Abstract
The blue stingray Dasyatis chrysonota is an important and endemic coastal inhabitant of southern African coastal waters however, it is listed as Near Threatened with declining populations. Understanding it’s spatial ecology in the context of current Marine Protected Areas (MPAs) is vital to not only inform population dynamics and ecological roles, but to determine whether currently zoned MPAs can provide sufficient protection to this species. Twenty-seven individuals were monitored using the Acoustic Tracking Array Platform (ATAP—a nation-wide collaborative network of acoustic receivers) for up to 4.5 years. Individuals displayed site affinity to defined regions of the coast, with the majority of detections for most individuals being recorded in the shallow bay/coastal shelf they were tagged in. However, important movement corridors linking different habitats were also identified (inshore vs offshore and coastal bay vs coastal shelf habitats). This habitat use varied monthly and was influenced by temperature, with individuals displaying restricted movements to the shallow bay habitat in summer when deeper waters were much colder. The large collaborative nature of the ATAP allowed for the monitoring of a benthic ray across large spatial scales for the first time, challenging preconceived notions that small undulatory batoids cannot travel large distances (many individuals were found to travel up to 200 km). Insights can also assist in local management of this species, and highlight that current MPA zonation may not be sufficient to protect blue stingrays from further population declines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rays (superorder: Batoidea) are considered one of the most threatened but under-researched vertebrate groups globally. A recent global review found 36% of ray species were considered at risk of extinction by the IUCN Red List of Threatened Species (Dulvy et al. 2021). In order to inform population dynamics, understand ecological roles and guide best management and conservation practices for rays, information on their movement ecology over multiple temporal and spatial scales is necessary. Although studies of ray movement have increased in recent years (for example Davy et al. 2015; Brinton and Curran 2017; Elston et al. 2021), there is still a lack of knowledge on their basic movement strategies. For example, the extent to which species display site affinity (defined as residency or a return to an area after migration) is still unclear, as well as their potential to conduct long-distance movements and connect multiple habitats, as studies over large-scales of expansive coastal corridors are lacking (Flowers et al. 2016) (although there are some limited examples from larger pelagic ray species, for e.g. Lowerre-Barbieri et al. 2021; DeGroot et al. 2021).
Determining the degree of site affinity is important because it can structure coastal populations on fine geographic scales (Chapman et al. 2015). Furthermore, knowledge on both local habitat use, as well as larger-scale habitat connectivity, is required to understand habitat dependence and the roles that rays may play within and across these habitats through ecological niche dynamics and energy flow (Beck et al. 2001; Murray et al. 2018; Frisk et al. 2019). Some pelagic rays have been found to be important in connecting different habitats such as islands, reefs and estuaries along continental shelves (for example Ajemian and Powers 2014; Braun et al. 2015; Ogburn et al. 2018; DeGroot et al. 2021). Conversely, benthic rays have only been shown to display site affinity to shallow and protected ecosystems, such as bays and estuaries (for example Cerutti-Pereyra et al. 2014; Davy et al. 2015; Brinton and Curran 2017), and it remains unclear whether these species may disperse from, or move between, these habitats (i.e. display habitat connectivity), an artefact of the small and constrained acoustic receiver arrays used in these studies.
It is also important to understand how environmental conditions may influence these movement dynamics. Animals will move, sometimes across large distances, to locate optimal conditions and resources (Doherty et al. 2017; DeGroot et al. 2021). Abiotic factors, specifically temperature, can be a major driver of movement in rays (Hight and Lowe 2007). They are ectothermic and consequently, temperature can influence their physiology and biology, specifically growth and metabolic rates, embryonic development, parturition, and the rate of digestion (Fangue and Bennett 2003; Hight and Lowe 2007; DiGirolamo et al. 2012; Dale et al. 2013). Thermoregulatory behaviours will often manifest over seasonal time scales, given that temperature changes across this temporal scale, particularly at temperate latitudes (Hopkins and Cech 2003; Vaudo and Lowe 2006; Bassos-Hull et al. 2014).
Passive acoustic telemetry offers a highly suitable method to assess the site affinity of a species, as well as habitat use and connectivity over small and large scales. Additionally, this can be coupled with long-term environmental data series (for example ocean temperature), allowing for the assessment of how environmental variables can influence movement behaviour. Of great advantage has been the recent development of several large-scale (hundreds to thousands of kilometres) tracking arrays consisting of acoustic receivers across the globe. Examples include the Integrated Marine Observing System’s (IMOS) Animal Tracking Facility (Steckenreuter et al. 2017; Taylor et al. 2017), the USA’s Animal Telemetry Network ATN (Block et al. 2016) and South Africa’s Acoustic Tracking Array Platform ATAP (Cowley et al. 2017). Specifically, the ATAP consists of an expanded network of marine and estuarine acoustic receivers placed along the South African coastline, providing the opportunity to assess large-scale movements and the connectivity of animals between various habitats.
The blue stingray (Dasyatis chrysonota) is an ideal candidate to further the understanding of how benthic rays may display site fidelity and habitat connectivity because of its relative abundance and postulated seasonal movements. It is a medium-sized (up to 75 cm disc width (DW), size at maturity is 41cm and 50cm for males and females respectively), common, benthic stingray endemic to southern Africa. Blue stingrays are known to occur in shallow inshore habitats (bays, estuaries and sheltered sandy beaches) during the summer, but are hypothesized to move to deeper offshore waters (up to 110 m depth) during winter (Ebert and Cowley 2003; Last et al. 2016). However, it is unknown whether individuals display fidelity (i.e. return to the same location) or dispersal (i.e. do not return to same location) and to what extent they may connect coastal habitats. Additionally, it is thought that the range of this species has shifted over the past three decades, from the wide shelf area of the Agulhas Bank off the south coast of South Africa to the narrower shelf area to the east (Currie et al. 2019). This range shift is partially attributed to rising sea water temperatures associated with climate change and may represent a loss of habitat to this species (Currie et al. 2019). It is therefore important to determine to what extent temperature influences the movement and behavior of this species. Furthermore, the blue stingray is currently listed as ‘Near Threatened’ by the IUCN Red List of Threatened Species with a declining population trend (Pollom et al. 2020). Although it is not caught for consumption purposes, it is captured as bycatch by trawl, commercial line, beach seine and gill net fisheries where it is discarded (da Silva et al. 2015). It is also particularly targeted by recreational anglers during competitions when they are abundant inshore (da Silva et al. 2015). Together, these fisheries account for the population reduction (da Silva et al. 2015). South Africa currently has a network of 41 Marine Protected Areas (MPAs), covering 5% of the Exclusive Economic Zone (EEZ), however the coverage of these MPAs have not yet been examined in relation to the spatial ecology of stingrays. Therefore, knowledge on their movement behaviours can inform whether the current MPA network is sufficient to protect this species from further declines.
