Abstract
Oil sludge degradation studies were conducted using individual bacterial strains and their formulated consortium “Oil busters” isolated from tank bottom oil sludge. Four potential strains were selected on the basis of biosurfactant production, presence of catabolic gene and aromatic fraction utilization which further characterized using hydrocarbon compounds as the carbon source. Surface tension, emulsification index and bacterial adhesion towards hydrocarbons (BATH) test were also performed. All four strains were selected for consortium preparation and degradation studies were conducted for 20 days in Minimal media inoculated with individual strains and consortium. The strains used after identification on the basis of 16 s rDNA sequencing were named as AAJ1; AAJ2, AAJ3 and AAJ4. At the end of the study, consortium showed effective degradation of oil and its components as compared to individual strains. The consortium degraded a maximum of 78 % of oil, followed by strains AAJ1 70 %, AAJ3 58 %, AAJ2 57 % and AAJ4 56 %. In the total petroleum hydrocarbons (TPH), highest degradation was found by consortium (75 %) followed by AAJ2 (57 %), AAJ3 (56 %), AAJ1 (55 %) and AAJ4 (54 %) respectively. The strains AAJ1 and AAJ2 were identified as Bacillus sp. and AAJ3 and AAJ4 as Pseudomonas sp., respectively. A total of nine polycyclic aromatic hydrocarbons (PAHs) were detected in aromatic fraction of oil sludge and gas chromatography-mass spectroscopy (GC–MS) profile confirmed the complete removal of these compounds in the presence of consortium. The findings come up with a potential bacterial formulation, i.e. “Oil buster” for planning bioremediation strategy for oil sludge/PAH contaminated sites.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Environmental contamination by crude oil and its various processing products is becoming a common phenomenon which severely damages soil and ground water resources. During routine operations in refining, oil sludge generated in huge quantity is improperly disposed off without following any regulatory guidelines. These wastes are composed of saturated alkanes, aromatics, heavy metals, NSO (nitrogen–sulfur–oxygen compound) and insoluble fraction, asphaltene [1]. Among the constituents of oil sludge, polycyclic aromatic hydrocarbons (PAHs) are of environmental concern because of their toxic, mutagenic and/or carcinogenic effect. Lower molecular weight PAHs (2-ring) are degraded and volatilized more rapidly than heavier PAHs (>3-ring). As molecular weight increases, hydrophobicity/lipophilicity increases, water solubility decreases, vapor pressure decreases, and the compound will become recalcitrant and will persist in the environment for a longer time [2]. A geno-toxicity study of PAHs [3] confirmed that these compounds affect the mammalian cell culture causing DNA damage and chromosomal aberration.
Microbial degradation of PAHs represents the major and potentially viable natural process through which these organic compounds are detoxified from contaminated environments. But the multi-component oil sludge requires more than single microbial species for effective degradation [4, 5]. A number of studies have been reported on the role of multi-bacterial formulation in remediation/removal of toxic compounds from oil sludge/crude oil contaminated soils [6, 7]. But very scanty reports are available on remediation of total fractions of oil sludge. In a microbial degradation process, the poor distribution ratio of PAHs in solid to water, restricts the bioavailability to microbial cells and ultimately affects their activity which can be overcome by using microbial surfactants. Biosurfactant producing microbes are suitable for solubilizing hydrophobic compounds and enhancing the degradation rates through increasing the mass transfer drive [8]. The PAH degrading potential microbes having biosurfactant producing capacity, which can therefore be effectively used for speedy bioremediation of oil-contaminated sites [5, 9]. The PAH/oil sludge contaminated sites are enormous source of biosurfactant producing strains and the application of these indigenous microorganisms in designing the experiment for oil sludge contaminated sites remediation will be a safer and viable approach. Further, biodegradation pathways of higher ring compounds are supported by a number of enzymes, particularly, catechol dioxygenase, which uses dihydrodiols pathways and can act as an indicator of biodegradation [10]. Therefore, the selection of potential microbes possessing biosurfactant producing ability, catabolic gene and aromatic fraction degradation capability is the key factor for bacterial cocktail preparation, assuring every chance of complete mineralization.
