Abstract
Little information is available on the movements and behaviour of tropical rays despite their potential ecological roles and economic value as a fishery and a tourism resource. A description of the movement patterns and site fidelity of juvenile rays within a coral reef environment is provided in this study. Acoustic telemetry was used to focus on the use of potential nursery areas and describe movement patterns of 16 individuals of four species monitored for 1–21 months within an array of 51 listening stations deployed across a lagoon, reef crest, and reef slope at Mangrove Bay, Ningaloo Reef, Western Australia. Juveniles used a small (< 1 km2), shallow (1–2 m depth) embayment where three receivers recorded 60–80 % of total detections of tagged animals, although individuals of all species moved throughout the array and beyond the lagoon to the open reef slope. Detections at these primary sites were more frequent during winter and when water temperatures were highest during the day. Long-term use of coastal lagoons by juvenile rays suggests that they provide an important habitat for this life stage. Current marine park zoning appears to provide an effective protection for juveniles within this area.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tropical rays are more vulnerable to exploitation than teleosts due to their life history traits of low fecundity, late sexual maturity, long gestation and relatively slow grow rates (Frisk 2010). Although rays are targeted for fisheries throughout the world and are frequently taken as by-catch (Dulvy et al. 2000; Heupel and Heuter 2001; Romanov 2001; Dulvy and Reynolds 2002; Stevens 2002; Ward et al. 2009), little is known about their ecology and movement patterns. Although ideally, the most vulnerable life-stage of the species of concern should be first identified so management measures could be implemented to protect it (Kinney and Simpfendorfer 2009), the low fecundity of tropical rays suggests that survival during juvenile stages may be key element of population persistence, and therefore, behavior and movement patterns during the juvenile phase are an important focus for research (Heupel et al. 2007).
Many sharks and rays are thought to use nursery areas (Castro 1993; DeAngelis 2008; Ache de Freitas et al. 2009), which are defined as areas where gravid females give birth and where the young spend their first weeks, months or years (Castro 1993; Garla et al. 2006a; DeAngelis 2008; Ache de Freitas et al. 2009). Heupel et al. (2007) proposed that higher abundances of juveniles less than 1 year old should be found within a nursery area, and that these animals should display long residency times across years to such area. Such nurseries often occur in estuarine and lagoon systems and are thought to provide enhanced feeding opportunities and protection from predation during early stage of life. Lower predation rates can occur in nurseries because predators enter these environments infrequently and higher relative turbidity protects young from detection and attack (Holland et al. 1993; Yokota and Lessa 2006; Yeiser et al. 2008). As individuals grow, food demands and activity space increase to meet this need (Morrisey and Gruber 1993; Collins et al. 2007), so that young are eventually forced to venture beyond the nursery area.
Although there is unequivocal evidence for the use of nursery areas in a number of species of sharks (e.g. Heupel 2007; Chapman et al. 2009; Speed et al. 2010) there have been few studies of this phenomenon in rays. Based on catches in artisanal and small-scale fisheries, Yokota and Lessa (2006) found evidence for the use of nursery areas by eight species of ray along the shallow coast of north-eastern Brazil. Their study supported the idea that nursery areas were most critical for slow-growing species which remained at higher risk of predation for longer than fast-growing species (Branstetter 1990; Kinney and Simpfendorfer 2009).
For many sharks the use of nurseries coincides with changes in water temperatures. For example, a rise in water temperatures in early summer elicits a movement of juvenile sandbar sharks, Carcharhinus plumbeus (Nardo, 1827)) into coastal nursery areas for the summer (Grubbs et al. 2005). Similarly, juvenile reef sharks such as Carcharhinus amblyrhynchos (Bleeker, 1856) occupy a small bay at Ningaloo Reef when water temperatures are warmest over the summer (Speed et al. unpublished data). Such studies and the large amount of evidence for the effects of temperature on diel movements, seasonal migrations, distribution and abundance of sharks and rays (Springer 1960; Hopkins and Cech 2003; Heupel 2007; Conrath 2008; DiGirolamo et al. 2012) suggest that water temperature may be an important factor determining the temporal scales of use of coastal nurseries by rays. Residency and movement patterns of rays can also be related to water temperature because it affects the growth, embryonic development, parturition, and feeding rates of sharks and rays (Economakis and Lobel 1998; Heupel and Heuter 2001; Fangue et al. 2003; Hight and Lowe 2007; Kinney and Simpfendorfer 2009)
Recent advances in tagging technology have made studies of movement patterns of sharks and rays in coastal waters more tractable. Acoustic telemetry has been used to examine the movement and residency patterns of sharks and rays in shallow coastal waters over temporal scales ranging from hours to years (Klimley et al. 2005; Dewar et al. 2008; Speed et al. 2010). In contrast to catch data from fisheries, acoustic monitoring is non-destructive and provides multiple records of position for an individual assuming that tagged animals remain within the bounds of the acoustic receiver array. This is useful given the vulnerability of many species to over-exploitation and the restrictions on destructive sampling in marine protected areas. Additionally, the information provided by acoustic monitoring studies can inform management by identifying critical habitats such as nurseries and by assessing the effectiveness of protected areas (Heupel et al. 2004; Simpfendorfer and Heupel 2004; Hight and Lowe 2007).
