Abstract
The spotted eagle ray, Aetobatus narinari, is listed on the IUCN Red List of Threatened Species as Near Threatened with a decreasing population trend, but many aspects of this ray’s biology and population status are unknown. Aerial and on-water surveys were conducted in the eastern Gulf of Mexico off southwest Florida 2008–2013, to document seasonal occurrence and life history characteristics of this species. Aerial surveys documented spotted eagle rays mostly in spring, summer, and autumn months with larger aggregations observed near inlet passes. Boat-based surveys documented rays on 152 out of 176 survey days, mostly as solitary individuals but sometimes in aggregations of up to 60. More rays were observed when water temperatures were 23-31 ºC. A total of 393 rays (231 males, 161 females, 1 unrecorded sex) were captured, measured, sampled, tagged, and released. Sizes ranged 41.4–203.0 cm disc width (DW) and weight 1.1–105.5 kg. Male size at 50 % maturity was 127 cm DW. Five percent (19) of tagged rays were recaptured after 5–1,293 days at liberty and recaptured rays exhibited faster growth than previously estimated from vertebral readings. Based on observations of rays relative to survey effort, numbers of observed rays declined after 2009 for reasons not yet understood. This observation, together with concerns about sustainability of fisheries targeting these rays in nearby Mexico and Cuba, underscore the need for investigations into stock structure, population trends, growth, and critical habitat of spotted eagle rays throughout the Gulf of Mexico, Caribbean Sea, and elsewhere in their range.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The spotted eagle ray is a large marine batoid found circumglobally in tropical and warm-temperate waters (Bigelow and Schroeder 1953; Last and Stevens 1994). Recent genetic evidence indicates these rays may comprise at least two separate species: Aetobatus narinari (Euphrasen, 1790) found in the Western Atlantic and Eastern Pacific, and Aetobatus ocellatus (Kuhl, 1823) found in the Western and Central Pacific (Richards et al. 2009; White et al. 2010). Further evidence using unique mitochondrial NADH2 gene sequences has distinguished seven Aetobatus species worldwide (Naylor et al. 2012). In Southeast Asia and some portions of the Gulf of Mexico and Caribbean Sea, Aetobatus spp. are targeted in fisheries or caught as bycatch (Trent et al. 1997; Dubick 2000; Stevens et al. 2000; White and Dharmadi 2007; Schluessel et al. 2010; Cuevas-Zimbrón et al. 2011; Tagliafico et al. 2012). The genetics of these rays and their potential vulnerability to localized fishing pressure call for focused, regional studies to better understand their life history and population status (Sellas et al. 2011).
Life history information on spotted eagle rays is limited, derived mainly from dead specimens obtained from fisheries (Dubick 2000; Schluessel 2008; Schluessel et al. 2010; Cuevas-Zimbrón et al. 2011; Tagliafico et al. 2012). Aetobatus spp. are live bearers with low fecundity, giving birth to 1–4 pups each year (Bigelow and Schroeder 1953; Kyne et al. 2006). These rays may take several years to reach sexual maturity and possibly live as long as 25 years or more (Dubick 2000; Schluessel et al. 2010), traits that make them susceptible to stock depletion where they are taken in targeted fisheries or as bycatch. The IUCN (International Union for Conservation of Nature) Red List of Threatened Species categorizes the spotted eagle ray as ‘Near Threatened’ with a decreasing population trend in most of its range and ‘Vulnerable’ in the Southeast Asia region, primarily due to local fisheries impacts (Kyne et al. 2006; IUCN 2013). In Florida U.S.A. it is unlawful to harvest, possess, land, purchase, sell, or exchange spotted eagle rays in state waters (FFWCC 2013), primarily due to concerns about vulnerability of the species if harvested for bait. In other U.S. states there currently are no regulations limiting the catch of spotted eagle rays.
No published field studies exist on spotted eagle ray population status, life history, or behavior in U.S. coastal waters. The few observations and field studies of free-swimming spotted eagle rays come from the Marshall Islands (Tricas 1980), Bahamas (Corcoran and Gruber 1999; Silliman and Gruber 1999), and Bermuda (Ajemian et al. 2012). In the Bahamas, spotted eagle ray movements along sandflats coincide with the tidal cycle (Silliman and Gruber 1999). Researchers in the Bahamas used spot patterns on the rays’ dorsal surface to reliably and repeatedly identify 157 individuals over eight months and found rays aggregate in mixed sex and size groups (Corcoran and Gruber 1999). In Bermuda, acoustically tagged rays select for areas highest in calico clam density and prefer waters less than 10 m deep (Ajemian et al. 2012).