The overall aim of this study was to present information on the spatial and temporal aspects of movement by adult blue stingrays along the South African coastline collected using acoustic telemetry. Specific objectives included:
-
Assessing whether there was site affinity to, or dispersal from shallow coastal ecosystems
-
Determining if any important movement corridors exist to inform habitat connectivity
-
Assessing habitat use and whether this was was influenced by season and temperature
-
Relating this information to the current MPA design in South Africa
Materials and methods
Study site and acoustic receiver array
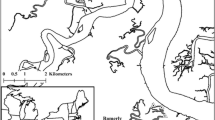
Acoustic monitoring of blue stingrays occurred along the South African coastline and was facilitated by the ATAP receiver network (Supplementary Fig. 1). This network consists of just over 300 acoustic receiver locations that spans \(\approx\)2200 km from the Berg Estuary on the west coast of South Africa, up to Ponta do Ouro in southern Mozambique on the east coast. At any given time, approximately 220 receivers (Innovasea, model VR2W and VR2AR) are active, depending on which research projects are currently underway. ATAP receivers are located within coastal bays, along shallow coastal shelves, and within certain estuaries across a highly diverse array of ecosystems and environmental conditions (Cowley et al. 2017). Broadly speaking, South Africa’s coastline is strongly influenced by two contrasting boundary currents, the warm Agulhas Current on the east coast and the cool Benguela Current on the west coast. These contrasting currents give rise to three major marine biogeographical zones: a cool temperate west coast zone, a warm temperate south coast zone, and a subtropical east coast zone (Turpie et al. 2000). The coastline is largely exposed, but a few embayments and a number of estuarine inlets exist.
Acoustic tagging
Tagging of blue stingrays occurred on the south coast biogeographical zone of South Africa in three different areas (Fig. 1). The majority were tagged in coastal bays while some were tagged along a shallow coastal shelf. Most individuals were tagged on the eastern section of the south coast (SEC) at two sites in Algoa Bay (n = 21, a coastal bay) in October 2017, and the remainder were tagged on the western section of the south coast zone (SWC) in Mossel Bay (n = 1, a coastal bay) in February 2016 and the De Hoop MPA (n = 5, a coastal shelf) in December 2018 (Fig. 1B). Individuals were caught by shore anglers using conventional fishing tackle and landed. Individuals were restrained in tonic immobility on wet PVC sheets to reduce the possibility of loss of external mucous coating. Innovasea acoustic transmitters were surgically implanted into their abdomens. All tags were V13, barring one V16 tag (individual dc14). Tag battery life ranged from 991 to 3650 days. All individuals were safely released after the tagging procedure at their capture locations.
Map of the Acoustic Tracking Array Platform receivers along the south coast of South Africa, with receivers denoted by circles and each colour representing defined areas. Defined habitat types are also represented by colour. Insets show more detailed maps of tagging areas; inset A shows the De Hoop Marine Protected Area, inset B shows Mossel Bay, and inset C shows Algoa Bay. Pink stars represent exact tagging locations. Black dotted polygons represent existing Marine Protected Areas
Average daily inshore and offshore sea temperatures for Algoa Bay between October 2017 and May 2020. The inshore logger was situated at 10 m depth in the north-western corner of the bay while the offshore logger was situated at 70m depth in the middle of the bay. Temperatures were recorded every hour by loggers
Data analysis
All analyses were conducted in R (version 4.0.2) (R Core Team 2020). Despite the nation-wide network of receivers spanning all three biogeographic zones of South Africa, individuals were only detected on marine receivers in the south coast zone (see results) and thus only receivers in this zone were considered in data analyses. These receivers were divided into defined areas (e.g. bays or sections of the coastal shelf) and were further divided by habitat type (Fig. 1). Habitat type for each receiver was extracted from the South African Marine Ecosystem Map developed during the National Biodiversity Assessment (Sink et al. 2019). Habitat types in the region included bay, deep rocky shelf, deep soft shelf, shallow rocky shelf, and shallow soft shelf. Receivers were classified based on both the area they occurred in and the habitat type of the receiver.
Site affinity and dispersal
Detection indices (DIs) were used to assess the level of site affinity to an individual’s tagging location. Two separate DIs were calculated: 1) a regional DI that considered presence on all receivers in the south coast zone and 2) a tagging location DI that considered presence only on receivers in the defined area that individuals were tagged in (i.e. specific bay or stretch of coastal shelf). The comparison of these two DIs provided information on whether individuals were detected more in the area they were tagged (i.e. displaying site affinity) or if they were detected more in other locations along the coastline (i.e. dispersal). The DI was calculated as number of days detected / number of days monitored. Days monitored was defined as the period between the date of tagging and 31 July 2020 (as most of the tags expired shortly after this date). An individual was considered to be present in an area if it was detected one or more times on a receiver in that area over a 24-h period. The minimum threshold of 1 detection, instead of the more commonly used 2 detections, was chosen because of the sparse nature of the receiver array resulting in fewer detections. Detections were manually inspected to remove poor quality or potentially spurious detections to reduce the likelihood of false detections. Spurious detections were defined as those recorded by a receiver that had washed up prior to the official retrieval, or when detections were recorded once on a receiver that was significantly out of range of the individual’s normal space-use.