The movement patterns of juvenile rays within a coral reef environment is described here. Restricted patterns of movement and long-term (months to years) residency within defined habitats would be expected if nurseries are important to this life history stages. The extent to which residency and movement patterns of rays were related to water temperature was also examined. Predictable patterns in habitat use that are strongly correlated with water temperatures would be expected if water temperature drives patterns of distribution.
Materials and methods
Study site
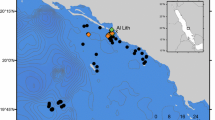
Ningaloo Reef extends for 320 km along the north-west coast of Western Australia (Fig. 1). It is a fringing coral reef that has been managed as a marine park since 1996 (Leprovost Dames and Moore 2000), with no-take sanctuary zones where fishing is prohibited incorporating approximately 33 % of the reef. The reef crest encloses a shallow, sandy lagoon that is between 0.2 and 7 km wide. Mangrove Bay (21° 58.035′S, 113° 56.487′E) one of the northern-most sanctuary zones in the Ningaloo Marine Park, is a broad sandy bay bordered by a fringing coral reef at the seaward edge with small areas of mangrove forest lining the shore. The bay includes a sanctuary zone that covers 11.35 km2 and is adjacent to recreational zones where fishing is permitted (Leprovost Dames and Moore 2000; Department of Conservation and Land Management 2005). Mangrove bay also has a tidal embayment adjacent to the mangrove forest (Fig. 1d)
Acoustic technology and passive monitoring
An array of 51 receivers (VR2w VEMCO©, Halifax, Canada) was deployed at Mangrove Bay (Fig. 1) as part of the Ningaloo Reef Ecosystem Tracking Array. These receivers form part of the Australian Animal Tagging and Monitoring System (www.imos.org.au/aatams.html), a national network of acoustic receiver stations. The receivers were downloaded every 4 to 6 months. The receiver array covered the Mangrove Bay area (2–15 m depth), the entrance of the tidal embayment adjacent to the mangrove forest (2 m depth) (Fig. 1d), channels from the lagoon to the open shelf (10–15 m depth) (Fig. 1e), and the open shelf (up to 40 m depth) (Fig. 1). Range testing showed the maximum detection range for receivers in Mangrove Bay was ~300 m and in the channel was ~ 500 m (Fig. S1). Temperature loggers (Minilog –II-T submersible temperature data logger, accuracy: ± 0.1 °C from −5 °C to 35 °C; range: −30 °C to +80 °C; VEMCO©, Halifax, Canada) were also attached to some receivers in the lagoon of Mangrove Bay and open-shelf to record temperature at 30-min intervals during the study period (Fig. 1c).
Rays were tagged in February and November 2008 near receiver 3 (Table 2). Gill and throw nets were used to catch rays in shallow (0.5 m) water. Each individual was then immobilized in a hand net, photographed, measured (cm disc width, DW) and externally tagged with a spaghetti tag (FD-94 Anchor tags, Floy Tag & Manufacturing) to avoid the possibility of double-tagging with acoustic tags. Species were identified based on Last and Stevens (2009) and tissue samples were taken from the right pelvic fin for genetic analyses to confirm initial identification of tagged species (Cerutti-Pereyra et al. 2012). Rays were then turned upside-down and an acoustic tag (VEMCO v13-1H transmitter; battery life approximately 16 months; transmitting interval: 120–240 s) was inserted in the abdominal cavity following the methods of Heupel et al. (2006). Prior to implantation, each tag was coated with paraffin to reduce any possibility of an immune response to the presence of a foreign body within the ray (Heupel et al. 2006). Measurement and tag insertion required between 5 and 10 min, after which individuals were released at the site of capture and monitored until recovery for 10 min. All of the 16 rays tagged were juveniles based on published sizes at maturity (Last and Stevens 2009). Rays were monitored within the array until May 2009.
Analysis
Site fidelity
Numbers and proportions of detections from each ray at each receiver were used to estimate site fidelity. The receiver that recorded the most detections was identified as ‘primary’ receivers for each individual (Garla et al. 2006b). The number of days each ray was recorded within the array was used to calculate the minimum percentage of days it was present at its primary receiver (Garla et al. 2006b).