To increase our understanding of spotted eagle ray biology in the wild and address questions about the life history and status of this species for resource management, we initiated a multi-year study of A. narinari in the eastern Gulf of Mexico. Our objectives were to reveal trends in distribution and seasonal occurrence of spotted eagle rays, using a combination of aerial and on-water surveys. Furthermore, we sought information on sex-specific trends in life history characteristics of size, growth, age, and maturity using capture, tag, and release of live rays in their natural environment. Our goal was to establish baseline data on the spotted eagle ray, capitalizing on our accessibility to this species along the southwest Florida coast, and thus provide critical information for the conservation of this species throughout its range.
Methods
Study location
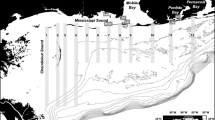
Spotted eagle rays were observed in the eastern Gulf of Mexico through aerial and boat-based surveys along the southwest Florida coast from May 2008 through June 2013 (Fig. 1). This area consists mostly of fringing barrier islands and passes and inlets (200–2,500 m wide) connected to the estuaries of Tampa Bay, Sarasota Bay, and Charlotte Harbor/Pine Island Sound. The coastal habitat along the barrier islands consists of mostly sand/shell bottom ranging 1–7 m in depth out to 1 km offshore. Most passes and inlets are relatively shallow but can range to 20 m deep and often have large, shallow shoals on the Gulf side composed of coarse sand/shell bottom. Estuarine bay-side habitat contains large beds of seagrass that includes turtle grass (Thalassia testudinum), manatee grass (Syringodium filiforme), shoal grass (Halodule wrightii), widgeon grass (Ruppia maritime), and star grass (Halophila engelmannii) with finer sediment on the bottom (Culter and Leverone 1993). Common invertebrates that are potential prey for spotted eagle rays in this habitat include macro-gastropods such as whelks and conch, and bivalves such as scallops and clams (Abbott 1974; Felder and Camp 2009; Schluessel et al. 2010; Ajemian et al. 2012).
Aerial surveys
Aerial surveys (n = 37) were opportunistically conducted to document the presence of spotted eagle rays and other large marine vertebrates in the study area between May 2008 and December 2012. These surveys covered approximately 180 km of coastline, following approximately the same flight path from John’s Pass to the north (latitude 27.8°N, longitude −82.8°W) to San Carlos Pass east of Sanibel Island to the south (latitude 26.5°N, longitude −82.0°W) (Fig. 1a). Planes used for the surveys were single-engine high-wing aircraft with two or three trained observers and the pilot. Flights were conducted once per month, with up to nine surveys per year, and were flown from 09:30–12:00 for optimal viewing conditions. Observations were made from an altitude of 120–250 m. Surveys ran north to south approximately 300–400 m seaward of the coastline. In addition to the along-shore flight pattern the pilot circled the area around each pass or major inlet. Observers identified spotted eagle rays by their distinctive diamond shape, protruding rostrum, and grayish-black coloration. Each sighting location was recorded onto a mapping GPS (Garmin 276C) and later downloaded and plotted in ArcGIS 10.0 (ESRI Inc.). Water temperature data were collected from local weather buoys near Sarasota Bay and plotted against spotted eagle ray count data for each flight to estimate a spotted eagle ray abundance-temperature relationship. We estimated the spotted eagle ray-temperature relationship using zero-inflated Poisson (ZIP) regression as it produces less-biased parameter estimates for count data with many zero observations (Martin et al. 2005). Because approximately the same flight path was taken, detection probabilities of spotted eagle rays were assumed to be equal across all flights. The Vuong test (Vuong 1989) was used to compare the ZIP model with an ordinary Poisson regression model to test for model selection.
Boat-based surveys
On-water surveys (n = 138) of spotted eagle rays were conducted between July 2009 and June 2013, primarily April through November each year. Sampling occurred within a concentrated portion of the aerial survey area between north Longboat Key (latitude 27.4°N, longitude −82.7°W) and south Siesta Key (latitude 27.2°N, longitude −82.5°W) (Fig. 1b). Surveys were opportunistic and targeted regions where spotted eagle rays could be feasibly captured. The vessel was a modified 8.5 m low-profile, mid-engine boat outfitted with a live well (1.8 m × 2.4 m and 0.8 m deep) constructed to hold spotted eagle rays. Surveys were conducted between 09:00 and 16:00 to optimize sun angle, sea state, and water visibility. Two trained observers stood atop a 2 m tower and scanned 180° arcs from starboard to port, searching for spotted eagle rays. Surveys covered the bounds of the entire boat-based study area within each survey day when conditions allowed. All ray sightings and survey tracks were recorded onto a Garmin 76× GPS (beginning in September 2009) and later downloaded into ArcGIS 10.0 (ESRI 2011). The size of free-swimming rays (in cm disc width [DW]) was estimated by the observers and classified into two categories: pups (≤80 cm DW) and juveniles/adults (>80 cm DW). Catch-per-unit-effort (CPUE) of ray sightings was calculated for each month in which boat-based surveys occurred. Because survey effort varied by month, “catch” was the total number of spotted eagle rays sighted in a month and “effort” was the total number of kilometers of coastal habitat surveyed that same month under good to excellent visibility.
