Abstract
The Atlantic stingray Dasyatis sabina is found along the Atlantic and Gulf coasts of the United States. It consumes commercially important shrimp and crabs, and its diet overlaps with recreationally valuable red drum Sciaenops ocellatus and pompano Trachinotus spp. Despite the potential economic impact of this species, it is unclear whether the Atlantic stingray is present year-round or only seasonally in coastal habitats. The objective of this study was to assess the seasonal residency patterns of Atlantic stingrays in two creek systems in the Savannah River estuary. Forty stingrays were tracked using acoustic telemetry, and a seasonal residence index was calculated for each individual. Atlantic stingrays were present year-round in the Savannah River estuary, as 15 % (n = 6) of the tagged rays remained in the study areas throughout the year. This is the northernmost region where this species has been documented to be present all year. Of the 85 % (n = 34) of the rays that migrated during winter, 38 % (n = 13) of those were detected within the estuary less than 20 km away. Had trawls or mark-recapture been used instead, the few animals remaining in the creek systems and/or those that migrated may not have been collected. Acoustic telemetry is a more accurate means of studying the residency of fishes than periodic sampling that requires capture, and researchers should consider incorporating this technology in future studies about fish-environment interactions. Underestimating the presence of a species could result in miscalculation of its economic and ecological impact and, by extension, result in the implementation of ineffective or even detrimental management strategies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Atlantic stingray Dasyatis sabina is a demersal elasmobranch that inhabits salt, fresh, and brackish waters along the Atlantic and Gulf coasts of the United States from the Chesapeake Bay to the Gulf of Mexico (Dahlberg 1975; Johnson and Snelson 1996; Kells and Carpenter 2011). This species is tolerant of a wide range of environmental conditions, surviving in salinities as low as 0 ppt (Funicelli 1975; Schwartz 2000) and as high as 41 ppt (Snelson et al. 1988), and water temperatures ranging from 15 °C (Funicelli 1975; Snelson et al. 1988) to 35 °C (Schwartz and Dahlberg 1978; Snelson et al. 1988).
Dasyatis sabina is preyed upon by larger elasmobranchs, including the bull shark Carcharhinus leucas (Snelson et al. 1984), great hammerhead shark Sphyrna mokarran (C. Cotton, pers. comm.), sharpnose shark Rhizoprionodon terraenovae, and blacktip shark Carcharhinus limbatus (Hoffmayer and Parsons 2003). The Atlantic stingray is an opportunistic predator of commercially important crustaceans such as shrimp Penaeus spp. and blue crabs Callinectes sapidus, as well as grass shrimp Palaemonetes spp. (Funicelli 1975; Howard et al. 1977; Blonder and Alevizon 1988). Its diet overlaps with those of recreationally valuable red drum Sciaenops ocellatus and pompano Trachinotus spp. (Cook 1994). The Atlantic stingray is also an ecosystem engineer; when it feeds, it excavates pits (Howard et al. 1977) and significantly reduces the abundance of meiofauna in the benthos (Reidenauer and Thistle 1981; Cross and Curran 2000; Cross and Curran 2004; Ebanks 2005). Despite the trophic significance of this species and its potential to impact both commercial and recreational fisheries, it is unclear whether the Atlantic stingray is present year-round or only seasonally in its coastal habitats.
Atlantic stingrays have been reported to be present year-round in some regions (Schwartz and Dahlberg 1978; Snelson et al. 1988; Fangue and Bennett 2003), but only seasonally in others (Funicelli 1975; Lewis 1982; Schwartz 2000), although there may be confounding effects of sampling gear (Table 1). Atlantic stingrays are present year-round on the Atlantic coasts of Georgia (Schwartz and Dahlberg 1978) and mid-Florida (Snelson et al. 1988) as well as the northern Gulf coast of Florida (Fangue and Bennett 2003). In contrast, less than 100 km east along the Gulf coast of Florida, Atlantic stingrays are thought to be present only seasonally (Lewis 1982). Additionally, Atlantic stingrays are considered to be present only seasonally off the coasts of North Carolina (Schwartz and Dahlberg 1978), South Carolina (Ogburn et al. 1988), and Texas (Sage et al. 1972).