The present investigation which was performed at CSIR-National Environmental Engineering Research Institute, Nagpur, India focuses on the screening and identification of effective bacterial strains and then applying those strains individually and in a group for degradation of all the fractions of hydrocarbons including asphaltene and higher PAHs.
Material and Methods
Sample Collection
Tank bottom oil sludge was collected from a disposal site of Gujarat refinery, Vadodara, India and stored in cold condition at 4 °C.
Chemicals and Media Used
AR grade (Across organics, Belgium) culture media, chemicals and chromatography grade (Merck Pvt. Ltd., Germany) solvents were used in the study. Modified minimal salt medium (MSM) used in the study were adopted as described by Verma et al. [11].
Oil Sludge Characterization
Oil sludge characterization was done using soxhlet apparatus (USEPA Method 3540C, 1996) and TPH fractionation was carried out on silica gel column chromatography [12, 13] and PAH compounds in aromatic fraction were quantified on GC–MS.
Enrichment and Screening Studies for Isolation of Effective oil Sludge Degraders
The oil sludge utilizing strains were isolated from oil sludge contaminated soil following five enrichment cycles. The resulting enriched liquid broth was spread on a nutrient agar plate for isolation of the bacterial colonies which were subsequently purified and stored in glycerol stocks. Screening of isolates was carried out based on biosurfactant producing capacity, presence of catechol dioxygenase gene and aromatic fraction utilization.
Biosurfactant Production
The isolated bacterial cultures were further supplemented with 1 % crude oil as the carbon source in modified mineral salt medium (MSM) [14] and incubated at 30 °C on a rotary shaker at 180 rpm.
Emulsification Index and BATH Assay
The surface tension of cell-free media was determined by tensiomat (Fisher Surface Tensiomat, USA) using the ring detachment technique. The emulsification index was determined by adding kerosene to the culture broth (1:1 V/V), vortexing for 2 min, and E-24 was calculated after 24 h. Additionally bacterial BATH assay was also performed using hexane [15].
Catechol Dioxygenase Gene and Assay
Genomic DNA was extracted from pure isolates and the catechol dioxygenase genes were amplified using dmpB and tcbC specific primers [16]. The selection of primers and PCR reactions was performed as per the protocol given by Dhote et al. [10]. Catechol 1, 2-dioxygenase (C1, 2DO), [EC 1.13.11.1] and catechol 2, 3-dioxygenase (C2, 3DO), [EC 1.13.11.2] activities were assayed in all the strains as described by Cenci et al. [17] using the colorimetric procedures. Based on the screening studies, the selected strains namely AAJ1 to AAJ4 were further tested for biosurfactant production capacity using different carbon sources like glucose, hexadecane and crude oil.
Molecular Identification of Oil Sludge Degraders
The 16S ribosomal ribonucleic acid (rRNA) gene was amplified by PCR using universal bacterial primers 27f and 907r universal primers. The amplified PCR products were sequenced and identified by using the BLASTN facility (http://www.ncbi.nlm.nih.gov/BLAST/).
Consortium Preparation and Biodegradation Studies of Oil Sludge
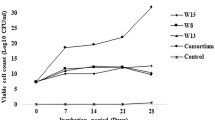
The consortium of four strains was prepared and degradation studies were performed for 20 days in 500 ml Erlenmeyer flask containing 100 ml sterile MSM with oil sludge [2 % (W/V)] which was incubated at 30 °C on an orbital shaking with 170 rpm. Individual strains were inoculated into four separate flasks having 108 CFU/ml and the active inocula of the consortium were aseptically inoculated in another flask. The un-inoculated control was also performed similarly. At every 5 days interval, degradation of oil, TPH and its fractions was estimated as the protocol used by Mishra et al. [18].
GC–MS Analysis of Aromatic Fraction
The aromatic fraction was determined on Varian 3800 GC coupled with ion trap mass spectrometer (model, Varian, Walnut Greek, USA) using DB-5 column (30 m-0.25 µm-0.25 mm). The oven temperature was 70–150 °C with a rate of 10 °C/min and further to 300 °C with 6 °C min and 10 min hold at 300 °C. Helium (ultra-pure) was employed as carrier gas at a flow rate of 1 ml/min. For quantification of PAHs, 16 mix standard (Dr. Ehrenstorfer, Merck Ltd. Germany) was used.