Animal captures, tagging and measurement
Subsets of spotted eagle rays were captured for life history and tagging studies. When rays were encountered in workable conditions of depth and current, a nylon seine net (500 m × 4 m) was deployed for medium to large rays or a 2.4 m cast net was thrown to capture smaller rays (typically pups). The seine was set rapidly in an approximately 50 m-diameter circle around the rays. One or two snorkelers then captured each ray with a scoop net and transferred it to the boat’s live well. Very large rays (>180 cm DW) were examined in a floating net pen (2.5 m diameter) off the side of the boat. This pen also was used to hold specimens temporarily when more than one ray was caught in a single set. A handheld dissolved oxygen meter (YSI Model Pro 2030) was used to measure salinity, water temperature, and dissolved oxygen at the set location.
Once a ray was restrained, a series of measurements and samples were taken. Males were identified by the presence of claspers, which were classified as non-calcified (soft and flexible claspers, immature), partially calcified (harder but partially flexible claspers, maturing), or fully calcified (large and rigid claspers, mature). DW and total length (TL) were measured to the nearest mm. Each ray then was placed temporarily on a floating mat and photographed to record spot patterns and any notable scars from shark bites, remoras, and human interactions such as hook and line entanglement or boat strikes. Each ray was scanned with a Biomark (model 601) passive integrated transponder (PIT) tag scanner to detect the presence of a previously applied tag. If no tag was found the ray was injected with a 22.0 mm HDX PIT tag in the central upper portion of the right pectoral fin. Rays <80 cm DW received a smaller, 12.0 mm HDX PIT tag. Beginning in 2011, individuals also were tagged with a uniquely numbered 10 cm nylon-headed dart tag (Hallprint Pty, Ltd., South Australia, Australia) in the central lower section of the left pectoral fin, with the exception of extremely small pups (<60 cm DW) deemed too small for dart tagging. Prior to release, each animal was weighed to the nearest 0.1 kg using a hoop net attached to a calibrated digital scale on a davit.
Sex/size composition and morphometrics
Chi-square test statistics (\( \chi \) 2) and a Monte Carlo significance test procedure (Hope 1968) were used to test for differences in counts by sex per month for each year from 2010–2012 and combined years. Data from 2009 and 2013 were not used due to incomplete monthly coverage. Welch’s t-test (Welch 1947) was used to determine mean differences between sexes for DW, TL, and weight.
A linear regression was fit to describe the TL-to-DW (both in cm) relationship for males and females separately, where a is the slope and b is the intercept, and the coefficient of determination (R-square statistic; see Sokal and Rohlf 1998) was calculated to examine goodness-of-fit, as follows:
Linear models fitted to male and female data separately were tested for homogeneity of variance and the slope and intercept of each model were then compared with an analysis of covariance (ANCOVA; see Wildt and Ahtola 1978) to determine differences between the sexes.
The weight-length equation below (Keys 1928) was used to predict weight (WT, kg) at DW (cm):
where a is the scaling coefficient and b the shape parameter. a and b were estimated by fitting the model to the available weight-length data (sexes combined) using non-linear regression analysis and a 95 % confidence interval was estimated for each parameter through bootstrapping (Efron and Tibshirani 1986). The coefficient a also was estimated with b held fixed at 3. The Akaike’s (1973) Information Criterion (AIC) was then used to evaluate whether the fit was significantly improved by estimating the additional parameter b. Linear regressions obtained after logarithmic transformation of weight and length data were then applied to male and female data separately and the slope and intercept of each regression were compared by means of an ANCOVA (see Wildt and Ahtola 1978).
Sexual maturity (males)
A logistic regression was used to predict the probability of maturity, where rays with non-calcified and partially calcified claspers were considered immature:
M is the proportion of mature males, a is the slope of M as a function of DW and b is the intercept. DW at 50 % maturity (DW50) was calculated by setting proportion of mature males to 50 % and solving for DW, thus DW50 = −b/a. The confidence interval at 95 % for DW50 was estimated by bootstrapping (Efron and Tibshirani 1986).
Recaptures, age and growth
Recaptures of individuals in our boat-based surveys were verified using three methods: PIT tag detection, dart tag re-sighting and/or photo-identification. PIT and dart tag recaptures were recorded in the field at time of capture. In the laboratory, photos of the dorsal surface of each individual’s head were organized into a catalog and photo-ID was conducted by trained staff. This was done to visually confirm recaptures identified in the field by matching spot patterns and to potentially match any recaptured individuals that were not identified in the field due to tag loss. We also used the Interactive Individual Identification System (I3S) spot-recognition software as another visual method to confirm recaptures (den Hartog and Reijns 2007).
