena 2015). As a result, the rate of degradation of the constituents of COS is impaired and such that it requires 6 to 8 months for the removal/degradation of hydrocarbons in oil sludge (Hassanshahian et al. 2012). Therefore, the management of COS in petroleum industry remains unsolved due to non-availability of proper technological solutions. Thus, there has been a constant research on the cost-effective microbial treatment system for the degradation of hydrocarbons present in the petroleum refining industry oil sludge (PRIOS) in short retention time.
Bioremediation of PRIOS using indigenous microorganisms is reported to be the most reliable method for the treatment of complex pollutants like oily wastes. The inherent characteristic adaptability of the microbial community and the ability to produce extracellular biomolecules i.e. biosurfactants and biocatalysts such as hydrolytic enzymes (lipase and esterase) and oxidoreductase (laccase), play a significant role in the degradation of toxic hydrocarbons into non-toxic TCA metabolic intermediates (Svendsen 2000; Liu 2005; Das and Chandran 2011; Frutos et al. 2012; Ferradji et al. 2014; Parthipan et al. 2017). In this context, the application of a mixed bacterial consortium has proved to be more beneficial in comparison to pure culture for the complete degradation of the hydrocarbons in the PRIOS (Mukred et al. 2008; Cerqueira et al. 2011a). Suganthi et al. (2018) reported the petroleum oil sludge bioremediation with intermittent addition of mixed microbial consortia for the treatment process of PRIOS to result in degradation of TPH by 96% within 1 month duration. However, it was concluded that the duration of treatment for the complete removal of hydrocarbons must be reduced significantly for the field level applications (Suganthi et al. 2018). The complex molecular configuration of petroleum oily waste sterically hinders their bioaccessibility to the conventional microbes used in the biodegradation process and also known to cause harmful effects on the environment through bioaccumulation and biotransformation (D’Costa et al. 2017). Hence, these conventional technologies more often fail to remove/degrade these immiscible and dispersed organic components due to their structural complexity (Aguelmous et al. 2019). Therefore, the treatment process of wastes generated from petroleum refining industries should be composed of interactive medium that can act as a stable microbial matrix and also to improve the surface contact between the oil sludge and the microbes (Chen et al. 2019b; Huang et al. 2019; Naeem and Qazi 2020). This would improve the accessibility of microorganisms towards the petroleum oil sludge in such a way that the metabolism of the constituents of oil sludge becomes more effective. Subsequently, the degradation of hydrocarbons at an enhanced rate can be achieved.
The focal theme of the present investigation is to develop a sustainable microbial-assisted biocarrier matrix system, using the agro waste (rice husk), for the complete degradation of petroleum hydrocarbons in the PRIOS at short retention time. It is well known, that the process optimization is essential for the successful development of any technology/treatment system. Therefore, in the present study, central composite design (CCD) mediated response surface methodology was employed to optimize the treatment conditions for achieving the maximum removal of TPH using MABC.
Materials and methods
All the chemicals and medium components used in this study were of analytical grade.
Collection of petroleum oil sludge and source for the petroleum hydrocarbon degrading microbes (PHC)
The petroleum refining industry oil sludge (PRIOS) was collected from the oil storage tank of a petroleum oil refining industry located in Chennai, Tamil Nadu. The indigenous hydrocarbonoclastic bacterial strains were isolated from diesel contaminated soil nearby generator (DC), crude oil contaminated soil from petroleum oil refinery (MS), soil acclimatized with petroleum oil sludge near the sewage treatment plant (STP) and soil around automobile workshop (AWS). Soil samples were stored at 4 ºC until further use.
Characterization of petroleum oil sludge
The PRIOS was characterised for its total petroleum hydrocarbon (TPH) content followed by the component analysis using Gas Chromatography–Mass Spectrometry (GC–MS) for the detection of hydrocarbons in oil sludge.
Total petroleum hydrocarbon (TPH) analysis
The TPH in the petroleum oil sludge was analysed in accordance with the procedure listed under EPA 418.1 analytical method with slight modifications. The oil sludge, 1 g was extracted with DCM to remove the dust and sand particles as precipitates. DCM was evaporated using rotary evaporator. Then the hexane was used to separate the TPHs from the DCM extract. This step was repeated for 3 times. The solvent layer having TPH was collected and evaporated in rotary evaporator to separate out the traces of hexane. The mass of TPH was determined gravimetrically from the initial and final weight of extracted hydrocarbon (Mishra et al. 2001; Tahhan and Abu-Ateih 2009). The experiment was carried out in triplicates. The percentage of hydrocarbon degradation was calculated using the formula (Suganthi et al. 2018).
Gas Chromatography–Mass Spectrometry (GC–MS)
The hydrocarbons present in the initial oil sludge (1 g) were extracted using n-hexane, 10 ml and the solvent layer was dried. The sample was injected into the GC–MS instrument (Agilent Technologies, USA, 7890B GC system coupled with 5977A MSD system) equipped with DB5MS column (30 m length, 0.25 mm internal diameter and 0.25 μ film thicknesses). The Flow rate of helium gas was maintained at 1.5 ml/min. The initial set temperature was 55 °C with 5 °C increase in temperature for every 1 min till 300 °C was reached and then held at the temperature for 40 min. The separated compounds were compared with reference spectra in NIST 2000 Library match (Gojgic-Cvijovic et al. 2012).
Isolation and enrichment of hydrocarbonoclastic microorganisms from soil sources
The indigenous hydrocarbonoclastic bacterial strains were isolated from hydrocarbon contaminated soil sources (mentioned under “Collection of petroleum oil sludge and source for the petroleum hydrocarbon degrading microbes (PHC)” section). For this, 1 g of each soil samples was individually inoculated into mineral salt medium (MSM) having composition (g/l): MgSO4, 1.0; K2HPO4 , 0.8; CaCl2·2H2O, 0.5; NaH2PO4 , 0.2; FeSO4·7H2O, 0.05; CuSO4·5H2O , 0.05; and NaCl, 0.1 and petroleum oil sludge 1% (w/v) as the carbon and energy source. The medium pH was adjusted to 7.0 and allowed for an incubation period for 7 days at 35 °C and agitated at 120 rpm for the growth and enrichment process. In 7 days interval, an aliquot of 10 ml of culture was transferred into fresh mineral salts medium with increasing concentration of oil sludge [2, 4, 6, 8, 10% (w/v)] subsequently and incubated under the same conditions. After which, the cultures endured in the oil sludge containing medium were isolated by serial dilution and spread platting method. Each colony of different morphologies was streaked to get pure isolates and stored in nutrient agar slants for further studies.
Development of microbial-assisted biocarrier matrix system onto agro-waste
After the enrichment process, the four enriched isolates individually as well as in a consortium (five different combinations of isolates) were allowed for the development of microbial-assisted biocarrier matrix (MABC) onto rice husk as 9 separate experimental setup in total and this was used as a support matrix for the growth of the organisms and to increase the bioavailability of hydrophobic crude oil sludge. Wherein, 10 g of rice husk was dispersed into the 100 ml of mineral salts medium containing 5% (v/v) microbial culture and incubated for 24 h at 37 °C for the development of microbial-assisted biocarrier matrix (MABC)”.