Results and Discussions
The oil sludge collected from Gujarat refinery Vadodara; India contained 2.4 ± 0.52 % moisture, 33 ± 0.025 solvent extractable fraction and 26 ± 0.055 % volatile organic matter. The oil composed of 20 % aliphatic, 43 % aromatic, 13 % NSO and 23 % asphaltene fractions respectively. Generally, the percentage of different fractions in oil sludge varies with the type of crude oil and processing efficiencies of the refinery. Effective biodegradation studies required precise selection of microorganisms which have the tremendous potential for degradation of recalcitrant compounds.
Enrichment and screening studies performed with oil sludge resulted in four promising strains, namely AAJ1 to AAJ4. The isolated strains were predominantly present in the soil of contaminated sites withstanding the toxic effects and capable of utilizing the recalcitrant compounds for their growth and survival [19]. All the four strains were found to be potential biosurfactant producers and capable of utilizing aromatic fraction as a carbon source as compared to un-inoculated control. The decline in prominent peaks of aromatic fraction indicated that the hydrocarbons are being utilized by the cultures and consortium (Fig. 1). Foaming was observed in MSM media inoculated with all the isolates, indicating the production of surfactants. Simultaneously, dioxygenase gene detection test resulted in amplification product of 238 bp (dmpB primer) which codes for catechol 2,3 dioxygenase (C 2,3 O) enzyme that initiates meta-cleavage pathway for aromatic compounds (Fig. 2). These strains were further characterized in details for biosurfactant production using hexadecane, crude oil and glucose as a sole carbon source.
All strains showed foam formation and growth after 108 h in the presence of hexadecane and crude oil (Table 1) and with glucose, foaming was observed after 36 h only. The delay may be attributed to the adaptation period of cultures using crude oil as the carbon and energy source for growth [20]. The surface tension reduction is a fundamental property of biosurfactant and maximum reduction was observed in case of strains AAJ1 and AAJ4 with glucose (Table 1). The produced surfactant reduces the interfacial surface tension of the growth medium and facilitates solubility and uptake of oil compounds by forming micelles [20].
The strains showed very high emulsification activity (54–72 %). A good bioemulsifier is one that has an index of >50 % [21]. The results of BATH assay (Supplementary Table 1) conformed higher hydrophobicity of biosurfacant producers towards hydrocarbons which indicates the involvement of such strains in hydrophobic compound emulsification and degradation. Therefore, the results of the present investigation suggest that the quality of biosurfactant produced by AAJ1 to AAJ4 has higher emulsifying activity and would be very useful in bioremediation. The results of the catechol dioxygenase assay (Supplementary Table 1) of biosurfactant producing strains indicate that the strains also encompass sufficient catabolic enzyme, required for aromatic degradation [10].
Based on 16 s rRNA sequencing AAJ1 and AAJ2 were identified as Bacillus sp and AAJ3 and AAJ4 as Pseudomonas sp. which were submitted to NCBI Gene Bank with following accession no (AAJ1; KU235492), (AAJ2; KU235494), (AAJ3; KU235493) and (AAJ4; KU235495). All the four were collectively used for consortia preparation and named as “Oil buster”.
The results of oil degradation in the presence of individual strains and consortium after 20 days revealed that (Supplementary Table 2) strain AAJ1 reduced the oil content from 323 ± 0.07 to 96 ± 0.01 g/kg oil sludge with 70 % degradation rate while in the presence of AAJ3, the removal of oil was from 346 ± 0.041 to 145 ± 0.04 g/kg (58 %). In case of strain AAJ4, the reduction was from 293 ± 0.025 to 137 ± 0.02 g/kg of oil sludge. The strain AAJ2 was found to reduce 57 % oil content from 270 ± 0.03 to 115 ± 0.05 g/kg of oil sludge. Although, highest significant reduction of 78 % was observed in the presence of consortium (p < 0.001) over the period of 20 days as against the control (30 %). The biosurfactant producing strains emulsify the oil and ultimately enhance solubility and degradation rates. However, consortium showed impressive results than individual strains and control. This might be due to production of dual surfactant i.e. rhamnolipid by Pseudomonas sp and surfactin by Bacillus sp. which accelerate the degradation rate in comparison to individual strains.