Temporal patterns
Temporal patterns in numbers of detections at the primary receivers were analyzed in order to determine patterns of seasonal movement of rays. The number of monthly detections at the primary receivers was modeled as a function of month and species. Total detections per ray per month were used as the response variable rather than the proportion of detections per ray per month as the latter were not normally distributed and this problem was not improved by transformation. Temporal patterns in occurrence were the same for both total and proportional detections. A non-linear relationship between the number of detections and time (month) was hypothesized, therefore a quadratic term (the square of month) was also included in some models. Due to low sample sizes, data from Himantura uarnak (Forskål, 1775) and detections from the final month of the study (May 2009) were excluded. The number of monthly detections was log-transformed to normalize distributions of data. Month was coded as an integer from 1 (February 2008) – 15 (April 2009). A suite of generalized linear mixed-effect models were then constructed using all combinations of the explanatory variables where the random effect was the individual ray. Temporal autocorrelation using the acf function in R was tested for but none was found. All models were fitted in R (R Development Core Team 2011) using package lme4 (Bates and Sarkar 2007), and ranked them according to weights of Akaike’s information criterion corrected for small sample sizes (wAIC c ). The wAIC c varies from 0 (no support) to 1 (complete support) (Burnham and Anderson 2002).
Differences by sex
A linear mixed-effects model was also applied to examine whether the number of monthly detections varied between the sexes, again comparing model AIC c weights. These models were fitted using the R package nlme (Pinheiro et al. 2011). The corAR1 function was used to account for temporal autocorrelation. The weight of the slope model (number of monthly detections ~ sex + random effect) was divided by that of the intercept-only model (number of monthly detections ~ 1 + random effect) to provide an information-theoretic evidence ratio (Burnham and Anderson 2002). The random effect was the individual ray as above.
Temperature analysis
The mean, minimum, and maximum temperature per hour and month were calculated (Table 1). Hourly wind speed data was collected by the Australian Government Bureau of Meteorology (BOM) climate station at Learmonth, the closest monitoring station to the study site (approx. 30 km). The data was requested from the website: http://www.bom.gov.au/. The spatial distribution of diel detections and the relationship between the mean proportion of hourly detections and the mean hourly temperature were examined by comparing the slope model to the intercept only model.
Wind analysis
Average wind speed per day and month were plotted against proportion of detections to look for any correlations between wind speed and receiver detection efficiency. High winds creating noisy surface waves and breakers would be expected to reduce receiver efficiency and thus reduce the likelihood of tag detections, particularly in the shallow waters of the lagoon, while calm weather would have the opposite effect, reducing ambient noise from waves and increasing receiver efficiency and the number of tag detections (Payne et al. 2010).
Cyclical patterns
Fast Fourier Transformations (FFTs) with Hamming window smoothing were used in the package MATLAB (MATLAB 2010) to search for cyclical patterns in detections. FFTs reveal cycles in the data as frequency peaks in a power spectrum (Meyer et al. 2009; Papastamatiou et al. 2009). Detections were summed up in each hour of every day for all individual per species and the FFT searched for monthly, weekly, and hourly patterns.
Results
Site fidelity
A total of 71,293 detections (Table 1) were obtained from the 16 individual rays tagged (Table 2) in February and November 2008. The majority (94 %) of all detections for all rays were obtained from three receivers (nos. 3, 15 & 19, Fig. 1) (Table 1) located in the shallowest area of the array (< 2 m water depth) on muddy sediments close to the mangrove forest (Fig. 1, Table 1) and within the sanctuary zone (Fig. S2). These three receivers were considered primary sites, although 82 % of the receivers had at least one detection of a tagged ray at some point during the study. Pastinachus atrus (Macleay, 1993) (n = 6) was detected on receivers within the sanctuary zone for 93 % of the total monitoring days. Three individuals of this species were never detected on receivers outside the sanctuary zone. Glaucostegus typus (Bennett, 1830) (n = 5) were detected for 92 % of the total monitoring days inside the sanctuary zone, while Urogymnus asperrimus (Bloch & Schneider, 1801) (n = 4) was detected for 98 % of the total monitoring days inside the sanctuary zone. Almost 99 % of all detections of one tagged H. uarnak occurred on receivers within the sanctuary boundaries.
Temporal patterns and temperature analysis
Water temperature at Mangrove Bay from February 2008 to May 2009 ranged from 15.5 to 33.4 °C, varying over different seasons by almost 18 °C (Table 1). Temperatures in the bay peaked in February 2008 at 33.4 °C and reached a low of 15.5 °C in June-August, 2008.
