Abstract
Sugarcane bagasse is a byproduct constituting more than 25 % processed matter after cane juice extraction and is thus a low-cost renewable substrate for value-added products such as bioethanol and xylitol due to its high content of hemicellulose and cellulose. In this study, a Box–Behnken response surface method design was used to optimize alkaline hydrogen peroxide pretreatment of dilute acid-treated sugarcane bagasse. Hydrogen peroxide concentration (2–6 % w/v), pretreatment time (10–40 h) and liquid/solid ratio (8–20 v/w) were tested in order to maximize glucose production in the enzymatic hydrolysis process. The optimum conditions obtained were 4.7 % w/v hydrogen peroxide concentration, 26.7-h pretreatment time, and 17.1 v/w liquid/solid ratio, producing 31.1 g/L glucose (40.2 % glucose yield) at 72-h hydrolysis. After optimizing alkaline hydrogen peroxide pretreatment, a second Box–Behnken design was used to evaluate the effects of cellulase loading (3.4–5.6 filter paper unit (FPU)/g solid), β-glucosidase loading (15–27 beta-glucosidase unit (CBU)/g solid) and Tween 80 concentration (0.11–1.7 % w/v) on glucose production during enzymatic hydrolysis. By analyzing response surface plots and time course hydrolysis, 50.1 g/L glucose (64.8 % glucose yield) was obtained at 120-h hydrolysis using 4.1 FPU/g solids for cellulase, 18.2 CBU/g solids for β-glucosidase and 0.95 % w/v for Tween 80. This yield corresponds to a 29 % improvement in glucose concentration compared to no Tween 80 addition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
About 80 % of the energy used worldwide comes from three types of fossil fuels: oil, coal, and natural gas which results in a strong energy dependence on them. Moreover, 98 % of carbon emissions come from the combustion of fossil fuels that contribute to global warming forcing countries to search for novel renewable energy sources, biofuels from agricultural wastes being the most outstanding [1]. In Mexico, the area planted with sugarcane is mainly in the state of Veracruz, where it is grown on an annual average of 264,000 ha, 35 % of the country's total [2]. Extraction of sugarcane juice yields a byproduct called sugarcane bagasse that represents about 25 to 40 % processed matter depending on fiber content and juice extraction efficiency [3]. In Veracruz, a minor fraction of bagasse production is used as fuel for the mills for the production of steam required for operation of the plant, leaving a large amount of waste sugarcane bagasse.
Sugarcane bagasse therefore emerges as a renewable cheap source with biotechnological potential for the production of bioethanol and other commercial products such as xylitol, both obtained from fermentation of sugars produced after fractionation of lignocellulosic biomass, making it an alternative for oil dependence reduction.
Several technologies for the pretreatment and fractionation of the lignocellulose matrix have been developed worldwide. Pretreatment strategies have generally been categorized into biological, physical, and chemical processes, or a combination of these approaches. Although the pretreatment can be performed following a number of different principles, its goals are to remove or alter the hemicellulose and/or lignin and also to increase pore volume and internal surface area prior to enzymatic attack to cellulose [4, 5].
The combination of two or more chemical pretreatments can achieve efficient fractionation of lignocellulosic biomass carbohydrate components. Delignification with alkaline hydrogen peroxide with a previous stage of dilute sulfuric acid hydrolysis might be used to separate hemicellulose and lignin in two sequential fractionation steps in order to obtain a solid with high cellulose content. Mild sulfuric acid conditions hydrolyze hemicellulose to xylose, while delignification with hydrogen peroxide at high pH values is capable of removing 50 % of the residual lignin by generation of active radicals such as HO and O2 − that participate in phenolic polymer degradation [6–8].
Enzymatic saccharification is a promising strategy to convert cellulosic biomass into glucose because of low-energy requirements, absence of side products, and pollution reduction. However, the main challenges in cellulose enzymatic hydrolysis are its low accessibility due to residual lignin after pretreatment and high cellulase and β-glucosidase loadings that constitute a significant contribution to the overall ethanol production cost. It is therefore important to find methods of reducing enzyme loading and increasing conversion rate [9–13].
One strategy to achieve higher hydrolysis yields at lower cellulase and β-glucosidase loading is the addition of emulsifiers like Tween 80 to the reaction volume prior to enzyme addition [14, 15].