Degradation of oil sludge using microbial-assisted biocarrier system
The developed MABC in single (four individual isolates) and mixed cultures (five combinations of isolates) were used for the treatment of oil sludge. The oil sludge (PRIOS) was layered in between the MABC as a stacking layer with the ratio of 1:1 (i.e. 5 g of oil sludge to 5 g of MABC). To this, 40 ml of mineral salt medium (pH 7) was added and air at ambient condition was uniformly supplied in the entire process to initiate the degradation. The efficiency of MABC in single as well as in mixed cultures for the treatment of oil sludge was monitored based on the production of biomolecules and TPH reduction profile. The reduction in TPHs (percentage degradation of hydrocarbons) was monitored for every 24 h and the procedure was mentioned in "Total petroleum hydrocarbon (TPH) analysis" section. The production of biosurfactant was monitored by emulsification index (E24) (Saranya et al. 2014). The activity of lipase was determined by titrimetric method (Ramani et al. 2012) and the laccase enzyme assay was estimated by spectrophotometric method using ABTS (2,2′-azino-bis (3-ethylbenzothiazoline-6-sulphonicacid) as the substrate (Kalcikova et al. 2014).
Lipase activity
Lipase activity was measured by titration method (Hepziba Suganthi and Ramani 2016). For the preparation of emulsion, Distilled water containing olive oil, 10% (v/v) and PVA, 2% (w/v) was mixed and sonicated using ultra sonicator. The emulsified oil, 5 ml was mixed with Triton X-100, 2 ml@ 0.03% (v/V), CaCl2 2H2O, 2 ml @ 0.075%, 3 M NaCl, 1 ml and phosphate buffer, 4 ml (pH 7) and agitated in shaker for 5 min at 100 rpm. To this, cell free supernatant, 1 ml was added and incubated for 15 min in an orbital shaker. Then acetone: ethanol, 1:1 ratio was added to arrest the enzyme reaction. The lipase activity was calculated using the formula (Ramani et al. 2012).
Laccase activity
The cell free supernatant (crude enzyme solution), 100 μl was added to 0.1 M sodium acetate buffer, 2.8 ml (pH 4.5) and 0.5 mM ABTS, 100 μl. The optical density of the mixed solution was measured using UV–Visible spectrophotometer (Cary 60 UV–Vis Spectrophotometer Agilent technologies, USA) at λ420 nm at an interval of 2 min. Laccase activity was calculated as follows (Kalcikova et al. 2014).
where ΔE change in extinction of light (/min), ɛ molar absorption coefficient of ABTS (/M/cm), d layer thickness (/cm), Vt & Vs total volume measured (ml) & volume of sample (ml).
Esterase activity
In order to estimate the esterase enzyme activity, solution A: 50 mM Potassium Phosphate Buffer was prepared in deionised water and adjusted to pH 7.5 and Solution B: 100 mM O-Nitrophenyl Butyrate solution (ONPB) was prepared using dimethylsulfoxide. Assay sample containing solution A, 2.87 ml and solution B, 0.03 ml was mixed well by inversion. The absorbance was measured at λ420 nm. Then the cell free supernatant (crude enzyme solution), 0.10 ml was mixed with this mixture and the increase in OD at λ420 nm was noted for 5 min using Spectrophotometer (Cary 60 UV–Vis Spectrophotometer Agilent technologies, USA). Esterase activity was calculated as follows (Parthipan et al. 2017).
where 3 Total volume (in milliliters) of assay, 5.0 Millimolar extinction coefficient of o-Nitrophenol at 420 nm at pH 7.5, df Dilution factor, 0.1 Volume (in milliliter) of enzyme used for the assay.
Quantitative analysis of biosurfactant
Emulsification index (E24)
The biosurfactant activity was analysed by evaluating E24 of the cell free supernatant. For this, 2 ml of hydrocarbon (kerosene) was mixed to the same volume of cell free supernatant and then subjected to vortex for 2 min. The height of emulsified layer was measured after 24 h of incubation under quiescent condition. The E24 index (%) was calculated using the formula (Guangming et al. 2005; Saranya et al. 2014).
Surface tension and CMC measurement
The surface tension of the biosurfactant produced by the microbial-assisted biocarrier system was measured by Behring et al. (2004) methodology (Behring et al. 2004). Initially, the biosurfactant was extracted using solvent extraction method adopted from Garcia-Reyes and Yanez-Ocampo (2016). The calibration was done with varying concentrations of sodium dodecyl sulphate (SDS) solutions and tested with pure water before estimating the biosurfactant surface tension and CMC. The measurements were performed in triplicate.
Confirmation of microbial growth onto biocarrier matrix (rice husk) using SEM analysis
The morphology of the carrier matrix [i.e. rice husk (RH)] before and after the impregnation of efficient mixed microbial consortium (labelled as SW1, SW2, and SW3 strains) (RH-SW) that showed higher efficiency in the treatment process (Fig. 1) was analysed by scanning electron microscopy (SEM) to confirm the aggregation of the microbial consortium onto rice husk (RH-SW) which could be mediated by the secretion of extracellular matrix.
Identification of potential hydrocarbonoclastic microorganisms by 16SrRNA sequencing
The three mixed (SW1, SW2, and SW3 strains) microbial consortia present in microbial- assisted biocarrier matrix system were observed to be capable of producing biocatalysts and biosurfactants and also effectively degraded hydrocarbons within short duration (7 days). The synergistic effect of the mixed microbial consortia was found to be higher in degrading hydrocarbons and producing the biocatalysts as well as biosurfactants than the individual strains. Hence, the three strains were identified by 16S rDNA sequencing. The target 16S rRNA region of isolated bacterial DNA was amplified using colony polymerase chain reaction (PCR) method. The forward primer, 27F (5′-AGA GTT TGA TCC TGG CTC AG-3′) and reverse primer 1492R (5′-CGG CTA CCT TGT TAC GAC TT-3) and PCR 262 Master Mix (Thermo Scientific Phusion Flash High-Fidelity) were used to execute the amplification. The agarose gel electrophoresis (1%) was performed to monitor the molecular weight of amplicons. PCR products were purified with Biorad PCR 269 Clean-UpKit. The obtained 16S rRNA gene sequences were lined-up by means of the Bioedit and CLUSTALW software (Mohan et al. 2020). Then, the deduced sequence was transferred to the 16S rDNA data base system to obtain other closely related sequences, Further, the phylogenetic tree was constructed using neighbour joining method.
Optimization of process parameters for the simultaneous production of biomolecules and oil sludge biodegradation through one variable at a time approach using MABC system
After the preliminary screening on the oil sludge degradation and production of enzymes and biosurfactant, three mixed microbial consortia having (SW1, SW2 and SW3 strains) was found to be efficient among the other isolates and it was selected for the studies on the optimization of treatment conditions for the enhanced production of biomolecules (biocatalyst such as lipase, esterase and laccase and biosurfactant) and degradation of TPH present in the oil sludge using the three mixed (SW1, SW2 and SW3 strains) microbial-assisted biomatrix system. The important parameters such as time (0–8 d), pH (3–10), and amount of oil sludge (10, 20, 30 g) and mass of RH-SW (10, 20, 30 g) in the ratio (1:1, 1:2, 1:3, 2:1 and 3:1) were selected. The optimization of treatment conditions were studied using one variable at a time. The biosurfactant and biocatalyst production (as lipase, esterase and laccase) and TPH degradation profile were monitored.