It is possible that Atlantic stingrays are present all year in some regions and only seasonally in others. However, it is also possible that they are present throughout the year in all regions but not collected using certain sampling methods. For example, benthic irregularities can reduce the effectiveness of bottom trawls and long-lines historically used to study residency. Both of these sampling gears can become entangled in or damaged by rocks, driftwood, oyster rakes, abandoned crab pots, and other debris. Strong winds can also interfere with the deployment of trawls, as occurred when McQuinn and Nellis (2007) trawled for lake sturgeon Acipenser fulvescens in the middle St. Lawrence estuary. Acoustic telemetry is also more effective than mark-recapture for assessing residency because movement data can be obtained even if the tagged animal is never encountered again (Heupel et al. 2006). For example, Schmid (1988) marked 203 Atlantic stingrays in the Indian River Lagoon but only recaptured 75 (37 %). However, 100 % of the Atlantic stingrays tagged by Brinton (2015) were “recaptured” by acoustic receivers at least 1 day after release. In addition, data can be collected by acoustic receivers even when weather conditions make it unsafe for researchers to be on the water (Heupel et al. 2006) and allow for greater temporal and spatial coverage of a study area than periodic sampling that requires capture (e.g., trawls, hooking gear, entanglement gear). Furthermore, the longevity of acoustic tags allows for the observation of animal movements over multiple years, such that researchers can determine the repeatability of movement patterns (Hussey et al. 2015). Passive acoustic telemetry does not require constant close proximity to a study organism, which could influence its behavior (Heupel et al. 2006). Finally, animals can be detected using acoustic telemetry even in regions of complex bathymetry where the deployment of trawls or long-lines is difficult or inefficient (Ramsden 2015; Brinton 2015).
Despite the effectiveness of passive acoustic telemetry in assessing the home ranges, habitat use, distribution, and site fidelity of many aquatic species (Hussey et al. 2015), there are few studies in which this technology has been used to assess the long-term movement patterns of batoid elasmobranchs. In Australia, acoustic telemetry has been used to investigate the activity patterns and habitat use of freshwater whiprays Himantura dalyensis (Campbell et al. 2012), mangrove rays Himantura granulata (Davy et al. 2015), shovelnose rays Glaucostegus typus, and reticulate whiprays Himantura uarnak (Vaudo and Heithaus 2012). Acoustic telemetry has been used to study the foraging behaviors of southern stingrays Dasyatis americana (Corcoran et al. 2013) and pink whiprays Himantura fai (Gaspar et al. 2008), and the seasonal residency patterns of round stingrays Urobatis halleri have also been studied using this technology (Vaudo and Lowe 2006). To date, the long-term movement patterns of the Atlantic stingray have not been studied using acoustic telemetry. The objective of the present study was to assess seasonal patterns in residency of Atlantic stingrays in two creek systems in the Savannah River estuary on the coast of Georgia using acoustic telemetry.
Materials and methods
Study sites
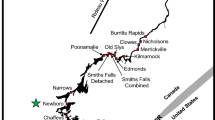
Two creek systems were studied on the northern coast of Georgia (Fig. 1). The Romerly Marsh Creek system is approximately 4.5 km in length from its mouth (31°55′46.45″ N, 80°59′4.66″ W) to its farthest tributary (31°55′32.81″ N, 81°1′45.13″ W), and, at mean low tide, the depth in the main channel ranges from 0.0 m in some places to 17.8 m at its deepest point. Salinity in Romerly Marsh Creek ranges from 20 to 30 ppt. The Herb River Creek system is approximately 10.0 km in length from its mouth (32° 0′ 31.57″ N, 81° 3′ 18.11″ W) to its farthest tributary (32° 1′ 22.16″ N, 81° 3′ 10.84″ W), and, at mean low tide, the depth of the main channel ranges from 0.0 in some places to 10.0 m at its deepest point. This creek system is farther from the open ocean than Romerly Marsh Creek, so its salinity is typically lower, ranging from 10 to 20 ppt. Both creeks experience semi-diurnal tides, ranging from −0.97 m to +2.91 m above mean lower low water. The banks are primarily vegetated with the smooth cordgrass Spartina alterniflora. The benthos of these systems is dominated by mud and sand flats with beds of the eastern oyster Crassostrea virginica. For the remainder of this manuscript, the terms ‘Romerly Marsh Creek’ and ‘the Herb River’ refer to both the main channels and smaller tributaries making up these systems.