We used the method of Fabens (1965) to fit von Bertalanffy (1938) growth curves to males and females separately as well as both sexes combined:
where K and L ∞ are the standard von Bertalanffy parameters, L t and L t+∆t are the lengths at release and recapture, respectively, and ∆t is the time spent at liberty prior to recapture. We bootstrapped the data to obtain estimates of the bias and standard error. These recapture data were applied to growth models estimated by Dubick (2000) and Schluessel (2008) to compare our estimates of growth to published values. Both Schluessel and Dubick estimated growth from the readings of vertebral rings. Schluessel estimated Gompertz (1832) growth parameters for males (n = 55) and females (n = 56) sampled from the waters of Australia and Taiwan, and Dubick estimated von Bertalanffy growth parameters for males (n = 38) and females (n = 84) sampled in fisheries in southwest Puerto Rico. The relative age of each animal at the time of tagging (A t ) was estimated from DW at tagging (L t ) by inverting the von Bertalanffy (5.1) and Gompertz (5.2) growth equations:
The age at recapture was calculated as the age at tagging plus the time-at-liberty. Vectors of growth then were fitted to the Schluessel and Dubick models to judge the goodness-of-fit of the predicted growth from each model to the observed growth from our tagging data.
Results
Seasonal occurrence and sightings – aerial surveys
We documented 476 separate observations of a total of 1,140 spotted eagle rays. Observations were grouped into solitary individuals (61.3 %), pairs (19.7 %), small groups (3–10) (15.3 %), and large groups (11+) (3.6 %). Solitary rays were observed throughout the aerial survey area but pairing was relatively common and occasionally groups of 10 or more were seen, generally around passes and inlets. The largest group consisted of 76 individuals and was within 2 km of Redfish Pass (latitude 26.36°N, longitude −82.13°W). The numbers of rays observed per flight were greater in 2008–2009 compared to 2010–2012 in most months surveyed (Fig. 2). Rays were more prevalent in warmer months, generally between March and November (Fig. 2), when water temperature was 23 °C or above (Fig. 3). According to the Vuong test, the ZIP model was superior to the ordinary Poisson regression model (p = 0.044); thus, a spotted eagle ray abundance-temperature relationship was tested for using the ZIP model. The ZIP regression analysis showed an increasing trend in numbers of spotted eagle rays with increasing temperature (incidence rate ratio [IRR] 1.03; 95 % CI 1.02–1.05; p = 0.000128) (Fig. 3). Following the winters of 2009–2010 and 2010–2011, we observed fewer spotted eagle rays in the spring and summer months (Fig. 2). In February and March 2010, spotted eagle rays were observed inside Tampa Bay in a warm water plume from an electric power plant, a phenomenon not seen in other years of the aerial surveys.
Scatter plot of the number of spotted eagle rays observed during 2008–2012 monthly aerial surveys compared to recorded average monthly water temperatures (°C). Zero-inflated Poisson (ZIP) regression analysis suggests a significant increasing trend in spotted eagle ray abundance with increasing water temperature (incidence rate ratio [IRR] 1.03; 95 % CI 1.02–1.05; p = 0.000128)
Seasonal occurrence and sightings – boat-based surveys
In the on-water surveys, spotted eagle rays were observed within the Sarasota study area on 152 out of 176 (86 %) survey days (9,713.7 km total search effort) between 2009 and 2013. Rays were predominantly seen spring to autumn (April-November). The majority of boat-based spotted eagle ray sightings (84.1 %) were of solitary individuals while 15.1 % of sightings were groups of 2–10. Large aggregations (>11 individuals) of up to 60 individuals were observed in three instances (<1 % of all sightings) between the months of May and July. Annual juvenile/adult CPUE from boat-based surveys peaked in 2010 at 0.116 rays/km and pup CPUE peaked in 2009 at 0.040 rays/km (Table 1). Across-year patterns in juvenile/adult CPUE by month were either stable or declined. An overall across-year decline in juvenile/adult CPUE was observed (Fig. 4a). No apparent pattern was observed in pup CPUE (Fig. 4b). Juvenile/adult CPUE was generally highest in spring (April-June) and pup CPUE was highest in autumn (October-November) (Fig. 4).
Line plots of monthly “catch”-per-unit-effort (CPUE) of spotted eagle rays in boat-based surveys of the Sarasota study area. CPUEs were calculated as the monthly totals of observed spotted eagle rays divided by the total numbers of kilometers of coastal habitat surveyed during those months. Separate calculations were made for (a) juvenile/adult and (b) pup sightings
Sex/size composition and morphometrics
A total of 393 individual rays (231 males, 161 females, 1 unrecorded sex) were captured between July 2009 and June 2013 (Fig. 5). Average DW of all captured individuals for each month was consistent with size estimates of rays observed but not captured; larger individuals dominated the catch in April-September and pups dominated the catch during October and November (Fig. 6). Two pups were caught in January 2012 during an exceptionally mild winter. Males were more frequently caught April through July, and October through November, while slightly more females were captured during August and September. Sex composition of A. narinari was not significantly different by month except in 2011 (2010: Chi-square test, \( \chi \) 2 = 11.48, p = 0.075; 2011: \( \chi \) 2 = 17.17, p = 0.006; 2012: \( \chi \) 2 = 9.12, p = 0.157; combined years: \( \chi \) 2 = 8.36, p = 0.203). Overall, more males (2009: n = 52; 2010: n = 96; 2011: n = 59; 2012: n = 34) were captured than females (n = 46, 67, 37, 24, respectively) for a sex ratio of 1:0.7 M:F.