Several mechanisms have been proposed to explain the enhancement of enzymatic saccharification by surfactant addition which can be summarized as follows: (1) surfactants are capable of changing the nature of the structure, thereby increasing the available cellulose surface by the promotion of reaction sites for cellulases to be adsorbed [16–18]; (2) surfactants lower the non-biospecific and non-productive adsorption of enzyme onto the lignin [14, 15]; and (3) surfactants increase enzymatic stability and activity by reducing thermal or shear force denaturation [13, 17, 19]. Response surface methodology (RSM) is a mathematical and statistical analysis, useful for modeling and analyzing problems where the response of interest is influenced by several variables. RSM has been used extensively for optimizing different steps in biotechnological processes [20, 21] and also to optimize enzymatic hydrolysis. Box–Behnken design for three variables at three levels was chosen.
RSM showed that optimum enzymatic hydrolysis has enzyme concentration of 10 FPU/g substrate, 7 % substrate loading (cellulose), and 45 °C temperature. Using optimum conditions, 11.40 g/L glucose was achieved at 24 h and 13.52 g/L at 48-h hydrolysis time [22]. Saccharification of pretreated bagasse with NaOH was carried out with crude enzymes together using a two-factor experimental design. Under optimized conditions, the pretreated bagasse was saccharified up to 42.7 % in 24 h [23]. The conversion of lignocellulosic material into biofuels is complicated and not yet a commercial business because pretreatment conditions and saccharification are not optimized, although trends towards commercialization are evident [24]. Hence, the objective of this work was to optimize alkaline hydrogen peroxide pretreatment of sugarcane bagasse for enzymatic saccharification with addition of Tween 80 using response surface methodology in order to obtain a better saccharified yield from sugarcane bagasse as reported by others authors using enzymatic hydrolysis.
2 Materials and methods
2.1 Substrate and dilute acid pretreatment
Fresh sugarcane bagasse, manually harvested in January 2012, was provided by the “El Modelo” distillery located in Cardel, Veracruz, Mexico. It was first dried in sunlight and then stored in bags for 20 days at 30 °C. The moisture content was 9.44 % (w/w) and particle sized ranged from 0.71 to 2 mm. Prior to being used in the delignification process, sugarcane bagasse was submitted to a dilute acid pretreatment (2 % (v/v) H2SO4 solution in a 6:1 liquid/solid ratio, at 125 °C for 40 min) which was performed in a 20-L aluminum container in an autoclave (AESA, model CV-250) in order to remove hemicellulose from the bagasse. The liquid fraction was separated by filtration and then analyzed for glucose, xylose, acetic acid, and furfural contents and the solid phase was washed with water until a neutral pH was achieved, then dried at 70 °C. The oven-dried samples were stored in bags for further analysis and delignification.
2.2 Experimental design
A three-factor, three-level Box–Behnken design was used to optimize the delignification conditions in the subsequent enzymatic hydrolysis by deriving a second-order polynomial equation and constructing 3-day plots to predict responses. Box–Behnken designs are experimental designs for response surface methodology which explore the relationships between several explanatory variables and one or more response variables. Box–Behnken designs consist of a central point and the middle points of the edges of the cube circumscribed on a sphere [25]. The evaluated variables were: hydrogen peroxide concentration (X 1), pretreatment time (X 2), and liquid/solid ratio (X 3), the response variable being the glucose concentration at 72-h incubation.
For the enzymatic hydrolysis of delignified samples with Tween 80, a Box–Behnken design was used to evaluate cellulase loading (X 4), β-glucosidase loading (X 5), and Tween 80 concentration (X 6) as independent variables, the response variable being glucose concentration at 72-h incubation.
STATISTICA (version 10.0), a software used in statistical design used for data analysis, predictive modeling and construction of graphics, was used for regression and graph analyses of the data obtained [26]. The fitness of the polynomial equation was expressed by R-squared, the coefficient of determination, and its statistical significance was checked by an F test at a 95 % confidence level.