Locations of the receiver arrays deployed in the Herb River and Romerly Marsh Creek systems in the Savannah River estuary on the coast of Savannah, Georgia. Receivers are denoted by white triangles. The square and arrow on the inset map represent the location of these creek systems on the East Coast of the United States
Passive acoustic receiver arrays
Arrays of passive acoustic receivers (Vemco VR2W-69 kHz) were deployed in both Romerly Marsh Creek and the Herb River using the methods described by Smith (2012) and modified by Ramsden (2015). Smith (2012) attached his receivers to steel rebar mounted in a concrete block. In the present study, the receivers were instead attached to PVC pipes that would not rust. The mounted receivers were then moored to concrete blocks lodged on the marsh shore to minimize movement by strong currents. Fifteen receivers were deployed in Romerly Marsh Creek in September 2012 at low tide depths ranging from 0 to 15 m (Fig. 1). Eleven receivers were deployed in the Herb River in April 2013 at low tide depths ranging from 0 to 10 m (Fig. 1). Receivers placed in locations that would be dry during low tide were deployed upside down, so that the hydrophone remained in the water for as long as possible. The detection radii of receivers ranged from 200 to 300 m. However, there was a significant effect of tidal stage and tributary width on detection range, with receivers in the widest tributaries having the greatest detection radii during high tide and receivers in the narrowest channels having the shortest detection radii during low tide (Brinton 2015). Stingrays were probably not in the vicinity of receiver stations when there was no water and receivers were not collecting data. Therefore, it is unlikely that a ray present in the creek system would be counted as absent, even though some receivers were not collecting data during certain hours of the tidal cycle.
In May 2013, a Vemco MINILOG-II-T temperature logger was attached to one acoustic receiver near the center of the widest tributary of each creek system. In Romerly Marsh Creek, the temperature logger was deployed at a low tide depth of 3 m. In the Herb River, the temperature logger was deployed at a low tide depth of 2 m. Strong tidal currents result in rapid mixing and prevent stratification in coastal Georgia estuaries (Ragotzkie and Bryson 1955). Both Romerly Marsh Creek and the Herb River experience extreme tidal ranges and currents, resulting in the waters of both systems being well mixed. Accordingly, temperatures collected from each logger location were considered to be representative of their entire respective system. Loggers recorded bottom-water temperature every 90 s to the nearest 0.1 °C. Temperature data from the loggers and detection data from the receivers were downloaded every 1–2 months.
Stingray capture and tagging
Stingrays were captured via long-lining using barbed, size 4 circle hooks baited with commercially collected frozen squid (Loligo spp.). Mass and disk width (DW) were measured on deck, and then stingrays were returned to the water for 1–2 min prior to the tagging surgery. Surgical instruments were in sterile packaging or were washed with 70 % ethanol solution. Rays were placed on their dorsal side, which was intended to induce tonic immobility, as described by Henningsen (1994). However, stingrays often remained mobile during the beginning of the surgery, so the tail was immobilized by sheathing it in a PVC pipe. The incision site was swabbed with a povidone-iodine swabstick and a 2–3 cm incision was made in the ventral surface of the stingray on the anterior lateral edge of the peritoneal cavity. Incisions were always made in the left edge to avoid the spiral valve, located on the right side. A Vemco V16-4H (24 g) or Vemco V13-1H (11 g) acoustic transmitter tag was inserted through the incision so that it lay lateral to the internal organs. Tag sizes were selected so that the weight of the tag was <2 % of the body mass of the stingray (Smith 2012). Incisions were closed with braided absorbable sutures coated with polyglycolic acid (MedRep-Express, 24 mm, 75 cm) using 1–2 interrupted sutures secured with surgeon’s knots. The total handling time, including initial weight and length measurements, ranged from 10 to 15 min, with surgery times usually <5 min. After surgery, rays were moved through the water until they resumed independent swimming. Recovery times ranged from 0 to 15 min. All surgeons practiced the tagging procedure on dead organisms before being allowed to perform the procedure on a live stingray. This increased the speed of the surgical procedure and reduced mortality risk.