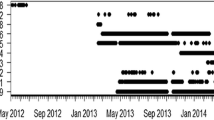
Modeling suggested that detections of tagged animals varied by month and species. Two plausible models described the number of detections per month: a model that had 50 % support and included month, its quadratic term, and species; and a model that had 49 % support and only included month and its quadratic term (Table 3). Detections increased from February 2008 until August and September of 2008 and then declined (Fig. 2a). Peak detections were similar for all species although P. atrus had the least detections. The highest number of detections (May-Aug, 2008) (Fig. 2a) coincided with the lowest wind speeds (Fig. 2c) and temperatures (Fig. 2d) of the year. Urogymnos asperrimus was detected for 7–15 months including both summer and winter months. Pastinachus atrus was detected in the array from 5 to 18 months after tagging also including winter and summer. The individual of H. uarnak was detected for 5 months and G. typus was detected from 2 to 15 months after tagging, the latter’s detections also included winter and summer months. The upper time limit for detections of these tags probably reflects the lifetime of the tag battery (around 16 months) (Fig. S3).
Generalized, linear mixed-effects modeling of the log-transformed number of detections vs species, month, and month squared (a), the number of log-transformed detections on the y-axis against month of study from February 2008 at Mangrove Bay. Fitted line for each species obtained by the top-ranked: dotted line is U. asperrimus, dashed line is G. typus (Note dotted and dashed lines are almost on top of one another) and solid line is P. atrus. Average hourly (b) and monthly (c) wind speeds (± se) at Ningaloo Reef. Average monthly temperature at the primary receivers, inside, and outside the lagoon in Mangrove Bay during the study period (mean ± se) (d)
Differences by sex
There was no evidence for a difference in monthly detections between male and female juvenile rays, with the intercept-only model (wAIC c = 0.60) having slightly more bias-corrected support than the slope model (wAIC c = 0.40)
Cyclical patterns
While the spectral analysis did not indicate weekly or monthly cycles of detections, there was a strong peak of detections for all species every 24 h and a secondary peak every 12 h (Fig. 3). Plots of hourly detections shows that these coincided with highest water temperatures (Fig. 4) and peaked between 15.00 and 19.00 h for P. atrus (Fig. 4b), U. asperrimus (Fig. 4d), and H. uarnak (Fig. 4e). Detections of G. typus peaked later around 23.00 h (Fig. 4c). Overall, there were more detections during the afternoon and night than the early morning and day. However, for all these species, around 80–90 % of detections at night occurred at only one or two receivers, while detections during the day were spread over more than two receivers. There was evidence for a positive relationship between the mean proportion of hourly detections and the mean hourly temperature (Fig. 4f) with the slope model (wAIC c = 1) having 100 % support over the intercept only model (wAIC c < 0.01). Both detections and water temperature were positively correlated with wind speed on an hourly basis (Fig. 4).
Detections and temperature at primary sites. Mean (± se) hourly temperatures at the primary receivers (3, 15 and 9) (a). The proportion of hourly detections (mean ± se) at all receivers for P. atrus (b), G. typus (c), U. asperrimus (d) and H. uarnak (n = 1) (e). The fitted line from the linear mixed-effects model of the relationship between mean proportion of hourly detections and mean hourly temperature (f)
Discussion
The use of nurseries appears to be a phenomenon common to many sharks and rays in coastal regions worldwide (Yokota and Lessa 2006; Heupel 2007; Speed et al. 2010). At Ningaloo Reef, juvenile rays were detected most frequently at three listening stations that were located in a small area of muddy habitat (approximately 1 km2) in shallow water (< 2 m depth) along the rim of a mangrove forest. Juveniles of P. atrus, H. uarnak, U. asperrimus and G. typus of both sexes were present here for many months over both years of the study. Evidence from a companion study that surveyed ray distributions across the reef and lagoon showed that juvenile rays were found only within this shallow bay (O’Shea et al. unpublished data). Additionally, males and females did not show differences in detections, likely because sexual segregation occurs with the onset of sexual maturity (e.g. Dale et al. 2011). These primary sites may, therefore, form part of a nursery as defined by Heupel et al. (2007).
The primary receivers at Mangrove Bay were at the entrance to a small embayment in the vicinity of mangroves that was accessible only during high tide. While this area might provide better opportunities for feeding, it was also more turbid than the waters of the reef nearby, which might offer some protection against predation (Holland et al. 1993). Vaudo and Heithaus (2009) described nurseries for juvenile P. atrus, H. uarnak and G. typus in shallow habitats in Shark Bay, 300 km to the south of Ningaloo Reef. They suggested that these functioned as predation refuges, reducing mortality of juveniles due to seasonally abundant predators such as tiger sharks, Galeocerdo cuvier (Peron & Lesueur, 1822). Surveys show that sharks, including blacktip, Carcharhinus melanopterus (Quoy & Gaimard, 1824), lemon, Negaprion acutidens (Rüppell, 1837) and tiger sharks, G. cuvier are abundant at Mangrove Bay (Stevens et al. 2009), suggesting that such embayment could offer some degree of protection against the threat of predation for relatively vulnerable juvenile rays in the lagoon habitat.