2.3 Delignification pretreatment
The acid-pretreated sugarcane bagasse was submitted to delignification treatment carried out in 20-L vessels using hydrogen peroxide solution as the oxidizing agent. Hydrogen peroxide concentration, pretreatment time, and liquid/solid ratio were evaluated in the subsequent enzymatic hydrolysis according to a Box–Behnken factorial design (Table 1) where the response variable was glucose production. Hydrogen peroxide treatment was prepared by adding H2O2 to distilled water and adjusting pH to 11.5 with 10-M sodium hydroxide solution. After delignification, the insoluble residue was collected by filtration, washed with distilled water until the pH was neutral, and then dried at 70 °C. The dried samples were stored in bags for further analysis and enzymatic hydrolysis.
2.4 Enzymatic hydrolysis of delignified samples
Enzymatic hydrolysis of the solid residues obtained after delignification was performed using a commercial cellulase preparation from Trichoderma reesei (Celluclast 1.5 L) with an activity of 70 FPU/g, supplemented with β-glucosidase from Aspergillus niger (Novozyme 188) with 250 CBU/g, both enzymes were obtained from Sigma Aldrich. For the reactions, 10-g dilute acid delignified bagasse were hydrolyzed in a liquid/solid ratio of 9:1 (v/w) with 50-mM sodium acetate buffer (pH 4.8), then a cellulase loading of 3.4 FPU/g dry pretreated solid was added, along with a β-glucosidase loading of 14.7 CBU/g dry pretreated solid.
Enzymatic hydrolysis was conducted in 250-mL Erlenmeyer flasks at 50 °C, 200 rpm in a shaker/incubator (Labtech, Daihanlabtech LSI-3016R). Samples were withdrawn at 72 h, and boiled for 10 min to terminate the reaction, then centrifuged (5425, Eppendorf) at 10,000 rpm for 10 min and the supernatants were analyzed for glucose by high performance liquid chromatography (Waters 600, TSP Spectra System, Waters, Milford, MA, USA) using a Bio-Rad Aminex HPX-87H column (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The temperature was 40 °C, 5-mM sulfuric acid mobile phase, 0.4 mL min−1 flow rate using an index refraction detector (Waters 2414, TSP Refracto Monitor V, Waters).
2.5 Enzymatic hydrolysis of delignified samples with Tween 80
The acid-treated delignified samples (at optimum conditions from statistical analysis of Table 1) were submitted to enzymatic hydrolysis in a liquid/solid ratio of 9:1 (v/w) with 50-mM sodium acetate buffer (pH 4.8) in 250-mL Erlenmeyer flasks at 50 °C, 200 rpm according to a Box–Behnken factorial design (Table 2) where the factors evaluated were cellulase loading, β-glucosidase loading and Tween 80 concentration while the dependent variable was glucose production. Samples were withdrawn at 72 h, and boiled for 10 min to terminate the reaction prior to glucose analysis.
The best enzymatic loading and surfactant concentrations predicted by the statistical analysis were submitted to experimental validation and samples were taken after 6, 12, 24, 48, 72, 96, and 120 h for glucose analysis.
2.6 Analytical procedures
The chemical composition of the substrates (cellulose, hemicellulose, and lignin) was determined following the detergent fiber technique [27]. Neutral detergent fiber (NDF), acid detergent fiber (ADF), and acid detergent lignin (ADL) were determined using the ANKOM Filter Bag method [28]. NDF quantifies cellulose, hemicellulose, and lignin and ADF quantifies cellulose and lignin; so, the difference between NDF and ADF determines hemicellulose and the difference between ADF and ADL determines cellulose.
Glucose and xylose were analyzed by HPLC (Waters 600 TSP Spectra System, Waters, Milford, MA, USA) using a Shodex SH1011 column (8 × 300 mm). Temperature was 50 °C, 5-mM sulfuric acid mobile phase, 0.6 mL/min flow rate using an index refraction detector (Waters 2414, TSP Refracto Monitor V, Waters).
3 Results and discussion
3.1 Composition of untreated and dilute acid-treated sugarcane bagasse
Dry sugarcane bagasse contained 39.3 % cellulose, 30.5 % hemicellulose, 11.8 % lignin, and 3.7 % ash. The lignin content was below the range of some reported data for sugarcane bagasse (21.8–42.4 %) [29, 30], possibly attributable to different chemical and biological soil properties in different regions of the world where sugarcane is grown. Treatment with 2 % v/v sulfuric acid at 125 ° for 40 min removed 86.51 % hemicellulosic fraction from the original fiber composition, producing solids that were 57.8 % cellulose, 4.1 % hemicellulose, and 23.6 % lignin.