The movements of 40 Atlantic stingrays were analyzed from December 2012–July 2014, with 16 rays tagged in Romerly Marsh Creek and 24 in the Herb River. Twenty-one animals were implanted with V16 tags that had a battery life of 863 days; 19 smaller animals were implanted with V13 tags that had a battery life of 372 days. Individuals were excluded from analysis after the date on which their tag battery was expected to expire. The tags do transmit signals after the predicted expiration date; however, the reliability of the rate and tonal frequency of the transmitter pings is unknown after the battery begins to expire. Analyzing data collected after the expected tag expiration date could result in incorrectly assuming that a ray is absent because of inconsistent pinging.
Data analysis
Presence or absence of a ray was tabulated by day instead of using number of detections because several environmental factors such as seasonal temperature changes, daily tides, episodic weather events (Mathies et al. 2014), and biotic noises such as snapping shrimp (Cotton 2010) can interfere with the detection range of acoustic receivers; thus, the absolute number of detections is not particularly informative. A ray was considered present on a given day if it was detected more than once on that day by any receiver in the array in which it was tagged. This was to avoid counting false positive detections that can be caused by biotic noise. Monthly residence indices were calculated for each stingray as follows, per Vianna et al. (2013):
Average bottom-water temperature was calculated each month. A linear regression analysis (α = 0.05) was used to determine if average bottom-water temperature was correlated with average monthly residence in either Romerly Marsh Creek or the Herb River.
Seasons from 2012 to 2014 were defined using equinox and solstice dates. A seasonal residence index was calculated for each stingray as follows, per Vianna et al. (2013):
An ANOVA with Tukey’s post hoc test (α = 0.05) was completed using the general linear model (GLM) procedure in the SAS statistical software program (Version 9.3; SAS Institute Inc., Cary, NC, USA, 2012) to determine if there was an effect of season on Atlantic stingray residence.
Results
The disk widths (DW) of rays from Romerly Marsh Creek ranged from 23.2–36.4 cm, with an average DW of 28.3 ± 1.00 cm (Table 2). The DW of rays from the Herb River ranged from 23.0–43.3 cm, with an average DW of 30.9 ± 1.25 cm (Table 3). There was no significant difference between the DW of rays from Romerly Marsh Creek (28.3 ± 1.00 cm) and those from the Herb River (30.9 ± 1.25 cm) (p = 0.11). Female rays (n = 29) were significantly larger (31.1 ± 0.99 cm) than males (n = 11) (26.8 ± 1.45 cm) (p = 0.02). However, there was no significant effect (p = 0.66) of the sex of stingrays on their monthly or seasonal residence.
Although number of detections can be affected by a variety of environmental factors, rays were detected between 2 and 125,979 times in Romerly Marsh Creek (Table 2) and between 1073 and 188,687 times in the Herb River (Table 3). The maximum number of consecutive days that rays were detected in Romerly Marsh Creek ranged from 1 to 321 days, with an average of 198.9 ± 25.8 days (Table 2). The maximum number of consecutive days that rays were detected in the Herb River ranged from 7 to 255 days, with an average of 112.7 ± 16.3 days (Table 3). A few rays remained within detection range of a single receiver for months at a time, contributing to the large numbers of detections for some individuals; however, seemingly stationary rays did eventually move during the study period and were detected by other receivers. No tagged animal remained stationary for such a long time that it was assumed to have died. In contrast, other rays appeared to be in constant motion, swimming from one receiver station to the next. A detailed analysis and description of the movement frequencies of the Atlantic stingray is presented in Brinton (2015).