The distance between an individual’s tagging location and each acoustic detection on a receiver was calculated. Distances were calculated using the dispersalSummary function in the ATT add-on to the VTrack R package (Campbell et al. 2012). Generalised Linear Mixed Models (GLMMs) were used to determine if distances travelled by individuals were influenced by sex, size or the tagging location of the individual. The distances best fit a log-normal distribution, therefore the Gaussian family with a ‘log’ link was used to create the model. The random effect in the model was ‘Individual’. An Information-Theoretic approach was adopted whereby the top performing models were determined through having the lowest AICc scores (if models had a delta AICc < 2 they were considered the best performing models).
Networks were created to visually assess the areas that individuals were traveling between. In this approach, receivers were considered as nodes and movements between receivers were considered as edges in the network.
Movement corridors and habitat connectivity
An Individual-Based Model (IBM) was developed to test the hypothesis that certain corridors of movement between receivers (i.e. edges identified in the constructed networks) were utilized more than expected based on model predictions and the distances between the receivers. The approach used by Jacoby et al. 2020 was adopted. As the model is based on the relationship between the frequency of movements between receivers and the distance between those receivers, maximum likelihood estimation (MLE) was used to determine if this relationship best followed a log-normal, power or exponential distribution. This was done using the poweRlaw package in R (Gillespie 2015).
The best fitting distribution was then used to parameterize a distance-decay IBM. A distance-decay model was chosen as it controlled for the tendency of nodes (receivers) that are closer together in space to be more connected within the network structure (Jacoby et al. 2020). The distances between receivers were calculated using the circuitous distance function in the VTrack R package (Campbell et al. 2012). This calculated the straight-line distance between a set of geographical coordinates with the use of waypoints to create indirect paths. This was chosen as the South African coastline does not represent a straight-line and waypoints were created at various features along the coast (for e.g. peninsulas) to better represent the path an individual would have to swim between receivers.
To run the IBM, a starting node from the area was randomly chosen and a number of ‘virtual stingrays’ (S) were simulated to move between the receivers in a given area for a number of transitions (N). Both the number of stingrays and the number of transitions mirrored the observed data. Transitions were weighted by the probability of that transition occurring based on the distance between receivers. Probabilities were drawn from the best fit distribution identified (log-normal, power or exponential). The simulation of S stingrays undertaking N transitions was repeated 1,000 times so that the observed number of transitions in the real network could be compared to the expected number of transitions in the simulated networks. These comparisons were performed using a Bonferroni corrected alpha (alpha of 0.05 divided by the number of possible transitions in the network). If a transition frequency between two receivers was statistically higher than the model predicted (confirmed through the Bonferroni corrected alpha), then this movement corridor was defined as being used more than expected and was mapped onto the network to visualize where these important movement corridors were located. Two separate IBMs were run for blue stingrays tagged in Algoa Bay (SEC) and in the De Hoop MPA (SWC).
Habitat use and the effect of season and temperature
The depth and substrate types over which blue stingrays were detected was determined. A Generalised Additive Model (GAM) was then used to determine if habitat use by stingrays was influenced by season and temperature. An additive model was chosen because it does not assume the underlying distribution of the data to be linear, and in this case, potential seasonal patterns would follow a cyclical (not linear) distribution. This model was run for individuals tagged in Algoa Bay only as there was a sufficient number of detections and temperature data to investigate trends. As individuals were hypothesized to use the inshore bay habitats on a seasonal basis, two separate data sets were created. One data set included presence/absence data for inshore bay habitats and the other included presence/absence data for offshore bay or coastal shelf habitats. A binomial model (with a logit link function) was developed whereby daily presence/absence to the defined habitat was the response variable. Covariates included month and two separate mean daily water temperatures (inshore and offshore). Water temperatures were recorded by HOBO temperature loggers (U22-001, Onset\(\circledR\), Cape Cod, Massachusetts, USA) in the two separate habitat types being investigated. The first logger was situated at 10 m depth on the inshore habitat of Algoa Bay (north-western corner), the second was situated at 70 m depth on the offshore habitat of Algoa Bay (centre of the bay). A smooth term was applied to each covariate, and the significance of each smooth was assessed to determine if season and temperature influenced habitat use. Autocorrelation of residuals was assessed using the default ACF function in R and it was determined there was no significant structure in the residuals.
Results
A total of 27 blue stingrays were tagged (18 female and 9 male, representing a skewed sex ratio of 2:1 in the favour of females). Tagged individuals ranged in size from 39 - 72 cm DW. One individual was not considered sexually mature at the time of tagging based on size estimates (dc01). Only two individuals were never detected on a receiver, and a total of 32,921 detections were recorded. However, after the removal of poor quality and potentially spurious detections, 32,451 detections remained.
Site affinity and dispersal
Individuals tagged in Algoa Bay, Mossel Bay and the De Hoop MPA were monitored for 2.5 years, 4.5 years and 1.5 years, respectively. Overall, the rate of detection along the entire south coast zone receiver array, and thus the calculated array DIs, were low. The majority of individuals (n = 22) were detected on less than 10% of days monitored while only three were detected for more than 10% of days monitored (dc03, dc15, dc17) (Table 1). Despite the sporadic nature of detections, most individuals were detected across a number of months and years (Fig. 2).
Individuals tagged in Algoa Bay (SEC) did not have overlapping ranges with individuals tagged in the SWC (i.e. Mossel Bay and De Hoop MPA) (Fig. 3). The exception was one individual (dc08) who was tagged in the SEC but dispersed to the SWC where detections ceased months later (Fig. 2). Furthermore, most detections occurred in the defined areas individuals were tagged in (that being defined coastal bays or shelves), with 15 individuals recording 100% of detections in their tagging area (Table 1). Five individuals (dc02, dc09, dc15, dc19, dc24) had the majority of detections in their tagging area, but were sometimes detected in other bays or sections of the coastal shelf. Interestingly, four individuals recorded less than half of their detections in their tagging areas (Table 1, Fig. 2). These included an individual who was tagged in the De Hoop MPA but was detected often in Mossel Bay (dc01, a distance of \(\approx\)200 km east), and individuals tagged in Algoa Bay, but were mostly detected in the neighboring St Francis Bay (dc05, dc07, a distance of \(\approx\)80 km west) or on the neighboring East London coastal shelf (dc10, a distance of \(\approx\)200 km east).