Sugar and other compound concentrations in the acid hydrolysate as a result of the pretreatment were measured and are shown in Table 3.
3.2 Enzymatic hydrolysis of delignified samples
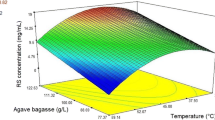
Glucose concentration obtained at 72-h incubation of enzymatic hydrolysis depended on the pretreatment conditions as shown in Table 4. The highest value (26.45 g/L) was obtained when conditions were applied at the medium level of experimental design (4 % w/v, 25 h and 14 (v/w)), while low glucose concentration was obtained at low liquid/solid ratio level of hydrogen peroxide (experiments 4, 6, and 13 in Table 4 with a mean value of 13.2 g/L) and low hydrogen peroxide concentration (experiments 10 and 12 in Table 4 with a mean value of 13.51 g/L).
The application of RSM yielded the regression Eq. (1) that represents an empirical relationship between the response (glucose concentration) and the tested variables (in uncoded units):
The statistical significance of each coefficient was checked by a Student t test and p value in Table 5 at a 95 % confidence level. The largest effect was observed for the quadratic term of time (X 2 2), followed by the quadratic term of liquid/solid ratio (X 3 2) and the quadratic term of pretreatment time (X 1 2). In this study, the linear and the interaction terms have a p value greater than 0.05, meaning they have little effect on the response.
The analysis of variance (ANOVA) performed on the model (Table 6) demonstrated that the regression is statistically significant with a p value lower than 0.05.
The model showed a good fit with the experimental data since the coefficient of determination (R-squared) had a value of 0.9037, implying that more than 90.37 % sample variation was attributed to the variables, and the model could not explain only 9.63 % of the total variation. The lack of fit is significant; however, R-squared value is high (0.9037) indicating that the model is well adapted to the response. Therefore, the model is suitable to predict glucose concentrations in accordance to pretreatment conditions with hydrogen peroxide.
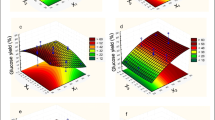
Analysis of the response surface plots (Figs. 1 and 2) reveals a well-defined region with best glucose concentration at the next working range: X 1 = 3.1–4.8 %, X 2 = 19–32.4 h, and X 3 = 12–17.2 (w/v).
An optimization analysis determined a maximum glucose concentration of 27.7 g/L for the following conditions: X 1 = 4.7 %, X 2 = 26.7 h, and X 3 = 17.1 (w/v). These conditions were validated experimentally obtaining 31 g/L glucose, an 11.9 % error from the prediction model. Optimum conditions using hydrogen peroxide at ambient temperature during pretreatment were set as pretreatment conditions prior to enzymatic hydrolysis with Tween 80.
One of the main advantages of using hydrogen peroxide as a pretreatment is that it can be performed at ambient temperatures and at low pressures thus avoiding the high cost of maintaining high temperatures that are necessary in other delignifying pretreatments that use agents such as sodium chlorite or peracetic acid [31].
Subsequent enzymatic hydrolysis using optimum pretreatment conditions for sugarcane bagasse with alkaline hydrogen peroxide produced 8.16 % less glucose concentration in comparison with Saha et al. [32] at 120-h incubation. This could be due to the lack of agitation with hydrogen peroxide solution during pretreatment, a condition that may not have removed all the lignin fraction; moreover, the lack of hemicellulase use and the lower cellulase and β-glucosidase loadings cause a less degrading effect on cellulose.
Saha et al. [31] showed that using hydrogen peroxide for pretreatment of rice hulls at 7.35 %, v/v, 35 °C, and 24 h resulted in 47.9 % less glucose yield in comparison with the present study. The difference in glucose yield may be attributed to the high solid concentration during pretreatment with H2O2 that didn't remove lignin efficiently, affecting subsequent enzymatic saccharification.
3.3 Enzymatic hydrolysis of delignified samples with Tween 80
Glucose concentration results from enzymatic hydrolysis of dilute acid-treated delignified samples (X 1 = 4.7 %, X 2 = 26.7 h, and X 3 = 17.1 (w/v)) are shown in Table 7.