Atlantic stingrays were present throughout the entire year in the Savannah River estuary, as 15 % (n = 6) of the 40 individuals tagged remained in the study sites during all seasons. Of the 34 rays (85 %) that migrated during winter, 13 were detected within the estuary less than 20 km away by receiver arrays deployed by the Georgia Department of Natural Resources (C. Kalinowsky, pers. Comm.). There were 24 rays at liberty during winter 2013–14. Nineteen (79 %) of those rays departed from the creek system in which they were tagged, and 10 of those (53 %) returned to their creek system of origin during the subsequent spring after being absent for 1–4 months.
Although some individuals were present year-round, Atlantic stingrays were detected for a lower percentage of days during winter than during all other seasons. In Romerly Marsh Creek, stingrays were present during winter 2013–14 for a significantly smaller percentage (p < 0.01) of days (20.8 ± 6.5 %) than during spring, summer, and fall 2014 (65.4–72.7 %) (Fig. 2a). In the Herb River, rays were present for a significantly (p < 0.01) smaller percentage of days during winter 2013–14 (9.1 ± 6.6 % of days) and winter 2014–15 (11.6 ± 5.6 %) than during any other season (38.0–86.3 %) (Fig. 2b). Bottom-water temperature over the course of the entire study ranged from 9.8–30.0 °C. There was a significant correlation between monthly Atlantic stingray residence and bottom-water temperature in Romerly Marsh Creek (p < 0.01) (R2 = 0.57) and the Herb River (p < 0.01) (R2 = 0.92), with stingrays using both creek systems more often when temperatures were warmer and less often when water temperatures were cooler (Fig. 3).
Average seasonal residence indices of Atlantic stingrays Dasyatis sabina tracked in (a) Romerly Marsh Creek and (b) the Herb River, Savannah, Georgia. Error bars represent +1 standard error. Different letters above bars indicate difference at the α = 0.05 significance level. For example, in the Herb River, Atlantic stingray residence during winter in both years was significantly lower than residence during all other seasons
Scatterplots of the relationship between the monthly residence indices of Atlantic Stingrays Dasyatis sabina and bottom-water temperature in Romerly Marsh Creek and the Herb River, Savannah, Georgia. The solid line represents the linear regression for Romerly Marsh Creek. The dashed line represents the linear regression for the Herb River
Discussion
The major finding of this study was that Atlantic stingrays were present year-round in the Savannah River estuary, as 15 % of the tagged rays were detected during all seasons. Additionally, many of the rays that did migrate out of the study areas during winter were detected within the estuary less than 20 km away. The rays that departed from, and then subsequently returned to, the creek systems in which they were tagged did so in as little as 1 month. The undulatory manner in which Atlantic stingrays swim is better suited for a slow-moving benthic lifestyle than fast, pelagic swimming (Rosenberger 2001). For an Atlantic stingray to return to the site at which it was tagged within 1 month of leaving, it could not have traveled far, further evidencing that this species probably remained in or near the estuary throughout the entire year.
The seasonal residency patterns of Atlantic stingrays have previously been attributed to changes in water temperature (Sage et al. 1972; Funicelli 1975; Lewis 1982). Although there was a significant correlation between bottom-water temperature and stingray presence in the present study, temperature probably did not directly limit the ability of Atlantic stingrays to remain in the Savannah River estuary system during the winter. This species has an extremely wide thermal tolerance of 0.7–43.3 °C, though this was based on only brief (<1 min) exposure (Fangue and Bennett 2003). Temperatures in our study sites (9.8–30.0 °C) never approached the threshold values calculated by Fangue and Bennett (2003), which could potentially explain why some stingrays remained in these creek systems during all seasons.
To date, the Savannah River estuary is the northernmost region in which the Atlantic stingray has been documented to be present year-round. South of this region, Schwartz and Dahlberg (1978) caught Atlantic stingrays throughout the entire year in St. Catherines and Sapelo sounds in Georgia, and Snelson et al. (1988) and Fangue and Bennett (2003) found Atlantic stingrays to be present year-round off the Atlantic and Gulf coasts of Florida, respectively (Table 1). North of the Savannah River estuary, Atlantic stingrays have only been collected seasonally during spring, summer, and fall in the Cape Fear estuary off the coast of North Carolina (Schwartz and Dahlberg 1978; Schwartz 2000) and the North Inlet estuary off the coast of South Carolina (Ogburn et al. 1988) (Table 1).