Although female maximum size (203.0 cm DW) and weight (105.5 kg) exceeded those of males (185.0 cm DW, 94.4 kg) (Figs. 6–8), there were no significant differences between average DW, TL, and WT of males vs. females (DW: t-test, t = 1.06, df = 344.3, p = 0.288; TL: t = 0.87, df = 347.8, p = 0.387; WT: t = 0.60, df = 267.3, p = 0.552). Of 230 males and 161 females measured, male DW ranged 42.0–185.0 cm (117.5 ± 37.0 cm) while females ranged 41.4–203.0 cm (121.8 ± 39.9 cm). Male TL ranged 29.0–138.4 cm (84.8 ± 26.5 cm), while females ranged 30.6–143.0 cm (87.3 ± 28.4 cm). A total of 83 pups were caught (49 males and 34 females) and ranged 41.0–73.2 cm DW (Fig. 5). Results from the ANCOVA demonstrated no difference between male and female TL-DW relationships (slope: F = 2.026, df = 1, 410, p = 0.155; intercept: F = 0.081, df = 1,411, p = 0.776) and the following strong linear relationship was found between TL and DW for the combined sexes (R2 = 0.98; Fig. 7) with 95 % confidence intervals for slope (0.700, 0.722) and intercept (−0.741, 1.93):
Male WT ranged 1.1–94.4 kg (32.7 ± 22.3 kg) and females ranged 1.3–105.5 kg (34.4 ± 25.3 kg). The following weight-length relationship was estimated for both sexes combined (Fig. 8):
with 95 % confidence intervals 2.684 × 10−05 − 2.838 × 10−05 and 2.870–2.873 for a and b, respectively. AIC values indicated the allometric model (AIC = 2086) was a slightly better fit to the data than the isometric model (AIC = 2089). Results from the ANCOVA suggest there was no significant difference in the slopes (F = 0.001, df = 1, 331, p = 0.992) and the y-intercepts (F = 0.001, df = 1, 332, p = 0.974) of the two regression lines between sexes.
Sexual maturity (males)
Of 151 males examined, 52 (34.4 %) had non-calcified claspers (immature), 15 (9.9 %) had partially calcified claspers (maturing), and 84 (55.6 %) had fully calcified claspers (mature) (Fig. 9). Male DW50 was determined to be 127.1 cm (Fig. 9) calculated using the following equation:
Proportion of mature male spotted eagle rays as a function of disc width based on clasper calcification. Red and light blue diamonds represent immature males and dark blue diamonds are mature males; black triangles are the proportion of mature males in each 20 cm disc width bin. DW50 equals 127 cm (dashed line) and the 95 % confidence interval for DW50 based on a bootstrapping method is shown (gray box)
The confidence interval (95 %) for DW50 ranged 122.9–131.6 cm. The three stages of male maturity all had a wide range for DW. Immature males (with non-calcified or partially calcified claspers) ranged 45–151 cm DW while mature males ranged 105–185 cm DW.
Recaptures, age and growth
A total of 19 individuals (13 males, 6 females) were recaptured between 5 days and 3.5 years after initial capture and release (Table 2). Three of these individuals (all males) were recaptured twice (SER 007, SER 042, and SER 143). Spot patterns on these recaptured animals remained relatively stable over time with only subtle changes noted, validating the consistency of photo-ID techniques for visual identification of individuals.
Estimated von Bertalanffy growth parameters and results from the bootstrapping analysis are presented in Table 3. Our relatively low sample size produced uncertainty in the results (Fig. 10, Table 3). In comparing growth observed in our recaptures to Dubick’s von Bertalanffy growth curves for A. narinari, we found the male and female models to be a poor fit to our data (Fig. 11a, b). Schluessel’s models were equally unsatisfactory (Fig. 11c, d). The recapture ends of the growth vectors consistently fell above the fitted von Bertalanffy curve; the rates of growth described by the recaptures were underestimated by all four models (Fig. 11).