Optimization of oil sludge treatment conditions using central composite design (CCD)-Response surface methodology (RSM)
The biodegradation rate of petroleum refining industry oil sludge (storage tank bottom oil sludge) was analysed based on the TPH removal (%). RSM is a mathematical modelling tool that was employed to study the significant factors such as time, pH, amount of oil sludge and mass of microbial-assisted biocarrier matrix and also their interaction among them on the TPH removal (%). The boundary parameters were selected in the range as time (5–9 d), pH (6.0–10.0), amount of oil sludge (0–20 g) and mass of RH-SW (10–40 g) for the effective TPH removal from oil sludge. The central composite design (CCD) in Design—Expert software, version 8.0.7.1 (Stat Ease Inc. Minneapolis, USA, trial version) was selected to design the experiment and to analyze the interaction of significant factors on TPH removal. The above mentioned independent variables were studied at three different levels and a series of experiments (n = 30) were carried out. The evaluation of the analysis of variance (ANOVA) was carried out using statistical analysis. The model equation used for the analysis was given below as Eq. (1)
where Y is the predicted response, k is the number of factors, α0 is the design factor of interest, αi and αij are coefficients. The significance of the model was analysed statistically using f-test of ANOVA and the coefficient of determination to measure the goodness of fit. The R2 value determines the accuracy and quality of the above polynomial model. The model was validated by performing the experiment for three times using the optimized conditions obtained from RSM. The high and low levels of significant factors tested in response surface methodology were provided in Table 1.
GC–MS analysis of petroleum oil sludge after the treatment process
The petroleum oil sludge, after the biodegradation using the microbial-assisted biocarrier matrix system at optimized conditions was subjected for component characterization (GC–MS analysis) to find out the conversion of hydrocarbons into the metabolic intermediates such as acids and alcohols. The metabolites (fatty acids and alcohols) formed after the treatment of oil sludge were analyzed using GC–MS (Suganthi et al. 2018). The treated sample (aqueous) was extracted using equal volume of hexane and the solvent was evaporated using rotary vacuum evaporator. The extracted sample was mixed with of methanol, 20 ml and of concentrated sulphuric acid, 1 ml and subjected for derivatization. After the derivatization, the sample was injected into the GC–MS instrument, Agilent Technologies GC–MS 5973 equipped with DB5MS column (length, 30 m ID, 0.25 mm and film thickness, 0.25 μ). The initial set temperature was 70 °C with incremental increase in temperature by 10 °C for every 10 min till 260 °C was reached and then held for 20 min. The separated compounds were identified, using NIST 2000 library match.
Molecular weight determination of extracted biomolecules (SDS-PAGE)
The molecular weight of the extracted crude biosurfactant (BS) and biocatalysts were determined by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS PAGE) (Laemmli 2011).
Results and discussion
Characterization of petroleum oil sludge
The petroleum refining industry oil sludge (PRIOS) was obtained from the petroleum oil refinery located in Chennai, Tamil Nadu and was analysed for total petroleum hydrocarbon content (TPH) content and GC–MS analysis. The presence of heavy metals was determined using ICP-OES analysis.
Total petroleum hydrocarbon content analysis (TPH)
Petroleum refining industry oil sludge (PRIOS) containing hydrocarbons were extracted using solvent extraction method. The solvent was evaporated and the hydrocarbons (TPHs) were quantified using gravimetric method (EPA 8015). The TPH presented in the initial oil sludge (before treatment) was found to be 40% (w/w). Few reports on storage tank bottom oil sludge stated that the TPH content of oil sludge usually ranges 14–40% and the TPH percentage depends on age of oil sludge in storage tank (Mishra et al. 2001; Suganthi et al. 2018).
GC–MS analysis
The presence of aliphatic and aromatic hydrocarbons in the PRIOS was confirmed using GC–MS analysis and they are presented in Fig. 8a, the peaks were numerous with higher abundance in the GC–MS chromatogram of untreated petroleum oil sludge (Fig. 8a), The GC–MS spectrum reveal that the initial oil sludge contains both heavy and light chain hydrocarbons (C14 to C34) such as Tridecane, 7 methyl Tetradecane, Pentadecane, Hexadecane, Heptadecane, Naphthalene, 2,3,6-trimethyl Pentadecane, 2,6,10-trimethyl Octadecane, Hexadecane, 2,6,10,14-tetramethyl Nonadecane, Heneicosane, Docosane, Tetracosane and Tetratriacontane. This result indicates that there are large numbers of long chain aliphatic hydrocarbons and few aromatic hydrocarbons are present in the oil sludge (PRIOS). Several reports also the specified the wide range of complex hydrocarbons (C14 to C34) in crude oil sludge (Gojgic-Cvijovic et al. 2012; Cheng et al. 2014; Bilen Ozyurek and Seyis Bilkay 2020).
Isolation and enrichment of hydrocarbonoclastic bacterial strains
The organisms were isolated from various oil-contaminated soil sources such as diesel contaminated soil nearby generator (DC), crude oil contaminated soil from petroleum oil refinery (MS), soil acclimatized with petroleum oil sludge near the sewage treatment plant (STP3) and soil around automobile workshop (AWS). (Wherein, 1.0 g of each soil sample was separately dispersed into 100 ml (pH 7.0) of mineral salt medium, containing 1.0 g of petroleum tank bottom oil sludge (as the sole source of carbon) and further, the process of enrichment and isolation of hydrocarbonoclastic bacterial strains were carried out as explained in the “Isolation and enrichment of hydrocarbonoclastic microorganisms from soil sources” section). In which, four distinct isolates were obtained from the above mentioned sources and were labelled as SW1, SW 2, SW3, SW4. These isolates were selected for further biodegradation studies and their combined effect was determined based on the biomolecules production and TPH degradation.
Development of microbial-assisted biocarrier matrix system onto agro-waste
The experimental set up for the bioremediation of petroleum oil sludge using the microbial-assisted biomatrix system was designed as mentioned under the subheadings “Development of microbial-assisted biocarrier matrix system onto agro-waste” and “Degradation of oil sludge using microbial-assisted biocarrier system” sections.
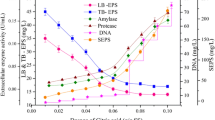
The enriched bacterial isolates individually and in the combinations of them with the culture density/optical density (OD) of 1.0/mL (w/v) were fixed and used for the development of microbial-assisted biocarrier system onto rice husk for the degradation of oil sludge. The treatment profile such as reduction percentage in TPH, biosurfactant activity, lipase and laccase enzyme activity were monitored at regular interval of time and they are presented in Fig. 1.
Amongst the four individual isolates collected from various sources and their five combinations, three mixed (labelled as SW1, SW2, and SW3) consortia, showed comparatively better TPH reduction by 60 ± 3%, Emulification index E24, (biosurfactant activity), 36 ± 1%; lipase activity, 110 ± 4 U/ml and laccase activity, 23 ± 1.5 U/ml. Therefore, the mixed microbial consortia, was selected for the degradation studies on PRIOS. The microbial production of biosurfactant and the extracellular enzymes such as lipase, laccase and esterase plays an important role in the biodegradation of hydrocarbons present in the petroleum oil sludge (Suganthi et al. 2018; Parthipan et al. 2017). The synergistic activity of mixed microbial consortia (SW1, SW2 and SW3 strains) could be highly favourable for the efficient bioremediation of hydrocarbons.
SEM analysis of biocarrier matrix
The morphology of rice husk (carrier matrix) before and after the formation of microbial-assisted biocarrier matrix was observed under SEM.