It is possible that Atlantic stingrays are present year-round in some regions, but only seasonally in others. However, it is also possible that they are present throughout the year in all regions but not collected using certain gear types. Small-scale seasonal movement patterns could further interfere with accurate assessments of abundance. Several batoid elasmobranchs including the Atlantic stingray utilize deeper parts of their habitats during the winter. For example, the short-tailed stingray Dasyatis brevicaudata in New Zealand (Le Port et al. 2008) and the thornback skate Raja clavata in the Thames River estuary in England (Hunter et al. 2005) migrate away from shallow waters and utilize deeper waters during winter. Snelson et al. (1988) observed that Atlantic stingrays in the Indian River Lagoon in Florida were often found during winter in deep channels that were 2–5 °C warmer than the surrounding shallower waters. Thirteen Atlantic stingrays in the present study were detected in deeper waters less than 20 km from the two study sites, but still within the Savannah River estuary, during winter. Due to their wide thermal tolerance, Atlantic stingrays may not have needed to take refuge from the cold; however, the rays could have been seeking out places of higher food abundance. Two prey items of the Atlantic stingray, grass shrimp (Welsh 1975) and blue crabs (Livingston 1976), move into deeper waters during the winter. These deeper or more topographically complex areas may be more difficult to sample using bottom trawls. Rays that do remain in an area, but move into deeper waters during winter, will be detected using acoustic telemetry. These same animals may not be caught during periodic capture surveys.
Conclusion
We found that the Savannah River estuary is the northernmost region in which the Atlantic stingray has been documented to be present during the entire year. There has been uncertainty about the residency patterns of this species throughout its geographic range in part because the sampling methods used historically may not have captured rays even when they were present. The results of our study were clear: rays were present in the monitored areas throughout the entire year, and many (38 %) of the rays that left those areas during winter were detected elsewhere within the estuary.
Incorrect assumptions about the residency of Atlantic stingrays are problematic because this species is an ecosystem engineer and has the potential to affect trophic dynamics and impact commercial fisheries. Other larger elasmobranchs may have even greater economic and ecological impacts. Falsely assuming a species is absent because it is not captured is an inherent bias in many sampling designs; therefore, researchers should consider incorporating acoustic telemetry into future studies to clarify the residency patterns of fishes for which there is inconsistent reporting. Underestimating the presence of a species could result in subsequent miscalculation of its ecological and economic impacts and, by extension, result in the implementation of ineffective or even detrimental management strategies.
References
Blonder BI, Alevizon WS (1988) Prey discrimination and electroreception in the stingray Dasyatis sabina. Copeia 1988:33–36
Brinton CP (2015) Tidal and diel movement patterns of the Atlantic stingray Dasyatis sabina in two creek systems near Savannah, Georgia. Thesis, Savannah State University
Campbell HA, Hewitt M, Watts ME, Peverell S, Franklin CE (2012) Short- and long-term movement patterns in the freshwater whipray (Himantura dalyensis) determined by the signal processing of passive acoustic telemetry data. Mar Freshw Res 63:341–350
Cook DA (1994) Temporal patterns of food habits of the Atlantic stingray, Dasyatis sabina (Lesueur, 1824) from the Banana River Lagoon, Florida. Thesis, Florida Institute of Technology
Corcoran MJ, Wetherbee BM, Shivji MS, Potenski MD, Chapman DD, Harvey GM (2013) Supplemental feeding for ecotourism reverses diel activity and alters movement patterns and spatial distribution of the southern stingray, Dasyatis americana. PLoS One 8:e59235