Growth trajectories of recaptured spotted eagle rays (solid lines [males], dashed lines [females]) overlain on theoretical growth curves (dotted lines) estimated by Dubick (2000) and Schluessel (2008). Dubick estimated a von Bertalanffy growth model for males (a) and females (b), while Schluessel estimated a Gompertz growth model for males (c) and females (d)
Discussion
Seasonal occurrence, trends in sightings and risk factors
Our survey data strongly indicate seasonal changes in inshore sightings of spotted eagle rays along the southwest Florida coast, with lower numbers in winter compared to spring, summer, and autumn. As with other elasmobranch species and migratory marine species in general, A. narinari in the eastern Gulf of Mexico likely has a preferred temperature range and undertakes seasonal movements as coastal waters cool in winter. This is consistent with findings of other researchers working on spotted eagle rays (Dubick 2000; Schluessel et al. 2010; Tagliafico et al. 2012) and other myliobatids, such as the longheaded eagle ray A. flagellum (Yamaguchi et al. 2005) and bat ray Myliobatis californica (Martin and Cailliet 1988; Gray et al. 1997; Matern et al. 2000), in other locations.
Off the southwest Florida coast, year-round underwater surveys have documented spotted eagle rays on offshore reefs May through October (A. Collins, Florida Fish & Wildlife Research Institute, unpubl. data). Along the northern Gulf and Texas coasts, spotted eagle rays are seen mainly in summer months (Hoese and Moore 1977; Parker and Bailey 1979; Shepard and Myers 2005; M. Ajemian, pers. comm.). During winter, when coastal water temperatures in the northern and eastern Gulf can drop to 13 °C, spotted eagle rays may migrate to warmer sites offshore or coastal areas to the south. Dive operators in the Florida Keys confirm the presence of spotted eagle rays on nearshore reefs during the winter (Elizabeth McNamee, pers. comm.). The species’ upper temperature threshold may be around 31 °C, as we observed few rays in summer months when temperatures reached that mark. During these warmer months the rays likely move offshore to cooler and deeper reef areas. This exodus from coastal waters during summer months with corresponding high water temperatures has been described in the Atlantic sharpnose shark (Rhizoprionodon terraenovae) in the north central Gulf of Mexico (Parsons and Hoffmayer 2005). Similar trends have been observed in the Bahamas, with more spotted eagle rays present in spring and lower numbers in summer (Silliman and Gruber 1999).
Our data also show an overall decrease in numbers of spotted eagle rays observed on a yearly basis in both our aerial and boat-based surveys from 2008 to 2013. Whether this reflects a true decrease in population abundance over time, a clustering phenomenon in our study area in 2008–2009, range-shifting away from the southwest Florida coastline in more recent years, or a sampling bias cannot be determined without further study. Two significant environmental perturbations occurred in the region in 2010: a record-breaking cold winter; and the Deepwater Horizon (DWH) oil spill (beginning in April). Vulnerable species of elasmobranchs inhabiting the northeastern Gulf of Mexico were at serious risk to the effects of the DWH spill (Campagna et al. 2011) but the direct impacts on spotted eagle rays are unknown. The winter of 2010 was one of the coldest on record in Florida, with January water temperatures dipping below 13 °C and massive fish kills occurring in southwest Florida and the Florida Keys (Adams et al. 2012; Matich and Heithaus 2012). This extremely cold winter may have had impacts on the distribution and/or health of the eastern Gulf of Mexico population of spotted eagle rays, especially if the Florida Keys is an overwintering site. An unusual mortality event occurred near our study area in winter 2010, when we observed spotted eagle rays using a warm-water refuge near an electric power plant in Apollo Beach, Florida (in northeastern Tampa Bay) during the cold winter months (K. Bassos-Hull, unpubl. data). At least 10 spotted eagle rays died and washed up on beaches near the power plant during this period. Whether these mortalities were due directly to a temperature threshold being exceeded or a lack of adequate food in the refuge is unknown, but the dead rays were visibly emaciated (K. Bassos-Hull and C. Luer, unpubl. data).
Another environmental factor possibly affecting post-2009 numbers of sightings was a prolonged harmful algal bloom of Karenia brevis, a toxic dinoflagellate, along the southwest Florida coast in 2012. Flewelling et al. (2010) documented a mass mortality of Atlantic sharpnose (Rhizoprionodon terraenovae) and blacktip (Carcharhinus limbatus) sharks in northwest Florida in 2000, due to red tide brevetoxins. Thus, elasmobranchs are indeed susceptible to these red tide events. In addition, severe red tide blooms off southwest Florida have long been known to cause mass mortalities of benthic invertebrates (Simon and Dauer 1972), affecting the potential food supply for spotted eagle rays in the region.
As spotted eagle rays move into coastal areas to feed on molluscs and other benthic invertebrates, they become susceptible to boat strikes and entanglement in fishing gear. Four of our captured rays showed signs of entanglement in hook and line gear and two rays had prop scars from boat strikes (K. Bassos-Hull, unpubl. data). When these rays inhabit nearshore waters, sand flats, inlets, lagoons and estuaries they are at risk of being taken in coastal fisheries, either as targeted catch or bycatch, and also may be injured by collisions with boats in high use areas such as inlets.