These trends were reflected in the distances that individuals travelled. Most individuals had the majority of detections less than 50 km away from their tagging locations. However, some individuals were often found to travel distances of 100–200 km, and the single individual who dispersed from the eastern to the western section of the south coast had most detections \(\approx\)450 km from their tagging location (Fig. 4). This individual was removed from GLMMs given this outlier behavior. Sex, size, and to a lesser extent tagging location significantly influenced the distances travelled by individuals. Although females had a slightly larger mean travel distance, males were found to have larger travel distances overall (a higher number of outlier movements). Travel distance significantly decreased with size. While the individual tagged in Mossel Bay displayed the highest mean travel distance, those tagged in Algoa Bay travelled further distances more frequently (Fig. 5).
Movement corridors and habitat connectivity
Upon comparing the simulated networks to the observed network for individuals tagged in Algoa Bay, most movements between receivers were not statistically different from what was expected based on the distance between receivers. However, a few important movement corridors were identified. Movements between inshore Algoa Bay receivers and offshore receivers, and to neighbouring shallow coastal shelf areas, occurred at higher rates than expected from the IBM (Fig. 6). Additionally, the north-western corner of Algoa Bay was shown to be particularly important as a movement corridor, despite these receivers being situated closely together.
Upon comparing the simulated networks to the observed network for individuals tagged in the De Hoop MPA, there were a number of important corridors identified between the coastal shelf and Mossel Bay, a large coastal bay \(\approx\)200 km away (Fig. 7).
Habitat use and the effect of season and temperature
Blue stingrays tagged on the SEC were only detected on 35 out of a possible 86 receivers on the SEC and most detections occurred in the inshore Algoa Bay habitat. Individuals also moved out of the bay and onto the deep soft shelf and the neighboring coastal shallow soft shelf and shallow rocky shelf. While many of the shallowest receivers did not record any detections, it still remains that the receivers occurring at depths less than 30 m (most notably at less than 15 m) recorded the largest number of daily detections. Individuals tagged in the SWC were only detected on 23 out of a possible 52 receivers on the SWC and most detections occurred in the inshore Mossel Bay habitat and on the coastal shelf of the De Hoop Nature Reserve. Three individuals travelled between these areas. Most detections occurred in the ‘Bay’ and ‘Shallow rocky shelf’ habitat types. There was one receiver in each of the ‘Deep soft shelf’ and ‘Shallow soft shelf’ habitat types and they both recorded a large number of detections too. Although there were a number of receivers in shallow waters < 20 m deep, most detections occurred on receivers at depths between 20 and 40 m (Supplementary Fig. 3).
Temperatures recorded in the inshore shallow habitat of Algoa Bay were on average higher than the temperatures recorded in the offshore deeper habitat year-round (Fig. 8). However, seasonal differences in temperature were apparent. Inshore temperatures were higher in the summer and lower in the winter months. Conversely, offshore temperatures were lower in summer and higher in the winter months. This resulted in very large temperature differences between these two habitats in the summer, but mostly overlapping temperature ranges in the winter (Fig. 8).
Offshore temperature and month were significant predictors of daily ray presence to the inshore bay habitat of Algoa Bay (Table 2). The probability of detection in this inshore habitat significantly declined in winter months and was significantly higher when offshore temperatures were lower. Although the influence of inshore temperature on inshore presence was not significant, there was a positive trend (Fig. 9).
Only month was a significant predictor of daily presence of blue stingrays at offshore and coastal shelf areas (Table 3). The probability of detection in these habitats peaked in winter and spring months, and was lowest during the summer months. Although the trend with temperature was not significant, the probability of detection at offshore and coastal shelf areas increased with increasing offshore temperatures and decreased with increasing inshore temperatures (Fig. 10).
Discussion
This study provides the first insight into the spatial ecology of the blue stingray in South Africa. The insights gained on this benthic stingray are novel, impactful and have important ramifications for future studies in this field. Although results revealed that blue stingrays displayed a high degree of site affinity (likely to be site fidelity specifically) to particular areas along the South African coastline, important movement corridors highlighting coastal habitat connectivity and large-scale movements were identified for the first time for a benthic stingray. Blue stingrays were found to travel much larger distances than have been previously recorded, and traditionally assumed, for small benthic, undulatory batoids, with a number of detections recorded up to 200 km away from individuals’ tagging locations. These novel insights into large-scale movements were only possible given the large spatial coverage of the ATAP receiver array and this study illustrates the value of a large collaborative acoustic receiver array with expansive spatial coverage.
Site affinity and habitat use
Although detections were sporadic given the sparse nature of the receiver array paired with the species’ biology, inter-annual site affinity to specific areas along the South African coast were identified. Blue stingrays tagged in different regions (i.e. SEC and SWC) did not display overlapping ranges. Furthermore, the majority of individuals had most of their detections, across multiple years, in the area in which they were tagged. Site affinity was reiterated through traditional tag-recapture data. The ORI-cooperative fish tagging project have tagged 1332 blue stingrays since 2010, but have only had 8 recaptures. Of these 8 recaptures, 7 were recaptured less than 10 km from the initial tagging location after a mean time-at-liberty of 92 days (range 0-271 days). The final individual was recaptured 97 km away from the tagging location after a time of liberty of 1217 days (Jordaan and Mann 2021).
There may, however, be some exceptions to this apparent site affinity; for example, one outlier male displayed dispersive behaviour whereby almost a year after tagging it left the SEC for the the SWC (De Hoop MPA) and appeared to remain in this region for over a year. Given the singular nature of this occurrence likely due to small sample size, it is difficult to reconcile. However, it suggests some individuals disperse to a different region, which facilitates the flow of genetic material. Individual variability in movements has been seen before in sharks (Matich and Heithaus 2015), but remains not well understood in elasmobranchs in general. Regardless, our evidence suggests sub-population structuring of blue stingrays along the south coast, as has been suggested for the white-spotted eagle ray Aetobatus narinari in Florida, which despite being a larger, pelagic and more mobile ray, displayed variable movement patterns related to tag location (DeGroot et al. 2021). Future genetic research would be notably helpful to confirm this potential fine-scale population structuring of blue stingrays along South Africa’s coast, information that is vital for the successful management of this species.