The results of TPH reduction and its fractions are presented in Fig. 3. In the presence of AAJ1 strain, the TPH content decreased from 279 ± 2.5 g/kg to 126 ± 6.8 (55 %) and with strain AAJ2, decrease was from 220 ± 4.2 to 94 ± 5.5 g/kg (57 %). After completion of the study, in the presence of strain AAJ3, removal was from 310 ± 3.9 g/kg to 137 ± 4.0 (56 %) and strain AAJ4 was responsible for 54 % reduction. It was very interesting to find that consortium showed the highest removal (75 %) of TPH (p < 0.05), while in control flask, 29 % reduction was found which might be due to physico- chemical losses and activity of native microflora of the sludge. Verma et al. [11] have reported 59 and 35 % oil sludge degradation by Bacillus and Pseudomonas sp., respectively and suggested that Bacillus sp. may have an efficient enzyme system for oil sludge degradation.
Ghazali et al. [4] reported that bacterial formulation of Bacillus and Pseudomonas sp. could effectively remove long and medium-chain alkanes from diesel contaminated soil after a period of 60 days. The study carried out by Bordoloi and Konwar [22] has also supported that bacterial strains are capable of producing biosurfactant which could utilize PAHs as the sole source of carbon and energy. Based on the findings and in light of available literature, it is concluded that the bacterial consortium acts more effectively than single culture on complex compounds like oil sludge. In case of a group of bacteria, when one species removes toxic compounds or produces metabolites partially, then second species acts on it and functions effectively. The recently conducted studies have also reported biodegradation of oil sludge but the issue of removal of all the fractions of tank bottom oil sludge including recalcitrant asphaltene and higher PAHs still remained to be uncovered which is the main highlight of the present study.
In the present study, consortium removed 89 % of aromatic content whereas strains AAJ1 and AAJ2 removed 76 and 64 % and in control removal was only 38 % (Fig. 3). Strains AAJ3 and AAJ4 showed 69 and 70 % reduction in aromatic contents, respectively. The synergetic effects of mixed cultures effectively degraded different fractions of oil sludge [23]. Cerqueira et al. [24] studied the removal of 51.8 % aromatic fractions in 1 % of oil sludge after 40 days but in finding 2 % oil sludge, 89 % removal was observed after 20 days of incubation with consortium. During the present studies, an increase of 88 and 16 % in non biodegradable asphaltene fraction was observed in the presence of AAJ2 and AAJ3 respectively, while an increase of 4 % in control was observed. Liao et al. [25] reported that increase may be due to enlargement of some resin molecules and increase in their polarity in order to precipitate by hexane as newly generated asphaltenes. In contrast to AAJ2 and AAJ3, a decrease in asphaltene fraction was found in the presence of AAJ1, AAJ4 and the consortium by 36, 56 and 80 %, respectively. The recalcitrant asphaltene compounds may be degraded by the co-oxidation process in the presence of alkane compounds. Recently, Tavassoli et al. [26] reported 48 % asphaltene degradation by consortium after 60 days whereas in the present study 80 % degradation was obtained in the presence of the consortium “Oil buster” after 20 days.
A quantitative estimation of PAHs in aromatic fraction presented in Table 2 showing the presence of nine compounds on initial day including sharp peaks showed the dominance some URP (Unresolved peaks).
The strains AAJ1, AAJ3 and AAJ4 as well as consortium were able to completely degrade fluoranthene, pyrene, benzo (a) anthracene, chrysene and benzo (b) fluoranthene respectively. The degradation was also noticed in control and AAJ2 but with reduced rate. Strain AAJ3 completely degraded fluorene and anthracene and 82 % for phenantherene while AAJ1 and AAJ4 totally removed the phenantherene. The results clearly indicated that no single strains could degrade all the fractions of oil sludge completely.