Sex/size composition and sexual maturity
More males than females were captured overall in the Sarasota surveys, but a month-to-month comparison showed a significant difference between the sexes only in 2011, with more males caught that year. Cuevas-Zimbrón et al. (2011) found possible spatial segregation by sex and size in spotted eagle rays in the southwestern Gulf of Mexico. More males and smaller individuals tended to be found in shallower waters and close to shore, whereas more females and larger individuals were found farther offshore in deeper waters. A similar pattern where smaller eagle rays were likely to remain in shallower water habitats was found in Puerto Rico (Dubick 2000). On the other hand, Ajemian et al. (2012) found mean maximum residency time in the relatively shallow waters of Harrington Sound, Bermuda to be significantly higher for female spotted eagle rays than males. These findings indicate variations by sex and size in usage patterns of spotted eagle rays in nearshore coastal waters, lagoons and estuaries, suggesting the need for further tagging studies.
In our study, no difference was found in morphometric measurements between males and females (DW/TL and TL/WT), however, Schluessel et al. (2010), Cuevas-Zimbrón et al. (2011), and Tagliafico et al. (2012) all observed sexual dimorphism in A. narinari, with females reaching larger sizes than males. If sexual dimorphism is genetically controlled in the spotted eagle ray, we would expect to observe it in our sampled population. Of the 12 largest individuals (176–203 cm DW) captured in our study, all were females with the exception of a 185 cm DW male. Our study did not include offshore surveys in deeper waters, so our observations of the total size range and average size of spotted eagle rays in the region are restricted to the inshore, coastal zone.
Size at maturity is a critical life history characteristic for analyzing the status of fish stocks. The DW50 of 127 cm found in our study for male spotted eagle rays is similar to other studies on A. narinari. Tagliafico et al. (2012) calculated DW50 to be 129.2 cm DW for male spotted eagle rays in Los Frailes Archipelago; Venezuela and Schluessel et al. (2010) estimated male maturity to be 130.6 cm DW. Size at maturity for females is less clear. Other studies examining fishery-caught specimens have concluded female DW50 is larger than in males (>150 cm DW, Schluessel et al. 2010; 135 cm DW, Tagliafico et al. 2012). Unfortunately, most methods for identifying female maturity, such as the presence of embryos or evidence of previous parturition, typically require sacrificing the animal. Our study focused on minimizing invasive procedures to collect measurements, take nonlethal samples such as blood and genetics (reported elsewhere) and release tagged rays, which was not conducive to determining stage of maturity for females. In the future we plan to use ultrasound to detect pregnancy, which will shed some light on female size at maturity.
Young pups in our study were observed and captured mainly during late summer and autumn months with the largest peak in October, similar to what has been observed in other myliobatid rays (Martin and Cailliet 1988; Gray et al. 1997; Dubick 2000; Matern et al. 2000; Yamaguchi et al. 2005; Schluessel et al. 2010; Tagliafico et al. 2012). Observed numbers of adult females compared to adult males increased in our study site during August and September, perhaps as the females were moving inshore for parturition. Measured pup size in our study area (41–73 cm DW) is within the range reported in other studies of spotted eagle rays (Schluessel et al. 2010; Tagliafico et al. 2012). Earlier studies observed slightly smaller DW at birth ranging 26–36 cm (Bigelow and Schroeder 1953; Last and Stevens 1994). Observations of spotted eagle rays born in captivity have reported a size at birth of 40–59 cm DW (Mahon et al. 2004).
Age and growth
Only two studies other than those of Dubick (2000) and Schluessel (2008) have investigated the age and growth characteristics of myliobatids. Martin and Cailliet (1988) provided estimates of growth for the bat ray (Myliobatis californica) of K = 0.229 and 0.100 for males and females, respectively, while Yamaguchi et al. (2005) provided estimates of growth for the longheaded eagle ray (A. flagellum) (K = 0.133 males, K = 0.111 females). Although our recapture sample size was small, we were able to obtain biologically realistic estimates of short-term growth for A. narinari (Fig. 11, Table 2). Our estimates of K for males fell within the range of growth rate estimates found in closely related species (Smith and Merriner 1987; Martin and Cailliet 1988; Neer and Thompson 2005; Yamaguchi et al. 2005). K estimated from female recaptures was, however, much less reliable due to small sample size. Our results suggest that with additional recaptures representing a wider range of sizes and times at liberty, we should be able to describe growth for the population more accurately.