“The SEM image of initial rice husk (RH) (Fig. 2a) depicts that the presence of rough surface and void space on the rice husk, which contributes for the attachment of bacterial strains. The SEM image of RH-SW (Fig. 2b) shows the prominent attachment/aggregation of microbial biomass onto rice husk. The attachment/aggregation of microbes might be contributed by the release of extracellular polysaccharides produced by the microbial cells (Costa et al. 2018). The RH-SW system could increase the utilization rate of oil sludge for the microbial degradation due to the increase in surface contact of oily medium and the microbial matrix (Huang et al. 2019). More particularly, the biocarrier matrix (rice husk) facilitates the fine attachment of microbes and could greatly assist in bioaugmentation process. Hence, these 3 bacterial strains (SW1, SW2 and SW3) were selected and identified by 16S rDNA sequencing.
Identification of potential hydrocarbonoclastic microorganisms by 16S rDNA sequencing
The organisms that exhibited better biodegradation ability of hydrocarbons as well as the production of biomolecules (biosurfactant and biocatalysts) in the microbial-assisted biocarrier matrix system (RH-SW) were identified as Enterobacter hormaechei, Serratia rubidaea, Stenotrophomonas acidaminiphila by 16S rRNA sequencing. The sequences of Enterobacter hormaechei, Serratia rubidaea, Stenotrophomonas acidaminiphila were submitted to NCBI GenBank and obtained the accession number as MK603175, MK816976 and MK816977, respectively. The phylogenetic tree was constructed using the Neighbour-joining method (Ref. Fig. S1 in the supplementary material).
There are few reports in the literature regarding the isolation of hydrocarbonoclastic bacteria from the oil sludge as well as hydrocarbon contaminated soil sources. Various strains such as Pseudomonas, Stenotrophomonas and Bacillus sp. were identified from the hydrocarbons-contaminated areas and were stated to have degradation capacity of oil sludge (Tuleva et al. 2005; Cerqueira et al. 2011a; Fernández-Luqueño et al. 2011; Thavasi et al. 2011; Suganthi et al. 2018). It has been reported that the strain Stenotrophomonas acidaminiphila was isolated from the anaerobic sludge used for the treatment of petrochemical effluents and used for bioremediation of oily wastes (Assih et al. 2002).
Studies on the optimization of process parameters using RH-SW-one variable at a time approach
The effect of parameters such as time (0–8 d), pH (3–10), and amount of petroleum oil sludge and mass of biocarrier matrix (RH-SW) ratio (1:1, 1:2, 1:3, 2:1 and 3:1) were studied for the production of biomolecules and degradation of hydrocarbons in oil sludge (TPH). The effect of time on the production of biomolecules and TPH degradation were monitored. All other parameters such as pH 7.0, oil sludge, 10 g and mass of biocarrier matrix (RH-SW) in the ratio of (1:1) with respect to oil sludge, were kept constant. The enzyme activity (lipase, laccase, and esterase), the biosurfactant activity in terms of emulsification index and the TPH reduction profile were determined from samples collected at different period of time using the protocols mentioned previously.
In the Fig. 3, the data illustrated that the enzyme activity such as lipase (128 ± 3 U/ml), laccase (48 ± 1.5 U/ml) and esterase (42 ± 1.5 U/ml) activity were gradually increased and attained the maximum value at day 7 and thereafter a slight decrease in the enzyme activity was observed. The biosurfactant activity (38 ± 2 (%) E24) was found to be the maximum on day 7 and remains stable as the time of degradation was increased. Hence, the maximum reduction in percentage of TPH (66 ± 2%) content was also occurred on 7th day beyond which the percentage degradation of TPH remains the same. Secondly, the effect of pH on biomolecules production and TPH degradation was analysed. The initial medium pH was adjusted from pH (3, 4, 5, 6, 7, 8, 9) and the mass of biocarrier matrix (RH-SW) and oil sludge ratio (1:1) were kept constant. The treatment profile was monitored at regular time interval. The enzyme activity (lipase, laccase, and esterase), the biosurfactant activity and the TPH reduction were estimated.
The Fig. 4 shows that the lipase activity was maximum at pH 8 (136 ± 4 U/ml) and the laccase enzyme (52 ± 1 U/ml) was higher at pH 7 and pH 8. The esterase activity (54 ± 1.5 U/ml) was found to be the maximum at pH 8. Similarly the biosurfactant activity, E24 was also increased to 44% at pH 8 and thereafter it was reduced. Therefore, the maximum TPH degradation, in oil sludge (68 ± 1%) was also recorded at pH 8. Hence, the initial medium pH was optimized to be pH 8 for further experiments. The studies have reported that in bioremediation process, the lipase acts as primary enzyme for the microbial uptake of oily substance is highly induced at pH 8 (Boran and Ugur 2010; Suganthi et al. 2018). Finally, by keeping the initial pH of the medium at 8 and time as constant, the amount of oil sludge and the mass of biocarrier matrix (RH-SW) ratio was varied from (1:1, 1:2, 1:3, 2:1 and 3:1). The degradation profile was analysed after day 7th day of inoculation. At the end of treatment process the pH of the fermented medium was around pH 6.5–6.8, due to the conversion of hydrocarbons into acid and alcoholic by-products.
From the Fig. 5, the data showed that the increase in oil sludge ratio results in the time delay for the production of biomolecules and reduction in TPH content. This is due to the physiological properties exhibited by the oil sludge that provides sterical and structural hinderers to the biomass, because of the insufficient amount of interactive medium (RH-SW) for the accessibility of oil sludge. Whereas, when the ratio of biocarrier matrix was increased, the enzymes and biosurfactant production were gradually improved. At 1:3 (oil sludge: RH-SW) ratio, the maximum TPH removal percentage, (87 ± 1) was attained.
The biomolecules produced by (biosurfactants and biocatalysts) microbial (Enterobacter hormaechei, Serratia rubidaea, Stenotrophomonas acidaminiphila) assisted biocarrier matrix system at the optimum culture conditions are presented in Table 2. The crude enzymes were extracted from the cell free supernatant at the optimum time and checked for their specific activity. The pH of culture supernatant was adjusted to 2.0 and subjected for 24 h incubation. The separated pellet was extracted using chloroform: methanol (1:1) ratio. The yield of biosurfactant was measured.
Surface tension and CMC of extracted crude biosurfactant
The critical micelle concentration (CMC) of the biosurfactant was determined by measuring the surface tension of BS (as mentioned under the subheading “Surface tension and CMC measurement” section). The extracted biosurfactant was dissolved in the 1 M phosphate buffer pH 7 and the surface tension was measured at varying concentrations of biosurfactant.
The Fig. 6 indicates, as the concentration of biosurfatant increases the surface tension decreased and reached a minimum surface tension of 24.12 mN/m occurred at 30 mg/ml of BS. Beyond this the surface tension remains same is due to the commencement of biosurfactant aggregation (micelle formation). Hence, the CMC of biosurfactant produced by RH-SW system was estimated to be 30 mg/ml.
Statistical optimization for the effective degradation of oil sludge (TPH)
The interactive effects between four significant parameters selected (time, pH, amount of oil sludge and mass of RH-SW) were analysed using RSM by employing CCD analysis. The multiple regression analysis was applied to get a second-order quadratic polynomial equation to predict TPH removal percentage with respect to coded factors as mentioned below,
where A, B, C, D are time (d), pH, amount of oil sludge (g) and mass of RH-SW (g) respectively.
The sign and value of the coefficient of each term in the polynomial equation exemplify the impact of respective term on the TPH removal/degradation.