Interestingly, site affinity to both the shallow areas of coastal bays and an exposed coastal shelf was identified. While coastal bays have often been reported to be important habitat types to benthic stingrays (Cerutti-Pereyra et al. 2014; Davy et al. 2015; Brinton and Curran 2017), only one other study has found a coastal shelf (specifically a coastal beach) to be an important habitat type (Vaudo and Lowe 2006). In the present example, inshore shallow coastal shelves, especially the surf zone of sandy beaches, have been identified as productive feeding grounds for blue stingrays (Ebert and Cowley 2003), which could be a factor driving the use of this habitat type. The potential value of shallow coastal shelves should be taken into consideration for future studies assessing site affinity and habitat use of stingrays to determine whether they could be a more commonly used habitat type than currently assumed. It is also worthwhile to note that while blue stingrays are reported to use estuarine habitats (Last et al. 2016), no detections were recorded in estuaries in this study, despite the presence of receivers in a number of estuaries on the south coast. It may be that estuary use is a rare phenomenon for this species or that individuals who may use estuaries were not tagged, based on the marine sampling approach.
Migration and habitat connectivity
Most blue stingrays had periods when they were often detected in their tagging area, interspersed with periods of absences. It is difficult to ascertain whether these individuals left their tagging area during these periods of absences (site fidelity), or whether they remained in the area but were simply not in range of a receiver (residency), however, evidence points to the former.
Firstly, blue stingrays were mostly detected within 50 km of their tagging location, indicating periods of residency to a core area. However, exceptional distances were often travelled, sometimes up to 200 km, indicating that individuals left their core areas. It is also worthwhile to note the one individual who dispersed almost 500 km from its tagging location. To the best of our knowledge, these are the largest movement distances recorded for a benthic undulatory ray. In previous studies, round stingrays Urobatis halleri were found \(\approx\)30 km away from their tagging beach (Vaudo and Lowe 2006) and a cowtail stingray Pastinachus ater was found to travel \(\approx\)90 km on a shallow oceanic bank in the Seychelles (Elston et al. 2020). One study has shown a small-eye stingray Megatrygon microps to make a return trip of 400 km, however, this species is very likely semi-pelagic in nature given it’s extensive size (Boggio-Pasqua et al. 2019). This previous lack of evidence for large distance movements in stingrays could be due to either the actual behaviour, but it is also likely a function of previous receiver array set ups that have been limited in their spatial coverage. Future studies should increase the extent of their receiver arrays where possible to determine the distances which stingrays may travel outside of their core areas.
Secondly, site fidelity rather than residency was evidenced through seasonal trends in habitat use. For blue stingrays tagged in Algoa Bay, there was a significantly higher probability of detection in the inshore bay habitat during summer, and in the offshore and neighboring habitats during winter, suggesting seasonal migrations. This corroborates previous work on the habitat use of the blue stingray, where adults were found to be commonly caught in the inshore zone in summer, but were caught in offshore habitats in winter (Ebert and Cowley 2003). Furthermore, important movement corridors were identified in the present study, particularly between different habitat types. Corridors between inshore and offshore habitats, and between shallow coastal shelves and bays were particularly important, highlighting critical habitat connections and potential migration corridors. Indeed, many individuals were found to leave their area of tagging for offshore or neighouring areas followed by a period of absence, and return to their tagging location, often via the same pathways. This absence means we cannot identify their overwintering grounds, but it may be that they move further offshore beyond the receiver array.
These are novel insights into the large-scale space use and coastal habitat connectivity of a benthic stingray. A number of marine vertebrates are known to connect widely separated ecosystems via large-scale movements such as seasonal migrations. Elasmobranchs, in particular, are often considered as important for ecosystem connectivity, yet large-scale movement studies have been focused on apex predatory sharks (Hammerschlag and Sulikowski 2011) with little effort to understand the role of mesopredatory rays in linking habitats. The vast majority of studies investigating ray movements have had receiver arrays limited to a single coastal bay, beach, or estuary. For example, round stingrays seasonally used a small sandy beach, but individuals were not tracked when they left this area (Vaudo and Lowe 2006). Similarly, juvenile cowtail stingrays and porcupine rays Urogymnus asperrimus had a higher probability of detection within a coastal bay during autumn and winter, but were not tracked outside of the bay (Cerutti-Pereyra et al. 2014). Small Atlantic stingrays Dasyatis sabina travelled between two estuaries, but these were located less than 20 km apart (Ramsden et al. 2016). More recently, a study utilized a collaborative network of receivers to highlight large-scale migration patterns for the white-spotted eagle ray A narinari; however, this is a pelagic species known to travel relatively far distances (DeGroot et al. 2021). The present study is the first to track large-scale coastal habitat connectivity and movements outside of a core area using acoustic telemetry for a benthic undulatory ray and was only possible through having collaborative nodes of receivers along the coastline. This has important ecological ramifications as species that move between habitats perform vital ecosystem services through trophic interactions (e.g., predator-prey relationships), as well as energy and nutrient transfers between habitat types (Griffin et al. 2018; Flowers et al. 2020).
Influence of temperature
Algoa Bay is known to be a fairly dynamic ecosystem in terms of temperature. During summer, prevailing wind conditions cause seasonal upwelling on both the eastern and western points of Algoa Bay. Additionally, intense thermoclines develop during this time (Goschen and Schumann 2011), resulting in deeper waters being much cooler than shallower waters, trends reflected in the temperature data presented in this study. These seasonal temperature patterns were found to significantly influence the habitat use of blue stingrays. In summer, as offshore deeper waters got colder, blue stingrays constrained their movements to the warmer inshore waters of Algoa Bay. Most notably, the north-western corner of Algoa Bay may represent a thermal refuge for these blue stingrays during summer, as the cold upwelled water doesn’t penetrate into this corner (Goschen and Schumann 2011). Indeed, most detections of blue stingrays occurred in this north-western corner and connections between receivers closely-placed in this area were more important than expected from random movements. Other ray species have also been shown to restrict their movements to certain temperature limits (Armstrong et al. 2020; DeGroot et al. 2021) and is unsurprising given the ectothermic nature of rays. The lower temperatures reached in deeper waters during summer (<12 °C) may represent the thermal limit for blue stingrays.