Here, individual strains act differently on different PAH compounds (3 to 5-ring PAH), but their consortium was more potent and promising in removing all the PAHs including URP at the end of study. In the degradation of complex compounds such as oil sludge, multifunctional activity of the consortium is required to support interlinked metabolic degradation pathways of PAHs. Some higher PAH compounds degrade co-metabolically in the presence of lower PAH compounds [27]. Similar trend has been reported by Das and Mukarjee [28] and Mukred et al. [29] that microorganisms attack alkane primarily and then utilize PAH compounds. The results of catabolic enzyme assay (supplementary Table 1) showed highest C 2,3 DO activity in the Bacillus sp. (AAJ1 and AAJ2) followed by Pseudomonas sp. (AAJ3 and AAJ4) which indicates that they followed meta cleavage pathways for PAHs degradation. These findings are contradictory with reports of Kumari et al. [30] who reported ortho cleavage pathways for oil degradation. Prabhu and Phale [31] and Cenci et al. [17] have also reported that the presence of sufficient C 2, 3 activity in Pseudomonas strains is required for degradation. Here, the consortium possessed catabolic activity and biosurfactant production capability, which could be the reason for complete degradation achieved with the consortium. In the present investigation, the applied bacterial consortia “Oil busters” for oil sludge degradation study showed excellent and promising results in removing hydrocarbon compounds completely and could play an important role in remediation of oil sludge and hydrocarbon contaminated sites.
Conclusion
The biodegradation of PAHs and other petroleum hydrocarbons in the environment is a complex process; preparation of the microbial consortia is of utmost importance, which dictates the rate of the overall microbial degradation processes. In the present studies, consortium “Oil buster” could degrade 100 % of all the fractions of oil sludge in 20 days of flask culture study. The important criteria for culture selection were biosurfactant production, aromatic fraction utilization and the presence of catechol dioxygenase gene, which ended up in complete degradation of oil compounds. These strains also showed the reduction in surface tension and high emulsification property in the presence of crude oil and its compounds. The highlight of the study is the selective culture selection criterion and application for complete degradation of oil and its compounds. Therefore it is concluded that the oil buster could be used as a better tool for in-situ bioremediation of oil sludge contaminated sites.
References
Hu G, Li J, Guangming Z (2013) Recent development in the treatment of oily sludge from petroleum industry: a review. J. Haz. Matter. 261:470–490
Elisa RN, José Perales-Vargas-Machuca A (2012) Microbial degradation of PAHs: organisms and environmental compartments. Microbial degradation of xenobiotics. Environ Sci Eng 12:263–290
Krishnamurthi K, Devi SS, Chakrabarti T (2007) The genotoxicity of priority polycyclic aromatic hydrocarbons (PAHs) containing sludge samples. Toxicol Mech Meth 17:1–12
Ghazali FM, Zaliha RN, Rahman A, Salleh AB, Basri M (2004) Biodegradation of hydrocarbons in soil by microbial consortium. Int Biodete Biodegrad 54:61–67
Moliterni E, Gómez R, Rodríguez L, Fernández FJ, Villaseñor J (2012) Biosurfactants production during diesel biodegranation by mixed microbial consortia selected from polluted spolls. Int J Environ Res 6(3):751–760
Mohamed ME, Al-Dousary M, Hamzah RY, Fuchs G (2006) Isolation and characterization of indigenous thermophilic bacteria active in natural attenuation of bio-hazardous petrochemical pollutants. Int Biodete Biodegrad 58:213–223
Alkhatib MF, Alam MZ, Muyibi SA, Husain IAF (2011) An isolated bacterial consortium for crude oil biodegradation. Afr J Biotechnol 10(81):18763–18767
Kumar M, Leon V, Materano ADS, Ilzins QA (2007) A halotolerant and thermotolerant Bacillus sp. degrades hydrocarbons and produces tensio-active emulsifying agent. W J Microbiol Biotechnol 23:211–220
Lafortune I, Juteau P, Déziel E, Pépine F, Beaudet R, Villemur R (2009) Bacterial diversity of a consortium degrading high-molecular-weight polycyclic aromatic hydrocarbons in a two liquid phase biosystem. Microbiol Ecol 57:455–468