Juvenile rays were detected more frequently in the primary sites during the late autumn – winter, suggesting that they may range less widely at this time of the year. However, this result should be treated with caution given the small sample and that seasonal changes in wind strength and direction are likely to alter receiver range (Huveneers et al. 2009; Payne et al. 2010), particularly in shallow water where the sites were located. The greatest number of detections generally coincided with the months of reduced wind speed, however detections peaked in August, some months after the lowest wind speeds in April and May. This lag is difficult to reconcile if the increase in detections was solely due to the improved ability of receivers to detect tags under low wind conditions. Assuming that temporal patterns in detections were not confounded by wind speed (see below), there was little evidence that use of the primary sites during autumn was due to behavioural thermoregulation, as water temperatures were similar among sites outside the reef, within the lagoon and in the primary sites. Interestingly, this seasonal pattern contrasts with many studies of the use of nurseries by sharks, which tend to occupy these habitats in summer months when water temperatures are warmest (Speed et al. 2010). The use of nurseries during summer is thought to provide optimum temperatures to increase rates of growth and thus reduce the duration of vulnerable life-history stages (Heupel et al. 2007).
The restriction of detections of juvenile rays both males and females to two or three receivers in a small area of the array contrasts with movement patterns by adults of these species. Additional tagging of mature animals showed that they routinely moved over the entire area of the array and in some cases, more than 125 km south along the Ningaloo Reef in only a few weeks (Cerutti-Pereyra et al. unpublished data). This broad-scale movement suggests a requirement for greater habitat diversity of adults than juveniles (Rousset 1990; Heupel et al. 2004; Vaudo and Lowe 2006). Alternatively, or in addition, juveniles may be restricted to feeding on the softer, muddy sediments present within Mangrove Bay, while adults can excavate coarser and harder sediments, allowing them to roam more widely over reef habitats (Marshall et al. 2008).
The overlapping nature of the diel patterns of detections among species suggests that there is little evidence for habitat partitioning at this temporal scale, with all juveniles showing 12 and 24-h patterns in detections. For all species, the lowest number of detections occurred around dawn or the early morning and the greatest numbers around dusk and the early evening. The consistency of these patterns suggest that these cycles in detections were not related to tides, as is the case in several other elasmobranchs (Medved and Marshall 1983; Ackerman et al. 2000), since the timing of high and low tides shift progressively during the day throughout the lunar cycle. No evidence was found that these daily cycles in detections were an artifact of ambient noise produced by wind and waves. The noise from wave chop and breakers can reduce the receiver ranges and would have resulted in a strong negative correlation between detections and the peak in wind speed during the day (Rousset 1990). Instead, the number of detections increased with the increase of wind speed, so that the greatest number of detections occurred in the late afternoon and early evening when average wind speed was highest. This implies that any noise generated by wind and waves on the surface had only a minor influence on receiver ranges. Furthermore, given that the range in daily wind speed (12–20 km/h) was very similar to that occurring on a seasonal basis, this provides further support for the idea that seasonal patterns in detections were not simply a consequence of variation in receiver efficiency due to ambient noise.
Over a 24-h cycle, rays tended to be found in primary sites when water temperatures were warmer at night time and they ranged more widely in the cooler hours of the early morning and at dawn. This pattern conflicts with the hypothesis that elasmobranchs obtain bioenergetic advantages by feeding in warm waters during daytime and resting during cooler hours for digestion (Matern et al. 2000; Sims et al. 2006). Such behavioural thermoregulation has been observed in other benthic elasmobranchs, such as bat rays, Myliobatis californica (Gill, 1865) that feed during the warmest times of the day (Matern et al. 2000) and the Atlantic stingrays, Dasyatis sabina, (Lesueur, 1824). However, the primary sites inhabited by rays at Mangrove Bay are known to be a focal point for intense feeding. Recent work suggests that at least half the sediment in the shallow, intertidal areas of the bay is overturned by stingray feeding each year and that feeding in the embayment occurs at a far greater rate than in other areas of the lagoon (O’Shea et al. 2012). Thus, restricted movement in the late afternoon may not necessarily indicate periods of resting, as suggested by other studies (Sims et al. 2006; Di Santo and Bennett 2011; DiGirolamo et al. 2012).
The delineation of essential habitat and nurseries for rays in a tropical reef environment has important implications for management and conservation, although due to low sample sizes, these results must be treated with caution. It is not known what proportion of the adult population is served by the primary sites at Mangrove Bay, or from what spatial extent the habitat draws neonates and juveniles. Given that Ningaloo Reef stretches over more than 320 km of coastline, it is unlikely that it is the only potential nursery, even though equivalent habitats with fringing mangroves are rare along this coastline. It is thus fortunate that Mangrove Bay is designated as a protected area and is of a sufficient size to protect major habitat use of juveniles within this habitat.