Cotton CF (2010) Factors affecting reception range of ultrasonic tags in a Georgia estuary. Mar Technol Soc J 44:17–24
Cross RE, Curran MC (2000) Effects of feeding pit formation by rays on an intertidal meiobenthic community. Estuar Coast Shelf Sci 51:293–298
Cross RE, Curran MC (2004) Recovery of meiofauna in intertidal feedings pits created by rays. Southeast Nat 3:219–230
Dahlberg MD (1975) Guide to coastal fishes of Georgia and nearby states. Athens
Davy LE, Simpfendorfer CA, Heupel MR (2015) Movement patterns and habitat use of juvenile mangrove whiprays (Himantura granulata). Mar Freshw Res 66:481–492
Ebanks DA (2005) The influence of physical factors and ray bioturbation on meiofaunal abundance in Chowan Creek, South Carolina. Thesis, Savannah State University
Fangue NA, Bennett WA (2003) Thermal tolerance responses of laboratory-acclimated and seasonally acclimatized Atlantic stingray, Dasyatis sabina. Copeia 2003:315–325
Funicelli NA (1975) Taxonomy, feeding, limiting factors, and sex ratios of Dasyatis sabina, Dasyatis americana, Dasyatis sayi, and Narcine brasiliensis. Dissertation, University of Southern Mississippi
Gaspar C, Chateau O, Galzin R (2008) Feeding sites frequentation by the pink whipray Himantura fai in Moorea (French Polynesia) as determined by acoustic telemetry. Cybium 32:153–164
Henningsen AD (1994) Tonic immobility in 12 elasmobranchs: use as an aid in captive husbandry. Zoo Biol 13:325–332
Heupel MR, Semmens JM, Hobday AJ (2006) Automated acoustic tracking of aquatic animals: scales, design and deployment of listening arrays. Mar Freshw Res 57:1–13
Hoffmayer ER, Parsons GR (2003) Food habits of three shark species from the Mississippi Sound in the northern Gulf of Mexico. Southeast Nat 2:271–280
Howard JD, Mayou TV, Heard, RW (1977) Biogenic sedimentary structures formed by rays. J Sediment Petrol 47:339–346
Hunter E, Buckley AA, Stewart C, Metcalfe JD (2005) Migratory behaviour of the thornback ray, Raja clavata, in the southern North Sea. J Mar Biol Assoc UK 85:1095–1105
Hussey NE, Kessel ST, Aarestrup K, Cooke SJ, Cowley PD, Fisk AT, Harcourt RG, Holland KN, Iverson SJ, Kocik JF, Mills Flemming JE, Whoriskey FG (2015) Aquatic animal telemetry: a panoramic window into the underwater world. Science 348:1255642
Johnson MR, Snelson FF (1996) Reproductive life history of the Atlantic stingray, Dasyatis sabina (Pisces, Dasyatidae), in the freshwater St. Johns River, Florida. B Mar Sci 59:74–88
Kells V, Carpenter K (2011) A field guide to coastal fishes from Maine to Texas. Baltimore
Le Port A, Sippel T, Montgomery J (2008) Observations of mesoscale movements of the short-tailed stingray, Dasyatis brevicaudata from New Zealand using a novel PSAT tag attachment method. J Exp Mar Biol Ecol 359:110–117
Lewis TC (1982) The reproductive anatomy, seasonal cycles, and development of the Atlantic stingray, Dasyatis sabina (Lesueur) (Pisces, Dasyatidae) from the northeastern Gulf of Mexico. Dissertation, Florida State University
Livingston RJ (1976) Diurnal and seasonal fluctuations of organisms in a north Florida estuary. Estuar Coast Mar Sci 4:373–400
Mathies NH, Ogburn MB, McFall G, Fangman S (2014) Environmental interference factors affecting detection range in acoustic telemetry studies using fixed receiver arrays. Mar Ecol Prog Ser 495:27–38
McQuinn IH, Nellis P (2007) An acoustic trawl survey of middle St. Lawrence estuary demersal fishes to investigate the effects of dredged sediment disposal on Atlantic sturgeon and lake sturgeon distribution. Am Fish Soc Symp 56:257–271
Ogburn MV, Allen DM, Michener WK (1988) Fishes, shrimps, and crabs of the North Inlet estuary, SC: a four-year seine and trawl survey. University of South Carolina Baruch Institute Technical Report 88:1–299
Ragotzkie RA, Bryson RA (1955) Hydrography of the Duplin River, Sapelo Island, Georgia. B Mar Sci Gulf Caribbean 5:297–314