Conversely, during winter, upwelling and stratification events are uncommon, and inshore and offshore temperatures are relatively similar. This likely facilitates the movement out of the inshore area to offshore and neighboring habitats during this time. However, temperature was not found to be a significant influence of this winter migration, suggesting there may be other reasons behind it. Various biotic factors such as prey availability, predator abundance and the presence of conspecifics have been shown to structure the movements of rays (Collins et al. 2007; Jaine et al. 2012). For blue stingrays, mating behaviours, such as ovulation and fertilization, occur in the inshore region during summer (Ebert and Cowley 2008). It may be that during winter when females are pregnant and have higher energetic demands, they expand their range of movement to larger or more energetically beneficial hunting grounds offshore. This could potentially be the reason behind females traveling a larger mean distance. However, having movement constrained to a nearshore environment because of temperature in the summer may represent a time of high levels of competition with other con- and hetero-specifics (rays and other bony fishes) for food resources, which could potentially be the reason that this species expands it’s range during winter.
Implications for management and conservation
The scale at which species are managed often do not adequately match the ecology of that species, hindering the success of management and conservation plans (Griffin et al. 2018). However, the integration of acoustic telemetry data into contemporary management of marine fishes has proven successful (Brooks et al. 2019). Blue stingrays contribute a significant proportion of the fish biomass to certain inshore coastal regions of South Africa (Cowley 1997), and as such likely play important ecological roles within these ecosystems. However, populations are in decline according to the most recent IUCN Red List assessment and current management initiatives do not appear to be sufficient to protect this species from further declines.
Although a large MPA already exists within Algoa Bay, the outcomes of this study suggest that this MPA likely has little benefit for blue stingrays in this region. The vast majority of detections were recorded in the north-western corner of the bay, outside of the boundary of the MPA. This north-western corner likely represents an important thermal refuge for blue stingrays in the summer months and as such, concentrated anthropogenic stressors in this area could have a disproportionate effect on the overall population. Most notably, blue stingrays are commonly caught by recreational anglers in this region during summer, and a large portion of these individuals are pregnant females. Abortions are common due to the stress of the capture (Adams et al. 2018, P. Cowley pers. comm.) and may be contributing to the population decline seen for this species. Conversely, the majority of detections for individuals tagged in the De Hoop MPA were inside the MPA, suggesting this MPA may be conferring local protection to these individuals. However, as individuals in both tagging regions were found to travel large distances to offshore and neighbouring habitats, this likely represents times when blue stingrays are vulnerable to capture; blue stingrays have been caught as by-catch in the offshore trawl fishery during winter months (Ebert and Cowley 2003).
Based on the outcomes of this study, future management plans that consider this species could include extending current MPAs, especially the current MPA in Algoa Bay, to include the areas determined as most important to blue stingrays. Alternatively, attempting to reduce the impact of recreational angling on this species through traditional management efforts, such as a closed season during the time when females are in the later stages of their pregnancy, may also benefit this species. Management likely needs to occur on local scales, given the potential for sub-population structuring and the observed differences in movement behaviour at a regional level.
Data availability
The datasets created and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Adams KR, Fetterplace LC, Davis AR et al. (2018) Sharks, rays and abortion: the prevalence of capture-induced parturition in elasmobranchs. Biol Conserv 217(October 2017):11–27. https://doi.org/10.1016/j.biocon.2017.10.010
Ajemian MJ, Powers SP (2014) Towed-float satellite telemetry tracks large-scale movement and habitat connectivity of myliobatid stingrays. Environ Biol Fishes. https://doi.org/10.1007/s10641-014-0296-x
Armstrong AJ, Armstrong AO, McGregor F et al. (2020) Satellite tagging and photographic identification reveal connectivity between two UNESCO World Heritage Areas for reef manta rays. Front Mar Sci 7(September):1–18. https://doi.org/10.3389/fmars.2020.00725
Bassos-Hull K, Wilkinson KA, Hull PT et al. (2014) Life history and seasonal occurrence of the spotted eagle ray, Aetobatus narinari, in the eastern Gulf of Mexico. Environ Biol Fishes 97(9):1039–1056. https://doi.org/10.1007/s10641-014-0294-z
Beck M, Heck K, Able K et al. (2001) The identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates. Bioscience 51(8):633–641
Block BA, Holbrook CM, Simmons SE et al. (2016) Toward a national animal telemetry network for aquatic observations in the United States. Anim Biotelemetry 4(1):4–11. https://doi.org/10.1186/s40317-015-0092-1
Boggio-Pasqua A, Flam AL, Marshall AD (2019) Spotting the “small eyes”: using photo-ID methodology to study a wild population of smalleye stingrays (Megatrygon microps) in southern Mozambique. PeerJ 7:1–20. https://doi.org/10.7717/peerj.7110
Braun CD, Skomal GB, Thorrold SR et al. (2015) Movements of the reef manta ray (Manta alfredi) in the Red Sea using satellite and acoustic telemetry. Mar Biol 162(12):2351–2362. https://doi.org/10.1007/s00227-015-2760-3
Brinton C, Curran M (2017) Tidal and diel movement patterns of the Atlantic stingray (Dasyatis sabina) along a stream-order gradient. Mar Freshw Res 68(9):1716–1725