A response surface regression was performed to fit the experimental data in Table 7 to a polynomial equation in encoded units (Eq. 2).
According to Table 8, at a 95 % confidence level, the largest effect was exhibited by the quadratic term of Tween 80 concentration (X 6 2), followed by the quadratic term of β-glucosidase loading (X 5 2) and the linear term of β-glucosidase loading (X 4 2). The analysis reveals that other linear and interaction terms have a p value greater than 0.05, meaning that there is little effect on the response.
The results of the second-order response surface model for glucose concentration in the form of ANOVA showed a p value lower than 0.05 (Table 9), implying that the model itself is significant.
The regression equation (Eq. 2) obtained after ANOVA indicated an R-squared value of 0.935, implying that the fitted model could explain 93.5 % of the total variability within the range of values studied. This ensured a satisfactory adjustment of theorical values to experimental data by this model. The p value for lack of fit was not significant, indicating that the regression equation was adequate for predicting glucose concentration during enzymatic hydrolysis with Tween 80.
Response surface plots (Figs. 3 and 4) shows that high glucose concentration (44 g/L) at 72-h incubation was obtained in the region from 4.1 to 5.1 FPU/g solid for X 4 (with an average value of 4.6 FPU/g solid), from 18.2 to 23.8 CBU/g solid for X 5 (with an average value of 21 CBU/g solid) and from 0.6 to 1.3 % (w/v) for X 6 (with an average value of 0.95 % (w/v)).
To confirm the validity of the response surface plots, two experiments (in duplicate) were conducted under the following predicted conditions: 4.6 FPU/g solid, 21 CBU/g solid, 0.95 % w/v Tween 80 (experiment 1) and 4.1 FPU/g solid, 18.2 CBU/g solid 0.95 % w/v Tween 80 (experiment 2). Experiment 1 employed average conditions for the three factors, while experiment 2 employed the lowest predicted level for cellulase and β-glucosidase loading and the average value for Tween 80 concentration.
According to glucose analysis at 72-h incubation, 43.9 ± 0.134 g/L was obtained from conditions in experiment 1, while 44.1 ± 0.141 g/L was obtained from experiment 2, showing no significant difference between them at a level of α = 0.05. This revealed that with experiment 2, it is possible to obtain approximately 44 g/L glucose (predicted glucose concentration) with a 10.8 % cellulase loading reduction and a 13.3 % β glucosidase reduction in comparison to conditions employed in experiment 1. These results indicate that response surface plots (Figs. 3 and 4) can be used to predict glucose concentration in the range of values selected for experimental design in Table 7.
3.4 Time course of enzymatic hydrolysis of delignified samples with Tween 80
Dilute acid-pretreated delignified sugarcane bagasse was enzymatically hydrolyzed with 4.1 FPU/g solid and 18.2 CBU/g solid with and without Tween 80 addition. Glucose concentration was determined at 12 h and then every 24 h during a 120-h period of enzymatic action. Results are shown in Table 10.
During the course of enzymatic saccharification, a continuous increase in glucose concentration was observed. The greatest increase in glucose concentration with respect to time was 24.4 % for Tween 80 sample and 20 % for control in the time interval from 24- to 48-h incubation; thereafter glucose increase started to reduce.
The positive effects of Tween 80 increased with hydrolysis time, reaching a 35 % glucose yield improvement with regard to control at 96-h hydrolysis. At 120-h hydrolysis, glucose concentration attained 50.1 g/L, corresponding to an increase of 24.9 % glucose yield compared to control after the same hydrolysis time.
Qi et al. [33] employed 0.5 to 1 % Tween 20 in the enzymatic hydrolysis stage to obtain an improvement of 13.1 % in glucose yield compared to control after 72-h hydrolysis, while this study showed, after the same hydrolysis time, a glucose yield improvement of 29 % with regard to control. This difference can be explained by higher lignin content in wheat straw after acid hydrolysis that makes the substrate less available for enzymatic attack compared to delignified sugarcane bagasse.