Analysis of variance (ANOVA)
The fitness of the proposed second-order response surface model by a mean square method was verified through analysis of variance (ANOVA) and summarized in Table 3. The F test and p values was assessed to verify the statistical significance of the equation as quoted in (Table 3). The p value represents the significance of each coefficient, which is used to understand the pattern of the mutual interactions among the best factors (Morando et al. 2014). Generally, the “prob > F” less than 0.0500 signify the model is significant and the values above 0.1000 indicate the model terms are not significant (Kuila et al. 2011). The ANOVA showed that, among the four selected factors, A (time), B (pH) and C (Amount of oil sludge) had considerable effects of the TPH removal (%).
The F value 106.12 for TPH removal (%) suggests the pure error is not significant and indicative of the goodness of the formulated model. The regression coefficient (R2) was used to analyse the precision of the model. The R2 value of 0.9900 designates the accuracy and satisfactory fitting of the proposed quadratic model to the data obtained. The above points signify the good competency of the second-order polynomial model proposed for elucidation of TPH removal/ degradation using RH-SW. The coefficient of variance is a statistical measure that explains the degree of precision of the experiment (Parameswaran et al. 1979). The coefficient of variance (%) value of 7.65 directs the further steering of the proposed model. The observed results were found to be nearer to the predicted values, which exhibits the relatedness of the model and performed experiments.
Localization of the optimum conditions
In the Fig. 7, the 3D-response surface plots were presented to investigate the interactions between two variables with respect to TPH removal. The 3D graphical response surface plot peaks indicates that the optimal value of the selected variables was within the design limits. The coordinates of the central point within the highest contour level denotes the optimum point for each significant factors (Puri et al. 2002). The plots showed that the TPH removal (88.78%) was attained the maximum, at the optimum conditions i.e. time, 7.23 d; pH, 7.95; amount of oil sludge, 11.2 g and mass of biocarrier matrix (RH-SW), 30.95 g. The TPH removal/degradation efficiency depends on the optimum treatment conditions of the RH-SW system.
In the context to the above results, one of the key factors affecting the biodegradation efficiency is the complexity of petroleum oil sludge and its rheological properties. This impairs the bioavailability of oil sludge towards microbes (Aguelmous et al. 2019; Singh and Kumar 2020) The development of microbial-assisted biocarrier matrix system (RH-SW) acts as a fixed contacting medium between the oil sludge and biomass that favours bioavailability of oil sludge to the microbial metabolism (Huang et al. 2019). Hence, rapid hydrocarbon degradation through hydrolysis and oxidation by 88.78% was achieved using CCD experimental design (RSM) which is significantly mediated by the enhanced production of biosurfactants. Yield of the biosurfactant through the microbial-assisted biocarrier matrix system was 220 mg/g of oil sludge, this is comparatively higher value than other researchers reported using suspension treatment system and other conventional biological treatment models (Zheng et al. 2012).
Biosurfactants are characterized by organic molecules containing a hydrophilic (head) and hydrophobic (tail) moieties, giving them the ability to act in the interface of different non-polar compounds which is greatly influenced by the uptake of hydrophobic micelles (hydrocarbons) (Nitschke and Pastore 2002; Sacramento et al. 2009). Das et al. (2009) reported the direct the influence of biosurfactant concentration on emulsification of hydrocarbons and the heavy metal removal at a specific critical micelle concentration of the biosurfactants (Das et al. 2009). The production of biocatalysts such as lipase, laccase and esterase that catalyze the conversion of toxic hydrocarbons into simpler form (acids and alcoholic by-products) which is possible for the microorganism to take up as nutrient thereby facilitating the complete degradation of hydrocarbons through intercellular metabolism (Svendsen 2000; Ferradji et al. 2014; Da Fonseca et al. 2015).
“The major bottle neck in the previously reported suspension culture system is the duration of treatment process (i.e. nearly 30 days) which could be due to the rheological nature (density, viscosity and surface tension) of PRIOS (Suganthi et al. 2018). This duration of treatment/Hydraulic Retention Time (HRT) was significantly reduced with the application of developed MABC system as the biocarrier matrix (rice husk) improves the surface contact between PRIOS and microbes and acts as the stable interacting medium during the solid state/attached growth type of treatment process, which is the major reason for the increase in degradation rate of oil sludge within short duration of time (i.e. 7 days). In addition to this, the use of mixed culture in the development of MABC system could provide synergistic effect in terms of attachment of microbes to the carrier matrix, biomolecules production yield and the rate of biocatalytic conversion of hydrocarbons when compared with the application of pure culture system (Fig. 1). Therefore, the observed results indicated that the use of mixed microbial consortia for the development of MABC system and its application for the treatment of PRIOS has greater advantages by means of greater efficiency of treatment in short duration over the use of suspension and pure culture system”.
Characterization of treated oil sludge using GC–MS analysis
The GC–MS spectrum of untreated petroleum oil sludge (Fig. 8a) reveals the presence of both heavy and light chain hydrocarbons (C14 to C34). In the untreated oil sludge, the peaks were numerous with higher abundance, whereas, after treatment with the RH-SW, peak height and the number of peaks were decreased after 7th day of inoculation. The initial chromatogram shows that the petroleum oil sludge contains the hydrocarbons ranges between C14 and C34. The GC–MS of treated oil sludge (Fig. 8b) indicated that the toxic hydrocarbons present in the crude oil sludge were converted into their respective metabolic intermediates such as fatty acids and alcohols represented in Table 4.
The metabolic intermediates/products listed in Table 4 were also shown in few reported GC–MS analysis for treated oil sludge literatures (Gargouri et al. 2011; Suganthi et al. 2018). From the GC–MS spectrum of treated oil sludge (Fig. 8b), it is inferred that the microbial consortia present in the microbial-assisted biocarrier matrix (RH-SW) system has degraded the hydrocarbons present in the oil sludge. Since, RH-SW system is capable of producing the required amount of biosurfactants and biocatalysts that facilitates degradation of hydrocarbon ranges from C14 and C34. Also, the mechanistic approach of MABC system, towards the degradation of hydrocarbons was represented in Fig. 9. The terminal/sub-terminal methyl group oxidation of hydrocarbons takes place during the degradation thereby converted into primary alcohols and then to respective aldehydes and finally converted into corresponding fatty acids (listed in Table 4). These fatty acids subsequently enter into intracellular β-oxidation pathway for the further oxidation into CO2 and H2O (complete mineralization) (Varjani 2017).
Therefore, the study confirms that the microbial-assisted biocarrier matrix (MABC) system is highly efficient, economical for the bioremediation of petroleum tank bottom oil sludge within short duration (7 days) when comparing to the previous conventional bioremediation studies (Yudono et al. 2010; Cerqueira et al. 2011b; Suganthi et al. 2018).
SDS-PAGE
The SDS-PAGE showed the molecular weight of the extracted biosurfactant and crude biocatalysts obtained from MABC.
In the Fig. 10a three intense bands were observed which corresponds to the molecular weight such as 50 kDa, 45 kDa and 35 kDa of biosurfactants, respectively. Similarly in Fig. 10b four bands were clearly observed in crude enzymes and their molecular weight was found to be 70 kDa, 52 kDa, 35 kDa and 22 kDa, respectively.