Dhote M, Juwarkar A, Kumar A, Kanade GS, Chakrabarti T (2010) Biodegradation of chrysene by bacterial strains isolated from oil sludge. W J Microbiol Biotechnol 26:329–335
Verma S, Bhargava R, Pruthib V (2006) Oil sludge degradation by bacteria from Ankleshwar, India. Int Biodeter Biodegrad. 57:207–213
USEPA (1996) Method 3540C, Soxhelet extraction. US Environ. protect agency, Washington, DC
USEPA (1996) Method 3630C, Silica Gel clean-up. US Environ. protect agency, Washington DC
Dubey K, Juwarkar A (2001) Distillery and curd whey wastes as viable alternative sources for biosurfactant production. W J Microbiol Biotechnol. 17:61–69
Škvarla J, Kupka D, Turčániová Ľ (2002) A complementary study of hydrophobicity and surface charge of Thiobacillus ferrooxidans: the effect of ionic surfactants. Acta Montanistica Slovaca Rocnik. 7:85–88
Narde G, Kaply A, Purohit HJ (2004) Isolation and characterization of Citrobacter strain HPC 255 for broad range substrate specificity for chlorophenols. Curr Microbiol 48:419–423
Cenci G, Caldini G, Boari L (1999) Dioxygenase activity and relative behaviour of Pseudomonas strains from soil in the presence of different aromatic compounds. W J Microbiol Biotechnol 15:41–46
Mishra S, Jyot J, Kuhad RC, Lal B (2001) In-situ bioremediation potential of an oil sludge-degrading bacterial consortium. Curr Microbiol 43:328–335
Ilori MO, Odocha J, Amobi AC (2005) Factors affect-ing biosurfactant production by oil degrading Aeromonas spp. isolated from a tropical environment. Chemosp 61:985
Cameotra SS, Singh P (2009) Synthesis of rhamnolipid biosurfactant and mode of hexadecane uptake by Pseudomonas species. Microb Cell Fact 8:16
Rahman PKSM, Pasirayi G, Auger V, Ali Z (2010) Production of rhamnolipid biosurfactants by Pseudomonas aeruginosa DS10-129 in a microfluidic bioreactor. Biotechnol Appl Biochem 55:45–52
Bordoloi NK, Konwar BK (2009) Bacterial biosurfactant in enhancing solubility and metabolism of petroleum hydrocarbons. J Hazard Matter 170:495–505
Moneke A, Nwangwu C (2011) Studies on the bioutilization of some petroleum hydrocarbons by single and mixed cultures of some bacterial species. Afr J Microbiol Res 5(12):1457–1466
Cerqueira VS, Hollenbach EB, Maboni F, Vainstein MH, Camargo FAO, Peralba MCR, Bento FM (2011) Biodegradation potential of oil sludge by pure and mixed bacterial cultures. Bioresour Technol 102:11003–11010
Liao Y, Geng A, Huang H (2009) The influence of biodegradation on resins and asphaltenes in the Liaohe Basin. Org Geochem 40:312–320
Tavassoli T, Mousavi SM, Shojaosadati SA, Salehizadeh H (2012) Asphaltene biodegradation using microorganisms isolated from oil samples. Fuel 93:142–148
Rehmann K, Noll HP, Steiberg CEW, Kettrup AA (1998) Pyrene degradation by Mycobacterium sp. Strain KR2. Chemosp. 36(14):2977–2992
Das K, Mukherjee AK (2007) Crude petroleum oil biodegradation efficiency of Bacillus subtilis and Pseudomonas aeruginosa strains isolated from a petroleum oil contaminated soil from North-East India. Bioresour Technol 98:1339–1345
Mukred AM, Hamid AA, Hamzah A, Yusoff WMW (2008) Development of three bacteria consortium for the bioremediation of crude petroleum-oil in contaminated water. OnLine J Biology Sci 8(4):73–79
Kumari B, Singh SN, Singh DP (2012) Characterization of two biosurfactant producing strains in crude oil degradation. Process Biochem 47:2463–2471
Prabhu Y, Phale PS (2003) Biodegradation of phenanthrene by Pseudomonas sp. strain PP2: novel metabolic pathway, role of biosurfactant and cell surface hydrophobicity in hydrocarbon assimilation. Appl Microbiol Biotechnol 61:342–351
Acknowledgments
Author is thankful to Council of Scientific and Industrial Research (CSIR), Govt. of India, for providing financial support in form of Senior Research fellowship (Grant No. P-81101).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dhote, M., Kumar, A. & Juwarkar, A. Petroleum Contaminated Oil Sludge Degradation by Defined Consortium: Influence of Biosurfactant Production. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 88, 517–523 (2018). https://doi.org/10.1007/s40011-016-0778-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-016-0778-z