Brooks J, Chapman J, Barkley A et al. (2019) Biotelemetry informing management: case studies exploring successful integration of biotelemetry data into fisheries and habitat management. Can J Fish Aquat Sci 76(7):1238–1252
Campbell HA, Watts ME, Dwyer RG et al. (2012) V-Track: software for analysing and visualising animal movement from acoustic telemetry detections. Mar Freshw Res 63:815–820
Cerutti-Pereyra F, Thums M, Austin CM et al. (2014) Restricted movements of juvenile rays in the lagoon of Ningaloo Reef, Western Australia—evidence for the existence of a nursery. Environ Biol Fishes 97(4):371–383. https://doi.org/10.1007/s10641-013-0158-y
Chapman DD, Feldheim KA, Papastamatiou YP et al. (2015) There and back again: a review of residency and return migrations in sharks, with implications for population structure and management. Ann Rev Mar Sci 7(1):547–570. https://doi.org/10.1146/annurev-marine-010814-015730
Collins AB, Heupel MR, Motta PJ (2007) Residence and movement patterns of cownose rays Rhinoptera bonasus within a south-west Florida estuary. J Fish Biol 71(4):1159–1178. https://doi.org/10.1111/j.1095-8649.2007.01590.x
Cowley PD (1997) Age and growth of the blue stingray Dasyatis chrysonota from the South-Eastern Cape coast of South Africa. S Afr J Mar Sci 18(1):31–38. https://doi.org/10.2989/025776197784161054
Cowley PD, Bennett RH, Childs AR et al. (2017) Reflection on the first five years of South Africa’s Acoustic Tracking Array Platform (ATAP): status, challenges and opportunities. Afr J Mar Sci 39(4):363–372. https://doi.org/10.2989/1814232X.2017.1399927
Currie JC, Thorson JT, Sink KJ et al. (2019) A novel approach to assess distribution trends from fisheries survey data. Fish Res 214:98–109. https://doi.org/10.1016/j.fishres.2019.02.004
Dale JJ, Drazen JC, Holland KN (2013) Stingray life history trade-offs associated with nursery habitat use inferred from a bioenergetics model. Mar Biol 160(12):3181–3192. https://doi.org/10.1007/s00227-013-2305-6
Davy LE, Simpfendorfer CA, Heupel MR (2015) Movement patterns and habitat use of juvenile mangrove whiprays (Himantura granulata). Mar Freshw Res 66(6):481. https://doi.org/10.1071/MF14028
da Silva C, Booth AJ, Dudley SFJ et al. (2015) The current status and management of South Africa’s chondrichthyan fisheries. Afr J Mar Sci 2338(September 2017):232–248. https://doi.org/10.2989/1814232X.2015.1044471
DeGroot BC, Bassos-Hull K, Wilkinson KA et al. (2021) Variable migration patterns of whitespotted eagle rays Aetobatus narinari along Florida’s coastlines. Mar Biol. https://doi.org/10.1007/s00227-021-03821-2
DiGirolamo AL, Gruber SH, Pomory C et al. (2012) Diel temperature patterns of juvenile lemon sharks Negaprion brevirostris in a shallow-water nursery. J Fish Biol 80(5):1436–1448. https://doi.org/10.1111/j.1095-8649.2012.03263.x
Doherty PD, Baxter JM, Godley BJ et al. (2017) Testing the boundaries?: Seasonal residency and inter-annual site fi delity of basking sharks in a proposed Marine Protected Area. Biol Conserv 209:68–75. https://doi.org/10.1016/j.biocon.2017.01.018
Dulvy NK, Pacoureau N, Rigby CL et al. (2021) Overfishing drives over one-third of all sharks and rays toward a global extinction crisis. Curr Biol 31(21):4773-4787.e8. https://doi.org/10.1016/j.cub.2021.08.062
Ebert DA, Cowley PD (2003) Diet, feeding behaviour and habitat utilisation of the blue stingray Dasyatis chrysonota (Smith, 1828) in South African waters. Mar Freshw Res 54:957–965
Ebert DA, Cowley PD (2008) Reproduction and embryonic development of the blue stingray, Dasyatis chrysonota, in southern African waters. J Mar Biol Assoc UK 89(04):809. https://doi.org/10.1017/S0025315408002907
Elston C, Cowley PD, von Brandis RG et al. (2020) Dietary niche differentiation in a mesopredatory dasyatid assemblage. Mar Biol 167(7):89. https://doi.org/10.1007/s00227-020-03695-w
Elston C, Cowley PD, Brandis RGV et al. (2021) Residency and habitat use patterns by sympatric stingrays at a remote atoll in the Western Indian Ocean. Mar Ecol Prog Ser 662:97–114. https://doi.org/10.3354/meps13632
Fangue NA, Bennett WA (2003) Thermal tolerance responses of laboratory acclimated and seasonally acclimatized Atlantic stingray. Dasyatis Sabina. Copeia 2:315–325
Flowers KI, Ajemian MJ, Bassos-Hull K et al. (2016) A review of batoid philopatry, with implications for future research and population management. Mar Ecol Prog Ser 562:251–261. https://doi.org/10.3354/meps11963
Flowers KI, Heithaus MR, Papastamatiou YP (2020) Buried in the sand: uncovering the ecological roles and importance of rays. Fish Fish. https://doi.org/10.1111/faf.12508
Frisk MG, Shipley ON, Martinez CM et al. (2019) First observations of long-distance migration in a large skate species, the winter skate: implications for population connectivity, ecosystem dynamics, and management. Mar Coast Fish 11:202–212. https://doi.org/10.1002/mcf2.10070
Gillespie CS (2015) Fitting heavy tailed distributions: the powerlaw package. J Stat Softw 64(2):1–16. https://doi.org/10.18637/jss.v064.i02
Goschen W, Schumann E (2011) The physical oceanographic processes of Algoa Bay, with emphasis on the western coastal region. Tech. Rep. April, South African Environmental Observation Network. https://doi.org/10.13140/RG.2.1.1823.3767
Griffin LP, Brownscombe JW, Adams AJ et al. (2018) Keeping up with the Silver King: using cooperative acoustic telemetry networks to quantify the movements of Atlantic tarpon (Megalops atlanticus) in the coastal waters of the southeastern United States. Fish Res 205(April):65–76. https://doi.org/10.1016/j.fishres.2018.04.008
Hammerschlag N, Sulikowski J (2011) Killing for conservation: the need for alternatives to lethal sampling of apex predatory sharks. Endanger Species Res 14(2):135–140. https://doi.org/10.3354/esr00354
Hight BV, Lowe CG (2007) Elevated body temperatures of adult female leopard sharks, Triakis semifasciata, while aggregating in shallow nearshore embayments: evidence for behavioral thermoregulation? J Exp Mar Biol Ecol 352(1):114–128. https://doi.org/10.1016/j.jembe.2007.07.021
Hopkins TE, Cech JJ (2003) The influence of environmental variables on the distribution and abundance of three elasmobranchs in Tomales Bay, California. Environ Biol Fishes 66:279–291. https://doi.org/10.1023/A:1023907121605