Conclusion
“The microbial-assisted biocarrier matrix (MABC) system was successfully developed and the significant factors such as time, pH, amount of petroleum oil sludge and mass of biocarrier matrix (RH-SW) responsible for the production of biomolecules and degradation of hydrocarbons presented in PRIOS were optimized in this study, using Response Surface Methodology (RSM). The treatment duration, sludge generation and the operational stability are considered as the major limitations of conventional treatment system which is due to the structural complexity of PRIOS that greatly affects its bioaccessibility. Therefore, the requirement of low-cost and environmentally friendly surface-contacting material between the enriched hydrocarbonoclastic microbial isolates and oil sludge is considered to be the most important phenomenon. On these attributes, the present study confirms that the MABC system works as the stable surface interacting medium which leads to the enhanced production of biomolecules (biosurfactant and biocatalysts) by increasing the bioavailability of petroleum oil sludge to the microbes, thereby achieving the efficient degradation of petroleum oil sludge in 7 days duration. Also, with the application of MABC system, the bioaccumulation of toxic hydrocarbons can be minimized and converted into non-toxic end products, this was confirmed by GC–MS analysis. Hence, the present study can be considered as a better alternate for the conventional bioremediation processes as it overcomes the hurdles faced by the existing treatment system. Moreover, the treatment process used in the present study would be highly helpful for the petroleum oil refining industries to manage the bulk amount of fuel tank bottom petroleum oil sludge/hydrocarbon containing wastes in the effective manner”.
References
Aguelmous A, El Fels L, Souabi S et al (2019) The fate of total petroleum hydrocarbons during oily sludge composting: a critical review. Rev Environ Sci Biotechnol 18:473–493. https://doi.org/10.1007/s11157-019-09509-w
Aguelmous A, Zegzouti Y, Khadra A et al (2020) Landfilling and composting efficiency to reduce genotoxic effect of petroleum sludge. Environ Technol Innov 20:101047. https://doi.org/10.1016/j.eti.2020.101047
Al Zubaidi I (2018) Heavy fuel oil recovery from oil sludge by multiple extraction processes. Prog Petrochem Sci 1:83–87. https://doi.org/10.31031/pps.2018.01.000517
Assih EA, Ouattara AS, Thierry S et al (2002) Stenotrophomonas acidaminiphila sp. nov., a strictly aerobic bacterium isolated from an upflow anaerobic sludge blanket (UASB) reactor. Int J Syst Evol Microbiol 52:559–568. https://doi.org/10.1099/00207713-52-2-559
Behring JL, Lucas M, Machado C, Barcellos IO (2004) Adaptation of the drop-weight method for the quantification of surface tension: a simplified apparatus for the CMC determination in the chemistry classroom. Quim Nova 27:492–495. https://doi.org/10.1590/S0100-40422004000300021
Besalatpour A, Hajabbasi MA, Khoshgoftarmanesh AH, Dorostkar V (2011) Landfarming process effects on biochemical properties of petroleum-contaminated soils. Soil Sedim Contam 20:234–248. https://doi.org/10.1080/15320383.2011.546447
Bilen Ozyurek S, Seyis Bilkay I (2020) Comparison of petroleum biodegradation efficiencies of three different bacterial consortia determined in petroleumcontaminated waste mud pit. SN Appl Sci 2:1–12. https://doi.org/10.1007/s42452-020-2044-5
Boran R, Ugur A (2010) Partial purification and characterization of the organic solvent-tolerant lipase produced by Pseudomonas fluorescens RB02-3 isolated from milk. Prep Biochem Biotechnol 40:229–241. https://doi.org/10.1080/10826068.2010.488929
Caliman FA, Robu BM, Smaranda C et al (2011) Soil and groundwater cleanup: benefits and limits of emerging technologies. Clean Technol Environ Policy 13:241–268. https://doi.org/10.1007/s10098-010-0319-z
Cerqueira VS, Hollenbach EB, Maboni F et al (2011a) Biodegradation potential of oily sludge by pure and mixed bacterial cultures. Bioresour Technol 102:11003–11010. https://doi.org/10.1016/j.biortech.2011.09.074
Cerqueira VS, Hollenbach EB, Maboni F et al (2011b) Bioresource Technology Biodegradation potential of oily sludge by pure and mixed bacterial cultures. Bioresour Technol 102:11003–11010. https://doi.org/10.1016/j.biortech.2011.09.074
Cheng L, Shi S, Li Q et al (2014) Progressive degradation of crude oil n-alkanes coupled to methane production under mesophilic and thermophilic conditions. PLoS One. https://doi.org/10.1371/journal.pone.0113253
Chen G, Cheng C, Zhang J et al (2019a) Synergistic effect of surfactant and alkali on the treatment of oil sludge. J Pet Sci Eng 183:106420. https://doi.org/10.1016/j.petrol.2019.106420
Chen L, Lei Z, Luo X et al (2019b) Biological degradation and transformation characteristics of total petroleum hydrocarbons by oil degradation bacteria adsorbed on modified straw. ACS Omega 4:10921–10928. https://doi.org/10.1021/acsomega.9b00906
Costa OYA, Raaijmakers JM, Kuramae EE (2018) Microbial extracellular polymeric substances: ecological function and impact on soil aggregation. Front Microbiol 9:1–14. https://doi.org/10.3389/fmicb.2018.01636
Cui B, Cui F, Jing G et al (2009) Oxidation of oily sludge in supercritical water. J Hazard Mater 165:511–517. https://doi.org/10.1016/j.jhazmat.2008.10.008
D’Costa A, Shyama SK, Praveen Kumar MK (2017) Bioaccumulation of trace metals and total petroleum and genotoxicity responses in an edible fish population as indicators of marine pollution. Ecotoxicol Environ Saf 142:22–28. https://doi.org/10.1016/j.ecoenv.2017.03.049
Da Fonseca FSA, Angolini CFF, Arruda MAZ et al (2015) Identification of oxidoreductases from the petroleum Bacillus safensis strain. Biotechnol Rep 8:152–159. https://doi.org/10.1016/j.btre.2015.09.001
Da Rocha ORS, Dantas RF, Duarte MMMB et al (2010) Oil sludge treatment by photocatalysis applying black and white light. Chem Eng J 157:80–85. https://doi.org/10.1016/j.cej.2009.10.050
Das N, Chandran P (2011) Microbial degradation of petroleum hydrocarbon contaminants: an overview. Biotechnol Res Int 2011:1–13. https://doi.org/10.4061/2011/941810
Das P, Mukherjee S, Sen R (2009) Biosurfactant of marine origin exhibiting heavy metal remediation properties. Bioresour Technol 100:4887–4890. https://doi.org/10.1016/j.biortech.2009.05.028
Dhote M, Kumar A, Juwarkar A (2018) Petroleum contaminated oil sludge degradation by defined consortium: influence of biosurfactant production. Proc Natl Acad Sci India Sect B 88:517–523. https://doi.org/10.1007/s40011-016-0778-z
Fernandez-Luqueño F, Valenzuela-Encinas C, Marsch R et al (2011) Microbial communities to mitigate contamination of PAHs in soil-possibilities and challenges: a review. Environ Sci Pollut Res 18:12–30. https://doi.org/10.1007/s11356-010-0371-6