Working with alkaline-treated maize straw, Chen et al. [34] found that adding 0.5 % (w/v) Tween 80 increased hydrolysis yield from 81.2 to 87.3 % at 48-h hydrolysis, an increase of 7.5 %, while in present study adding 0.95 % (w/v) Tween 80 the increase was from 41.4 to 48.8 %, an increase of 17.8 %. The difference in hydrolysis improvement is expected since enzymatic loading used for delignified sugarcane is lower than the one used with alkaline-treated maize straw.
The improvement in enzymatic hydrolysis using Tween 80 may be attributed to enzyme protection caused by the addition of the nonionic surfactant that prevents proteins from inactivation; this is more evident from 24-h hydrolysis.
4 Conclusions
Results in this study show that the combination of dilute acid pretreatment at 125 °C followed by alkaline peroxide delignification under optimized conditions (26.7 h, using 4.7 % (w/v) H2O2 in a 17.1 (w/v) liquid/solid ratio) was an efficient technique to enhance the recovery of cellulose from sugarcane bagasse. The cellulosic solid was then submitted to enzymatic saccharification as follows: cellulase loading of 4.1 FPU/g solid, β-glucosidase loading of 18.2 CBU/g solid and Tween 80 supplementation at 0.95 % (w/v) giving a 64.8 % glucose yield at 120-h hydrolysis compared to 51.8 % glucose yield without Tween 80 addition, this difference corresponding to a 24.9 % glucose yield improvement. These results indicate that Tween 80 addition increases glucose yield in enzymatic hydrolysis compared to control, resulting in a 50.1 g/L glucose solution that can be converted into value-added product such as ethanol.
References
Demirbas A (2009) Biofuels: securing the planet's future energy needs. Springer, Turkey
SFA SAGARPA (2011) Perspectivas de largo plazo para el sector agropecuario de México 2011–2020. Subsecretaría de Fomento a los Agronegocios. Available from: http://www.sagarpa.gob.mx/agronegocios/Documents/estudios_economicos/escenariobase/perspectivalp_11-20.pdf. Accessed 24 February 2013
Pernalete Z, Piña F, Suárez M, Ferrer A, Aiello C (2008) Fraccionamiento del bagazo de caña de azúcar mediante tratamiento amoniacal: efecto de la humedad del bagazo y la carga de amoniaco. Biogeosciences 20(1):3–10
Monte JR, Brienzo M, Milagres AMF (2011) Utilization of pineapple stem juice to enhance enzyme-hydrolytic efficiency for sugarcane bagasse after an optimized pre-treatment with alkaline peroxide. Appl Energy 88:403–408
Sun JX, Sun XF, Sun RC, Su YQ (2004) Fractional extraction and structural characterization of sugarcane bagasse hemicelluloses. Carbohyd Polym 56:195–204
Sun Y, Cheng J (2002) Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresour Technol 83:1–11
Sun JX, Sun XF, Zhao H, Sun RC (2004) Isolation and characterization of cellulose from sugarcane bagasse. Polym Degrad Stab 84:331–339
Sun RC, Fang JM, Tomkinson J (2000) Delignification of rye straw using hydrogen peroxide. Ind Crop Prod 12:71–83
Gupta R, Khasa YP, Kuhad RC (2011) Evaluation of pretreatment methods in improving the enzymatic saccharification of cellulosic materials. Carbohyd Polym 84:1103–1109
Zhao X, Zhang L, Liu D (2008) Comparative study on chemical pretreatment methods for improving enzymatic digestibility of Crofton weed stem. Bioresour Technol 99:3729–3736
Wooley R, Ruth M, Sheehan J, Ibsen K (1999) Lignocellulosic biomass to ethanol process design and economics utilizing co-current dilute acid prehydrolysis and enzymatic hydrolysis current and futuristic scenarios. Technical report, NREL/TP-580-26157
Gregg DJ, Boussaid A, Saddler JN (1998) Techno-economic evaluation of a generic wood-to-ethanol process: effect of increased cellulose yields and enzyme recycle. Bioresour Technol 63:7–12
Alkasrawi M, Eriksson T, Börjesson J, Wingren A, Galbe M, Tjerneld F, Zacchi G (2003) The effect of Tween-20 on simultaneous saccharification and fermentation of softwood to ethanol. Enzyme Microb Technol 33:71–78