References
Ache de Freitas RH, Rosa RS, Wetherbee BM, Gruber SH (2009) Population size and survivorship for juvenile lemon sharks (Negaprion brevirostris) on their nursery grounds at marine protected area in Brazil. Neotropical Ichthyol 7:205–212
Ackerman JT, Kondratieff MC, Matern SA, Cech JJ (2000) Tidal influence on spatial dynamics of leopard sharks, Triakis semifasciata in Tomales Bay, California. Environ Biol Fishes 58:33–43
Bates DM, Sarkar D (2007) lme4: Linear mixed-effects models using S4 classes, R package version 0.99875-6
Branstetter S (1990) Life style of sharks. In: Gruber SH (ed) One reason sharks are vulnerable to overfishing, a volume honoring the work of Stewart Springer. American Litoral Society Highlands, New Jersey, pp 23–29
Burnham KP, Anderson DR (2002) Model selection and multimodel Inference: a practical information-theoretic approach. Springer, New York
Castro JI (1993) The shark nursery of Bulls Bay, South Carolina, with a review of the shark nurseries of the southeastern coast of the United States. Environ Biol Fishes 38:37–48
Cerutti-Pereyra F, Meekan MG, Wei N-WV, O’Shea O, Bradshaw CJA, Austin CM (2012) Identification of Rays through DNA Barcoding: An Application for Ecologists. PLoS One 7:e36479
Chapman DD, Babcock EA, Gruber SH, Dibattista JD, Franks BR, Kessel SA, Guttridge T, Pikitch EK, Feldheim KA (2009) Long-term natal site-fidelity by immature lemon sharks (Negaprion brevirostris) at a subtropical island. Mol Ecol 18:3500–3507
Collins A, Heupel MR, Motta PJ (2007) Residence and movement patterns of cownose rays Rhinoptera bonasus within a south-west Florida estuary. J Fish Biol 71:1159–1178
Conrath CL (2008) Investigations into depth and temperature habitat utilization and overwintering grounds of juvenile sandbar sharks, Carcharhinus plumbeus: the importance of near shore North Carolina waters. Environ Biol Fishes 82:123–131
Dale JJ, Wallsgrove NJ, Popp BN, Holland K (2011) Nursery habitat use and foraging ecology of the brown stingray Dasyatis lata determined from stomach contents, bulk and amino acid stable isotopes. Mar Ecol Prog Ser 433:221–236
Dames L, Moore (2000) Ningaloo Marine Park (Commonwealth Waters) Literature review
DeAngelis BM (2008) First characterization of shark nursery habitat in the United States Virgin Islands: evidence of habitat partitioning by two shark species. Mar Ecol Prog Ser 358:257–271
Department of Conservation and Land Management (2005) Management plan for the Ningaloo Marine Park and Muiron Islands Marine Management Area 2005–2015. Management Plan no. 52. Department of Conservation and Land Management, Perth
Dewar H, Mous P, Domenier M, Muljadi A, Pet J, Whitty J (2008) Movements and site fidelity of the giant manta ray, Manta birostris, in the Komodo Marine Park, Indonesia. Mar Biol 151:121–133
Di Santo V, Bennett WA (2011) Is post-feeding thermotaxis advantageous in elasmobranch fishes? J Fish Biol 78:195–207
DiGirolamo AL, Gruber SH, Pomory C, Bennett WA (2012) Diel temperature patterns of juvenile lemon sharks Negaprion brevirostris, in a shallow-water nursery. J Fish Biol 80:1436–1448
Dulvy NK, Reynolds JD (2002) Predicting extinction vulnerability in skates. Predicción de la vulnerabilidad de extinción en rayas. Conserv Biol 16:440–450
Dulvy NK, Metcalfe JD, Glanville J, Pawson MG, Reynolds JD (2000) Fishery stability, local extinctions, and shifts in community structure in skates. Estabilidad de la pesquería, extinciones locales y cambios en la estructura de comunidades de rayas. Conserv Biol 14:283–293
Economakis AE, Lobel PS (1998) Aggregation behavior of the gray reef shark, Carcharhinus amblyrhynchos, at Johnston Atoll, central Pacific Ocean. Environ Biol Fishes 51:129–139
Fangue NA, Bennett WA, Douglas ME (2003) Thermal tolerance responses of laboratory-acclimated and seasonally acclimatized Atlantic stingray, Dasyatis sabina. Copeia 2003:315–325
Frisk MG (2010) Life history strategies of Batoids. In: Carrier JC, Musick JA, Heithaus MR (eds) The Biology of Sharks and their Relatives. CRC Press, Boca Raton, pp 283–316
Garla RC, Chapman DD, Shivji MS, Wetherbee BM, Amorim AF (2006a) Habitat of juvenile Caribbean reef sharks, Carcharhinus perezi, at two oceanic insular marine protected areas in the southwestern Atlantic Ocean: Fernando de Noronha Archipelago and Atol das Rocas, Brazil. Fish Res 81:236–241