Jacoby DM, Ferretti F, Freeman R et al. (2020) Shark movement strategies influence poaching risk and can guide enforcement decisions in a large, remote marine protected area. J Appl Ecol. https://doi.org/10.1111/1365-2664.13654
Jaine FR, Couturier LI, Weeks SJ et al. (2012) When giants turn up: sighting trends, environmental influences and habitat use of the manta ray Manta alfredi at a coral reef. PLoS ONE. https://doi.org/10.1371/journal.pone.0046170
Jordaan G, Mann B (2021) ORI-cooperative fish tagging project: summary of the tag and recapture data for the blue stingray (Dasyatis chrysonota) caught along the South African coastline : 2010–2021. Tech. Rep. December 2021, Oceanographic Research Institute, Durban
Last P, Naylor G, Séret B, et al. (eds) (2016) Rays of the world. CSIRO
Lowerre-Barbieri SK, Friess C, Griffin LP et al. (2021) Movescapes and eco-evolutionary movement strategies in marine fish: assessing a connectivity hotspot. Fish Fish 22(6):1321–1344. https://doi.org/10.1111/faf.12589
Matich P, Heithaus MR (2015) Individual variation in ontogenetic niche shifts in habitat use and movement patterns of a large estuarine predator (Carcharhinus leucas). Oecologia 178(2):347–359. https://doi.org/10.1007/s00442-015-3253-2
Murray TS, Cowley PD, Bennett RH et al. (2018) Fish on the move: connectivity of an estuary-dependent fishery species evaluated using a large-scale acoustic telemetry array. Can J Fish Aquat Sci 75(11):2038–2052
Ogburn MB, Bangley CW, Aguilar R et al. (2018) Migratory connectivity and philopatry of cownose rays Rhinoptera bonasus along the Atlantic coast, USA. Mar Ecol Prog Ser 602:197–211. https://doi.org/10.3354/meps12686
Pollom R, Bennett R, Da Silva C et al. (2020) Dasyatis chrysonota. Tech. rep, The IUCN Red List of Threatened Species
R Core Team (2020) R: a language and environment for statistical computing. https://www.r-project.org/
Ramsden S, Cotton CF, Curran MC (2016) Using acoustic telemetry to assess patterns in the seasonal residency of the Atlantic stingray Dasyatis sabina. Environ Biol Fishes. https://doi.org/10.1007/s10641-016-0498-5
Sink K, van~der Bank MG, Majiedt P, et al. (2019) South African national biodiversity assessment 2018 technical report volume 4: Marine realm. Tech. rep., South African National Biodiversity Institute, Pretoria
Steckenreuter A, Hoenner X, Huveneers C et al. (2017) Optimising the design of large-scale acoustic telemetry curtains. Mar Freshw Res 68(8):1403–1413. https://doi.org/10.1071/MF16126
Taylor MD, Babcock RC, Simpfendorfer CA et al. (2017) Where technology meets ecology: acoustic telemetry in contemporary Australian aquatic research and management. Mar Freshw Res 68(8):1397–1402. https://doi.org/10.1071/MF17054
Turpie JK, Beckley LE, Katua SM (2000) Biogeography and the selection of priority areas for conservation of South African coastal fishes. Biol Conserv 92(1):59–72. https://doi.org/10.1016/S0006-3207(99)00063-4
Vaudo JJ, Lowe CG (2006) Movement patterns of the round stingray Urobatis halleri (Cooper) near a thermal outfall. J Fish Biol 68(6):1756–1766. https://doi.org/10.1111/j.0022-1112.2006.01054.x
Acknowledgements
The Acoustic Tracking Array Platform (ATAP) hosted by the National Research Foundation-South African Institute for Aquatic Biodiversity (NRF-SAIAB), the Ocean Tracking Network (OTN) headquartered by Dalhousie University, Canada, the Department of Science and Innovation-Shallow Marine and Coastal Research Infrastructure (DSI-SMCRI) programme and the National Research Foundation-South African Environmental Observation Network Elwandle Node (NRF-SAEON Elwandle Node) are thanked for providing acoustic telemetry hardware that facilitated data collection for this study. Furthermore, the Save Our Seas Foundation (SOSF) and the African Coelacanth Ecosystem Programme (ACEP) are acknowledged for funding to maintain the national ATAP. In particular, the authors gratefully acknowledge the ATAP partners who assist in the deployment and maintenance of the ATAP receiver array in the regions described in the paper, particularly Enrico Gennari and his team in Mossel Bay, JD Filmalter in De Hoop, Barry Skinstad in Plettenberg Bay and NRF-SAEON Elwandle Node in St. Francis and Algoa Bay. We would like to thank those who assisted in the capture and tagging of blue stingrays, including Rhett Bennett, JD Filmalter and the South African Shore Angling Associations anglers. Funding for acoustic transmitters was provided by the Shark Conservation Fund (SCF) and implemented by the WILDTRUST, through its WILDOCEANS programme, and the National Research Foundation South African Institute for Aquatic Biodiversity (NRF-SAIAB).We also thank the Oceanographic Research Institute’s Cooperative Fish Tagging Project, who provided the traditional tag-and-recapture data for blue stingrays and the NRF-SAEON Elwandle Node for providing temperature data. We are also grateful to the two anonymous reviewers who provided helpful and insightful feedback which improved the quality of the manuscript.
Funding
Funding were provided by National Research Foundation, South African Institute for Aquatic Biodiversity, Save Our Seas Foundation, African Coelacanth Ecosystem Program and Shark Conservation Fund.
Author information
Authors and Affiliations
Contributions
PC conceived and developed the project. PC, MP and TM contributed towards data collection. CE performed data analyses and wrote the first draft of the manuscript. MP and TM edited the manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethics approval
The tagging and monitoring of animals was approved by the South African Institute for Aquatic Biodiversity Animal Ethics Committee (number 2017-07).
Additional information
Communicated by James Tony Lee.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to the Topical Collection: Coastal and marine biodiversity.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Elston, C., Cowley, P.D., Murray, T.S. et al. Novel insights into coastal site affinity and habitat connectivity of a benthic stingray with implications for management. Biodivers Conserv 32, 181–202 (2023). https://doi.org/10.1007/s10531-022-02494-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-022-02494-7