Ramsden S (2015) Seasonal patterns in residency and distribution of the Atlantic stingray Dasyatis sabina in two creek systems near Savannah, Georgia. Thesis, Savannah State University
Reidenauer JA, Thistle D (1981) Response of a soft bottom harpacticoid community to stingray (Dasyatis sabina) disturbance. Mar Biol 65:261–267
Rosenberger LJ (2001) Pectoral motion in batoid fishes: undulation versus oscillation. J Exp Biol 204:379–394
Sage MR, Jackson RG, Klesch WL, de Vlaming VL (1972) Growth and seasonal distribution of the elasmobranch Dasyatis sabina. Contrib Mar Sci 16:71–74
SAS software version 9.3 (2012) SAS Institute Inc., Cary, NC, USA
Schmid TH (1988) Age, growth, and movement patterns of the Atlantic stingray Dasyatis sabina in a Florida coastal lagoon system. Thesis, University of Central Florida
Schwartz FJ (2000) Elasmobranchs of the Cape Fear River, North Carolina. J Elisha Mitch Sci S 116:206–224
Schwartz FJ, Dahlberg MD (1978) Biology and ecology of the Atlantic stingray (Dasyatis sabina) (Pisces: Dasyatidae) in North Carolina and Georgia. Northeast Gulf Sci 2:1–23
Smith DT (2012) Using ArcGIS and acoustic telemetry to assess the distribution and movement of coastal shark species off the coast of Georgia, USA. Thesis, Savannah State University
Snelson FF, Mulligan TJ, Williams SE (1984) Food habits, occurrence, and population structure of the bull shark, Carcharhinus leucas, in Florida coastal lagoons. B M Sci 34:71–80
Snelson FF, Williams-Hooper SE, Schmid TH (1988) Reproduction and ecology of the Atlantic stingray, Dasyatis sabina, in Florida Coastal Lagoons. Copeia 1988:729–739
Vaudo JJ, Heithaus MR (2012) Diel and seasonal variation in the use of a nearshore sandflat by a ray community in a near pristine system. Mar and Freshwater Res 63:107–1084
Vaudo JJ, Lowe CG (2006) Movement patterns of the round stingray Urobatis halleri (Cooper) near a thermal outfall. J Fish Biol 68:1756–1766
Vianna GMS, Meekan MG, Meeuwig JJ, Speed CW (2013) Environmental influences on patterns of vertical movement and site fidelity of grey reef sharks (Carcharhinus amblyrhynchos) at aggregation sites. PLoS One 8:e60331
Welsh BL (1975) The role of grass shrimp, Palaemonetes pugio, in a tidal marsh ecosystem. Ecology 56:513–530
Acknowledgments
This research would not have been possible without funding from the Department of Education Title VII Grant, Award # P382G09003-12, nor without the help of Cameron Brinton, who patiently taught the first author everything from how to efficiently use data management software to how to surgically implant tags into stingrays. We would like to thank Chris Kalinowsky for notifying us when our stingrays ventured into receiver arrays deployed by the Georgia Department of Natural Resources. We would also like to thank the Nature Conservancy for loaning us additional receivers to expand our study area. We would like to give a special thanks to Amanda Kaltenberg for her edits and contributions as well as the anonymous reviewers for their suggestions. We would also like to thank Brigette A. Brinton, Michele B. Sherman, Jennifer A. Güt, and Dan McGarvey for their editing assistance, and the graduate and undergraduate students at Savannah State University who helped in the field. This publication is listed as Contribution Number 1812 of the Belle W. Baruch Institute for Marine and Coastal Science.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All research was conducted in compliance with protocols approved by the Savannah State University Institutional Animal Care and Use Committee (IACUC) and the Georgia Department of Natural Resources, collection permit #29-WJH-14-223.
Rights and permissions
About this article
Cite this article
Ramsden, S., Cotton, C.F. & Curran, M.C. Using acoustic telemetry to assess patterns in the seasonal residency of the Atlantic stingray Dasyatis sabina . Environ Biol Fish 100, 89–98 (2017). https://doi.org/10.1007/s10641-016-0498-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-016-0498-5