Garla RC, Chapman DD, Wetherbee BM, Shivji M (2006b) Movement patterns of young Caribbean reef sharks, Carcharhinus perezii, at Fernando de Noronha Archipelago, Brazil: the potential of marine protected areas for conservation of a nursery ground. Mar Biol 149:189–199
Grubbs RD, Musick JA, Conrath CL, Romine JG (2005) Long-term movements, migration and temporal delineation of a summer nursery for juvenile sandbar sharks in the Chesapeake Bay Region. American Fisheries Society Symposium:1–22
Heupel M (2007) Exiting Terra Ceia Bay: an examination of cues stimulating migration from a summer nursery area. Am Fish Soc Symp 50:265–280
Heupel M, Heuter R (2001) Use of a remote acoustic telemetry system to monitor shark movements in a coastal nursery area. In: Sibert JR, Nielsen JL (eds) Electronic Tagging and Tracking in Marine Fisheries. Kluwer Academic Publishers, Amsterdam, pp 217–223
Heupel M, Simpfendorfer CA, Hueter RE (2004) Estimation of shark home ranges using passive monitoring techniques. Environ Biol Fishes 71:135–142
Heupel M, Simpfendorfer CA, Collins AB, Tyminski JP (2006) Residency and movement patterns of bonnethead sharks, Sphyrna tiburo, in a large Florida estuary. Environ Biol Fishes 76:47–67
Heupel MR, Carslon JK, Simpfendorfer C (2007) Shark nursery areas: concepts, definition, characterization and assumptions. Mar Ecol Prog Ser 337:287–297
Hight BV, Lowe CG (2007) Elevated body temperatures of adult female leopard sharks, Triakis semifasciata, while aggregating in shallow nearshore embayments: Evidence for behavioral thermoregulation? J Exp Mar Biol Ecol 352:114–128
Holland KN, Wheatherbee BM, Peterson JD, Lowe CG (1993) Movements and distribution of hammerhead shark pups on their natal ground. Copeia 1993:495–502
Hopkins TE, Cech JJ Jr (2003) The influence of environmental variables on the distribution and abundance of three elasmobranchs in Tomales Bay. Calif Environ Biol Fish 66:279–291
Huveneers C, Harcourt R, Boomer A, Hobday AJ, Pederson H, Semmens JM, Stieglitz T, Vallee R, Webber D, Heupel M, Simpfendorfer C, Peddemors V (2009) The influence of environmental parameters (meteorological and oceanographic) on the performance of acoustic receivers. Papers from 2nd AATAMS Workshop AATAMS Workshop, Mosman, NSW, Australia
Kinney MJ, Simpfendorfer CA (2009) Reassessing the value of nursery areas to shark conservation and management. Conserv Lett 2:53–60
Klimley AP, Kihslinger RL, Kelly JT (2005) Directional and non-directional Movements of bat rays, Myliobatis californica, in Tomales Bay, California. Environ Biol Fishes 74:79–88
Last P, Stevens J (2009) Sharks and Rays of Australia, 2nd edn. CSIRO, Collingwood
Marshall AD, Kyne PM, Bennett MB (2008) Comparing the diet of two sympatric urolophid elasmobranchs (Trygonoptera testacea Muller & Henle and Urolophus kapalensis Yearsley & Last): evidence of ontogenetic shifts and possible resource partitioning. J Fish Biol 72:883–898
Matern SA, Cech JJ, Hopkins TE (2000) Diel movements of bat rays, Myliobatis californica, in Tomales Bay, California: evidence for behavioral thermoregulation? Environ Biol Fishes 58:173–182
MATLAB (2010) MathWorks, Natick, Massachusetts, USA, http://www.mathworks.com/products/matlab/
Medved RJ, Marshall JA (1983) Short-term movements of young sandbar sharks Carcharhinus plumbeus (Pisces, Carcharhinidae). Bull Mar Sci 33:87–93
Meyer CG, Clark TB, Papastamatiou YP, Whitey NM, Holland KN (2009) Long-term movement patterns of tiger sharks Galeocerdo cuvier in Hawaii. Mar Ecol Prog Ser 381:223–235
Morrisey JF, Gruber SH (1993) Habitat selection by juvenile lemon sharks, Negaprion brevirostris. Environ Biol Fishes 38:311–319
O’Shea OR, Thums M, van Keulen M, Meekan M (2012) Bioturbation by stingrays at Ningaloo Reef, Western Australia. Mar Freshw Res 63:189–197
Papastamatiou YP, Lowe CG, Caselle JE, Friedlander AM (2009) Scale-dependent effects of habitat on movements and path structure of reef sharks at a predator-dominated atoll. Ecol Soc 90:996–1008
Payne NL, Gillanders BM, Webber DM, Semmens JM (2010) Interpreting diel activity patterns from acoustic telemetry: the need for controls. Mar Ecol Prog Ser 419:295–301
Pinheiro J, Bates D, DebRoy S, Sarkar D, the R Development Core Team (2011) nlme: linear and nonlinear mixed effects models. R package version 3.1-101.