Eriksson T, Börjesson J, Tjerneld F (2002) Mechanism of surfactant effect in enzymatic hydrolysis of lignocellulose. Enzyme Microb Technol 31:353–364
Qing Q, Yang B, Wyman CE (2010) Impact of surfactants on pretreatment of corn stover. Bioresour Technol 101:5941–5951
Helle SS, Duff SJB, Cooper DG (1993) Effect of surfactants on cellulose hydrolysis. Biotechnol Bioeng 42:611–617
Kaar WE, Holtzapple MT (1998) Benefits from Tween during enzymatic hydrolysis of corn stover. Biotechnol Bioeng 59:419–427
Kristensen JB, Börjesson J, Bruun MH, Tjerneld F, Jørgensen H (2007) Use of surface active additives in enzymatic hydrolysis of wheat straw lignocellulose. Enzyme Microb Technol 40:888–895
Park JW, Takahata Y, Kajiuchi T, Akehata T (1992) Effects of nonionic surfactant on enzymatic-hydrolysis of used newspaper. Biotechnol Bioeng 39:117–120
Ahmed FM, Rahman RS, Gomes DJ (2012) Saccharification of sugarcane bagasse by enzymatic treatment for bioethanol production. Mal J Microbiol 8(2):97–103
Li W, Du W, Liu DH (2007) Optimization of whole cell-catalyzed methanolysis of soybean oil for biodiesel production using response surface methodology. J Mol Catal B Enzym 45:12–127
Thongkheaw S, Pitiyont B (2011) Enzymatic hydrolysis of acid-pretreated sugarcane shoot. World Acad Sci Eng Technol 60:454–458
Naveena BJ, Atlaf M, Bhadnah K, Reddy G (2005) Direct fermentation of starch to 1(+) lactic acid in SSF by Lactobacillus amylophilus GV6 using wheat bran as support and substrate: medium optimization using RMS. Bioprocess Biochem 40:681–690
Wu H, Hanna MA, Jones DD (2012) Fluidized bed gasification of dairy manure by Box–Behnken design. Waste Manag Res 30(5):506–511
Hamelinck C, Rijksuniversiteit te Utrecht, Copernicus Institute et al (2003) Production of FT transportation fuels from biomass technical options, process analysis and optimisation and development potential. Utrecht University, Utrecht
Van SPJ, Robertson JB, Lewis BA (1991) Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci 74:3583–3597
Vogel KP, Pedersen JF, Masterson SD, Toy JJ (1999) Evaluation of a filter bag system for NDF, ADF, and IVDMD forage analysis. Crop Sci 39:276–279
Lee YJ, Chung CH, Day FF (2009) Sugarcane bagasse oxidation using a combination of hypochlorite and peroxide. Bioresour Technol 100:935–941
Beukes N, Pletschke BI (2010) Effect of lime pre-treatment on the synergistic hydrolysis of sugarcane bagasse by hemicellulases. Bioresour Technol 101:4472–4478
Saha BC, Cotta MA (2010) Comparison of pretreatment strategies for enzymatic saccharification and fermentation of barley straw to ethanol. New Biotechnol 27(1):10–16
Saha BC, Cotta MA (2007) Enzymatic saccharification and fermentation of alkaline peroxide pretreated rice hulls to ethanol. Enzyme Microb Technol 41:528–532
Qi B, Chen X, Wan Y (2010) Pretreatment of wheat straw by nonionic surfactant-assisted dilute acid for enhancing enzymatic hydrolysis and ethanol production. Bioresour Technol 101:4875–4883
Chen M, Zhao J, Xia L (2008) Enzymatic hydrolysis of maize straw polysaccharides for the production of reducing sugars. Carbohyd Polym 71:411–415
Acknowledgments
Authors acknowledge the economic support from the National Council of Science and Technology, Mexico (CONACYT, scholarship 383152 and project # 128052) and the critical reading of Patricia Hayward Jones MSc. and Dulce María Barradas Dermitz MSc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morando, L.E.N., Gómez, C.X.D., Zamora, L.L. et al. Statistical optimization of alkaline hydrogen peroxide pretreatment of sugarcane bagasse for enzymatic saccharification with Tween 80 using response surface methodology. Biomass Conv. Bioref. 4, 15–23 (2014). https://doi.org/10.1007/s13399-013-0091-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-013-0091-5