Ferradji FZ, Mnif S, Badis A et al (2014) Naphthalene and crude oil degradation by biosurfactant producing Streptomyces spp. isolated from Mitidja plain soil (North of Algeria). Int Biodeterior Biodegrad 86:300–308. https://doi.org/10.1016/j.ibiod.2013.10.003
Ferrarese E, Andreottola G, Oprea IA (2008) Remediation of PAH-contaminated sediments by chemical oxidation. J Hazard Mater 152:128–139. https://doi.org/10.1016/j.jhazmat.2007.06.080
Frutos FJG, Pérez R, Escolano O et al (2012) Remediation trials for hydrocarbon-contaminated sludge from a soil washing process: evaluation of bioremediation technologies. J Hazard Mater 199–200:262–271. https://doi.org/10.1016/j.jhazmat.2011.11.017
Garcia-Reyes S, Yanez-Ocampo G (2016) Microbial biosurfactants: methods for their isolation and characterization. J Microbiol Biotechnol Food Sci 6:641–648. https://doi.org/10.15414/jmbfs.2016.6.1.641-648
Gargouri B, Karray F, Mhiri N et al (2011) Application of a continuously stirred tank bioreactor (CSTR) for bioremediation of hydrocarbon-rich industrial wastewater effluents. J Hazard Mater 189:427–434. https://doi.org/10.1016/j.jhazmat.2011.02.057
Gojgic-Cvijovic GD, Milic JS, Solevic TM et al (2012) Biodegradation of petroleum sludge and petroleum polluted soil by a bacterial consortium: a laboratory study. Biodegradation 23:1–14. https://doi.org/10.1007/s10532-011-9481-1
Guangming Z, Hua Z, Guohe H, Haiyan F (2005) Physicochemical and microbiological effects of biosurfactant on the remediation of HOC-contaminated soil. Prog Nat Sci 15:577–585. https://doi.org/10.1080/10020070512331342590
Hassanshahian M, Emtiazi G, Cappello S (2012) Isolation and characterization of crude-oil-degrading bacteria from the Persian Gulf and the Caspian Sea. Mar Pollut Bull 64:7–12. https://doi.org/10.1016/j.marpolbul.2011.11.006
Hassanzadeh M, Tayebi L, Dezfouli H (2018) Investigation of factors affecting on viscosity reduction of sludge from Iranian crude oil storage tanks. Pet Sci 15:634–643. https://doi.org/10.1007/s12182-018-0247-9
He S, Tan X, Hu X, Gao Y (2019) Effect of ultrasound on oil recovery from crude oil containing sludge. Environ Technol 40:1401–1407. https://doi.org/10.1080/09593330.2017.1422553
Hepziba Suganthi S, Ramani K (2016) Microbial assisted industrially important multiple enzymes from fish processing waste: purification, characterization and application for the simultaneous hydrolysis of lipid and protein molecules. RSC Adv 6:93602–93620. https://doi.org/10.1039/c6ra11867d
Hu G, Li J, Zeng G (2013) Recent development in the treatment of oily sludge from petroleum industry: a review. J Hazard Mater 261:460–490. https://doi.org/10.1016/j.jhazmat.2013.07.069
Huang Y, Pan H, Wang Q et al (2019) Enrichment of the soil microbial community in the bioremediation of a petroleum-contaminated soil amended with rice straw or sawdust. Chemosphere 224:265–271. https://doi.org/10.1016/j.chemosphere.2019.02.148
Ismail W, Al-Rowaihi IS, Al-Humam AA et al (2013) Characterization of a lipopeptide biosurfactant produced by a crude-oil-emulsifying Bacillus sp. I-15. Int Biodeterior Biodegrad 84:168–178. https://doi.org/10.1016/j.ibiod.2012.04.017
Janbandhu A, Fulekar MH (2011) Biodegradation of phenanthrene using adapted microbial consortium isolated from petrochemical contaminated environment. J Hazard Mater 187:333–340. https://doi.org/10.1016/j.jhazmat.2011.01.034
Johnson OA, Affam AC (2019) Petroleum sludge treatment and disposal: a review. Environ Eng Res 24:191–201. https://doi.org/10.4491/EER.2018.134
Kalcikova G, Babic J, Pavko A, Gotvajn AZ (2014) Fungal and enzymatic treatment of mature municipal landfill leachate. Waste Manag 34:798–803. https://doi.org/10.1016/j.wasman.2013.12.017
Karamalidis AK, Voudrias EA (2007) Cement-based stabilization/solidification of oil refinery sludge: leaching behavior of alkanes and PAHs. J Hazard Mater 148:122–135. https://doi.org/10.1016/j.jhazmat.2007.02.032
Karthick A, Roy B, Chattopadhyay P (2019) A review on the application of chemical surfactant and surfactant foam for remediation of petroleum oil contaminated soil. J Environ Manag 243:187–205. https://doi.org/10.1016/j.jenvman.2019.04.092
Kuila A, Mukhopadhyay M, Tuli DK, Banerjee R (2011) Accessibility of enzymatically delignified bambusa bambos for efficient hydrolysis at minimum cellulase loading: an optimization study. Enzyme Res. https://doi.org/10.4061/2011/805795
Kuo CH, Lee CL (2010) Treatment of oil/water emulsions using seawater-assisted microwave irradiation. Sep Purif Technol 74:288–293. https://doi.org/10.1016/j.seppur.2010.06.017
Kuznetsova NV, Kravchenko AA (2020) The problems of China as a major consumer of energy resources. Int J Energy Econ Policy 10:331–341. https://doi.org/10.32479/ijeep.8478
Laemmli (2011) Laemmli SDS PAGE Fanglian He Carnegie Institution at Stanford. Bio-ProtocolOrg 1:3–6
Li X, Zhang F, Guan B et al (2020) Review on oily sludge treatment technology. IOP Conf Ser Earth Environ Sci 467:e012173. https://doi.org/10.1088/1755-1315/467/1/012173
Lin C, He G, Dong C et al (2008) Effect of oil phase transition on freeze/thaw-induced demulsification of water-in-oil emulsions. Langmuir 24:5291–5298. https://doi.org/10.1021/la704079s
Liu L (2005) Modeling for surfactant-enhanced groundwater remediation processes at DNAPLs-contaminated sites. J Environ Inform 5:42–52. https://doi.org/10.3808/jei.200500045
Liu J, Jiang X, Zhou L et al (2009) Pyrolysis treatment of oil sludge and model-free kinetics analysis. J Hazard Mater 161:1208–1215. https://doi.org/10.1016/j.jhazmat.2008.04.072
Mansur AA, Pannirselvam M, Al-hothaly KA et al (2015) Recovery and characterization of oil from waste crude oil tank bottom sludge from Azzawiya oil refinery in Libya. J Adv Chem Eng 5:1–11. https://doi.org/10.4172/2090-4568.1000118
Mishra S, Jyot J, Kuhad RC, Lal B (2001) In situ bioremediation potential of an oily sludge-degrading bacterial consortium. Curr Microbiol 43:328–335. https://doi.org/10.1007/s002840010311
Mohan T, Sheik Farid NS, K V S, et al (2020) Sustainable biological system for the removal of high strength ammoniacal nitrogen and organic pollutants in poultry waste processing industrial effluent. J Air Waste Manag Assoc. https://doi.org/10.1080/10962247.2020.1731013
Morando LEN, Gómez CXD, Zamora LL, Uscanga MGA (2014) Statistical optimization of alkaline hydrogen peroxide pretreatment of sugarcane bagasse for enzymatic saccharification with Tween 80 using response surface methodology. Biomass Convers Biorefinery 4:15–23. https://doi.org/10.1007/s13399-013-0091-5
Mukred AM, Hamid AA, Hamzah A, Yusoff WMW (2008) Development of three bacteria consortium for the bioremediation of crude petroleum-oil in contaminated water. Online J Biol Sci 8:73–79. https://doi.org/10.3844/ojbsci.2008.73.79