R Development Core Team (2011) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Romanov EV (2001) Bycatch in the tuna purse-seine fishery in the western Indian Ocean. Fish Bull 100:90
Rousset J (1990) Population structure of thornback rays Raja clavata and their movements in the Bay of Douarnenez. J Mar Biol Assoc UK 70:261–268
Simpfendorfer CA, Heupel M (2004) Assessing habitat use and movement. In: Carrier JC, Musick JA, Heithaus MR (eds) Biology of Sharks and their Relatives. CRC Press, Boca Raton, pp 533–571
Sims DW, Wearmouth VJ, Southall EJ, Hill JM, Moore P, Rawlinson K, Hutchinson N, Budd GC, Righton D, Metcalfe JD, Nash JP, Morritt D (2006) Hunt warm, rest cool: bioenergetic strategy underlying diel vertical migration of a benthic shark. J Anim Ecol 75:176–190
Speed CW, Field I, Meekan MG, Bradshaw CJA (2010) Complexities of coastal shark movements and their implications for management. Mar Ecol Prog Ser 408:275–293
Springer S (1960) Natural history of the sandbar shark, Eulamia milberti. Fish Bull 61:1–38
Stevens J (2002) A review of Australian elasmobranch fisheries. Pages 122–126 in Elasmobranch, biodiversity, conservation and management: Proceedings of the international seminar and workshop. IUCN SSC Shark Specialist Group. IUCN, Gland and Cambridge, Sabah, Malayisia, July 1997
Stevens J, Last PR, White WT, McAuley RB, Meekan MG (2009) Diversity, abundance and habitat utilization of sharks and rays. Final report to Western Australian Marine Science Institute. CSIRO
Vaudo J, Heithaus M (2009) Spatiotemporal variability in a sandflat elasmobranch fauna in Shark Bay, Australia. Mar Biol 156:2579–2590
Vaudo JJ, Lowe CG (2006) Movement patterns of the round stingray Urobatis halleri (Cooper) near a thermal outfall. J Fish Biol 68:1756–1766
Ward RD, Hanner R, Hebert PDN (2009) The campaign to DNA barcode all fishes, FISH-BOL. J Fish Biol 74:329–356
Yeiser BG, Heupel MR, Simpfendorfer CA (2008) Occurrence, home range and movement patterns of juvenile bullshark (Carcharhinus leucas) and lemon (Negaprion brevirostris) sharks within a Florida estuary. Mar Freshw Res 59:489–501
Yokota L, Lessa R (2006) A nursery area for sharks and rays in northeastern Brazil. Environ Biol Fishes 75:349–360
Acknowledgments
This research was approved by the Animal Ethics Committee from Charles Darwin University (no. A07026). We thank to the Australian Institute of Marine Science (AIMS) and CSIRO for financially supporting this project, and CONACYT-Mexico for international grant. We thank the tagging and receiver-deployment crews, P. Last for species identification, Y. Berger for analytical assistance, D. Lindsay for comments on the manuscript, A. Tan and D. McGaffin for tagging and sampling equipment manufacture, and C. Speed, F. McGregor, S. Baccarella, K. Wenziker, P. Haskell, Exmouth Light Engineering and Ningaloo Dreaming for field assistance.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Mangrove Bay array range testing. Proportion of detections received by VR2 receivers at increasing distances from the test transmitter in the lagoon channel (filled circles, 4 m water depth) and at Mangrove Bay (white circles, 1 m water depth) (DOC 446 kb)
Fig. S2
Proportion of days spent by each species inside and outside of the Mangrove Bay sanctuary zone (DOC 190 kb)
Fig. S3
Number of daily detections per ray at Mangrove Bay. Individual plots of detections of juvenile rays primary receives (solid shapes) and other receivers (hollow shapes). (DOC 725 kb)
Rights and permissions
About this article
Cite this article
Cerutti-Pereyra, F., Thums, M., Austin, C.M. et al. Restricted movements of juvenile rays in the lagoon of Ningaloo Reef, Western Australia – evidence for the existence of a nursery. Environ Biol Fish 97, 371–383 (2014). https://doi.org/10.1007/s10641-013-0158-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-013-0158-y