Naeem U, Qazi MA (2020) Leading edges in bioremediation technologies for removal of petroleum hydrocarbons. Environ Sci Pollut Res 27:27370–27382. https://doi.org/10.1007/s11356-019-06124-8
Nanekar S, Dhote M, Kashyap S et al (2015) Microbe assisted phytoremediation of oil sludge and role of amendments: a mesocosm study. Int J Environ Sci Technol 12:193–202. https://doi.org/10.1007/s13762-013-0400-3
Nitschke M, Pastore GM (2002) Biossurfactantes: propriedades e aplicações. Quim Nova 25:772–776. https://doi.org/10.1590/S0100-40422002000500013
Parameswaran R, Box GEP, Hunter WG, Hunter JS (1979) Statistics for experimenters: an introduction to design, data analysis, and model building. J Mark Res 16:291. https://doi.org/10.2307/3150696
Parthipan P, Preetham E, Machuca LL et al (2017) Biosurfactant and degradative enzymes mediated crude oil degradation by bacterium Bacillus subtilis A1. Front Microbiol 8:1–14. https://doi.org/10.3389/fmicb.2017.00193
Puri S, Beg QK, Gupta R (2002) Optimization of alkaline protease production from Bacillus sp. by response surface methodology. Curr Microbiol 44:286–290. https://doi.org/10.1007/s00284-001-0006-8
Ramani K, Jain SC, Mandal AB, Sekaran G (2012) Microbial induced lipoprotein biosurfactant from slaughterhouse lipid waste and its application to the removal of metal ions from aqueous solution. Colloids Surf B 97:254–263. https://doi.org/10.1016/j.colsurfb.2012.03.022
Ramirez D, Collins CD (2018) Maximisation of oil recovery from an oil-water separator sludge: influence of type, concentration, and application ratio of surfactants. Waste Manag 82:100–110. https://doi.org/10.1016/j.wasman.2018.10.016
Sacramento V, Alberto J, Costa V (2009) Biodegradation of toluene and fish oil in impacted soil using chemical and biological surfactants. Química nova 32:394–400. https://doi.org/10.1590/S0100-40422009000200024
Saimmai A, Kaewrueng J, Maneerat S (2012) Used lubricating oil degradation and biosurfactant production by SC-9 consortia obtained from oil-contaminated soil. Ann Microbiol 62:1757–1767. https://doi.org/10.1007/s13213-012-0434-7
Saranya P, Ramani K, Sekaran G (2014) Biocatalytic approach on the treatment of edible oil refinery wastewater. RSC Adv 4:10680–10692. https://doi.org/10.1039/c3ra43668c
Sarika Saxena VP (2015) Treatment of oil sludge contamination by composting. J Bioremediat Biodegrad 06:9–19. https://doi.org/10.4172/2155-6199.1000284
Scala F, Chirone R (2004) Fluidized bed combustion of alternative solid fuels. Exp Therm Fluid Sci 28:691–699. https://doi.org/10.1016/j.expthermflusci.2003.12.005
Singh B, Kumar P (2020) Physicochemical characteristics of hazardous sludge from effluent treatment plant of petroleum refinery as feedstock for thermochemical processes. J Environ Chem Eng 8:103817. https://doi.org/10.1016/j.jece.2020.103817
Suganthi SH, Murshid S, Sriram S, Ramani K (2018) Enhanced biodegradation of hydrocarbons in petroleum tank bottom oil sludge and characterization of biocatalysts and biosurfactants. J Environ Manag 220:87–95. https://doi.org/10.1016/j.jenvman.2018.04.120
Svendsen A (2000) Lipase protein engineering. Biochim Biophys Acta 1543:223–238. https://doi.org/10.1016/S0167-4838(00)00239-9
Tahhan RA, Abu-Ateih RY (2009) Biodegradation of petroleum industry oily-sludge using Jordanian oil refinery contaminated soil. Int Biodeterior Biodegrad 63:1054–1060. https://doi.org/10.1016/j.ibiod.2009.09.001
Thavasi R, Jayalakshmi S, Banat IM (2011) Effect of biosurfactant and fertilizer on biodegradation of crude oil by marine isolates of Bacillus megaterium, Corynebacterium kutscheri and Pseudomonas aeruginosa. Bioresour Technol 102:772–778. https://doi.org/10.1016/j.biortech.2010.08.099
Tuleva B, Christova N, Jordanov B et al (2005) Naphthalene degradation and biosurfactant activity by Bacillus cereus 28BN. Zeitschrift fur Naturforsch 60:577–582. https://doi.org/10.1515/znc-2005-7-811
Varjani SJ (2017) Microbial degradation of petroleum hydrocarbons. Bioresour Technol 223:277–286. https://doi.org/10.1016/j.biortech.2016.10.037
Wang Y, Liang J, Wang J, Gao S (2018) Combining stable carbon isotope analysis and petroleum-fingerprinting to evaluate petroleum contamination in the Yanchang oilfield located on loess plateau in China. Environ Sci Pollut Res 25:2830–2841. https://doi.org/10.1007/s11356-017-0500-6
Ward O, Singh A, Van Hamme J (2003) Accelerated biodegradation of petroleum hydrocarbon waste. J Ind Microbiol Biotechnol 30:260–270. https://doi.org/10.1007/s10295-003-0042-4
Xiaogang Y, Wei T, Yu B (2009) Demulsification of asphaltenes and resins stabilized emulsions via the freeze/thaw method. Energy Fuels 23:481–486. https://doi.org/10.1021/ef800600v
Xu N, Wang W, Han P, Lu X (2009) Effects of ultrasound on oily sludge deoiling. J Hazard Mater 171:914–917. https://doi.org/10.1016/j.jhazmat.2009.06.091
Yudono B, Said M, Napoleon A et al (2010) Kinetics of petroleum-contaminated soil biodegraded by an indigenous bacteria Bacillus megaterium. HAYATI J Biosci 17:155–160. https://doi.org/10.4308/hjb.17.4.155
Zhang Y, Zhao H, Shi Q et al (2011) Molecular investigation of crude oil sludge from an electric dehydrator. Energy Fuels 25:3116–3124. https://doi.org/10.1021/ef200512c
Zheng C, Academy C, Yu L et al (2012) Microbial-enhanced treatment of oil sludge from oil-production plants using Rhodococcus ruber Z25. J Can Pet Technol 51(04):290–294
Zhou L, Jiang X, Liu J (2009) Characteristics of oily sludge combustion in circulating fluidized beds. J Hazard Mater 170:175–179. https://doi.org/10.1016/j.jhazmat.2009.04.109
Zubaidy EAH, Abouelnasr DM (2010) Fuel recovery from waste oily sludge using solvent extraction. Process Saf Environ Prot 88:318–326. https://doi.org/10.1016/j.psep.2010.04.001
Acknowledgements
The authors are highly thankful to the Department of Biotechnology, Ministry of Science and Technology, New Delhi for funding the project under the Scheme “Biosystems and Bioprocess Engineering scheme” (No. BT/PR20297/BBE/117/193/2016). SRM-DBT Partnership Platform for Contemporary Research Services and Skill Development in Advanced Life Sciences Technologies (No. BT/PR12987/INF/22/205/2015) and Dept. of Biotechnology, School of Bioengineering, SRMIST are acknowledged for extending the analytical services.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Swathi, K.V., Muneeswari, R., Ramani, K. et al. Biodegradation of petroleum refining industry oil sludge by microbial-assisted biocarrier matrix: process optimization using response surface methodology. Biodegradation 31, 385–405 (2020). https://doi.org/10.1007/s10532-020-09916-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10532-020-09916-9