Our preliminary estimates of growth differ from those found by Dubick (2000) and Schluessel (2008) (Fig. 11); their models predict an animal of 100 cm DW should take about 2–4 years to grow 10 cm, yet, according to our recapture data, such a magnitude of growth occurs in a matter of months (Table 2). The apparent discrepancy between observed growth in our recaptured animals and growth as predicted by Dubick and Schluessel could be explained by several factors: differing methodologies, individual growth variability, biased samples, and/or ageing error. According to Francis (1988), the von Bertalanffy parameters have slightly different meanings when age-length data are fitted to the model versus when mark-recapture data are used. These differences, however, are relatively small so it is likely that other factors are contributing to the observed differences. Eagle ray populations in Taiwanese and Australian waters, where Schluessel studied the rays, may follow different growth rates from those in the eastern Gulf of Mexico. Dubick’s studies were conducted in Puerto Rico and its geographic proximity to the Gulf make the possibility of differing growth rates less likely. In that case, limited sample size and age composition are likely to have contributed to the observed discrepancy. Schluessel had a disproportionately large amount of small individuals in her sample and Dubick did not have any individuals corresponding to ages 1–6. Furthermore, we considered the possibility that observed discrepancies in growth rate between our data and earlier studies might be explained by seasonal differences. A closer look into individual records showed this was not the case since most animals used in the growth assessment were recaptured and re-measured approximately whole numbers of years (i.e. 1.0, 2.0, etc.) later.
Ageing estimates using vertebral band data are not without error (Francis et al. 2007; Schluessel 2008). Although Dubick (2000) and Schluessel (2008) both used advanced techniques to visualize band pairs and annual band pair deposition has been verified in closely related species (Yamaguchi et al. 2005), Schluessel (2008) maintained that variability in band clarity is likely to have contributed to decreasing the accuracy of band count, pointing out the potential presence of false bands as a likely cause of bias. False bands are a common concern in elasmobranch age and growth studies (Campana 2001) and have been shown to occur in the closely related bat ray (Martin and Cailliet 1988). Aware of the issue, Schluessel (2008) took a second count of band pairs from the vertebrae of the largest eagle ray (female) in the study but this time excluded potential false bands and concluded the age of the specimen to be 18 years old versus the 34 years originally estimated. This observation, along with the apparent discrepancies between growth described by our tagging and vertebral band data, highlight the need for validation studies to be carried out to verify whether or not false bands exist in A. narinari, and thus reduce uncertainty in estimates derived from vertebral readings.
Conclusions
Our study contributes important life history and localized sighting information on the spotted eagle ray obtained from wild-caught rays off the southwest Florida coast. Our recapture data indicate faster growth rates in the wild than previously estimated through vertebral band models of age and growth. Further tag-recapture studies and validation of vertebral band patterns in this species are needed to explain this discrepancy. Fisheries-dependent and captive studies can contribute to our understanding as well: by comparing our data to those from rays taken in fisheries or maintained in aquariums, we can gain a better understanding of age and growth, diet, reproduction, and parasite loads, and the factors affecting them (Uchida et al. 1990; Smale et al. 2004).
Our observations of seasonal occurrence of spotted eagle rays strongly indicate a preference for coastal water temperatures above 23 °C, with an upper limit of around 31 °C. Thus, the rays are relatively common off southwest Florida in spring, summer and autumn but not in the warmest days of summer or in winter. Our data signal a decline in numbers of observed rays after 2009, for reasons as yet unknown. Explanations may include a natural cyclical pattern in population abundance, environmental perturbations such as extreme cold, red tide, or oil spills, or actual depletion due possibly to fishery removals at a distant location. Other risk factors faced by spotted eagle rays include predation by sharks, entanglement in fishing gear, and possible impacts of parasites and remoras.
Since the initiation of our research in the eastern Gulf of Mexico in 2008, two studies have been published on target fisheries for A. narinari in the western Atlantic: one off Mexico in the southern Gulf of Mexico (Cuevas-Zimbrón et al. 2011); and another off Venezuela (Tagliafico et al. 2012). The species also is taken in commercial fisheries along the coast of Cuba (R. Hueter, pers. obs.). These reports have contributed to our understanding of life history characteristics and also have documented localized fishery impacts to A. narinari stocks. Considering the uncertain conservation status of spotted eagle rays, further research is needed to understand their long-term population trends, ecological relationships with other species, and impacts on their health and habitats (Simpfendorfer et al. 2011), as well as the capacity of this elasmobranch to support sustainable target fisheries. This species’ relatively low abundance, extremely low reproductive capacity, and vulnerability to net, harpoon, and other fishing gears should place eagle rays on a worldwide watchlist for signs of population decline.
References
Abbott TR (1974) American seashells. Van’Nostrand Reinhold Company, New York
Adams AJ, Hill JE, Kurth BN, Barbour AB (2012) Effects of a severe cold event on the subtropical, estuarine-dependent common snook, Centropomus undecimalis. Gulf Caribb Res 24:13–21
Ajemian MJ, Powers SP, Murdoch TJT (2012) Estimating the potential impacts of large mesopredators on benthic resources: integrative assessment of spotted eagle ray foraging ecology in Bermuda. PLoS One. doi:10.1371/journal.pone.0040227
Akaike H (1973) Information theory and an extension of the maximum likelihood principle. In: Petrova BN, Csaki F (eds) Proceedings of the 2nd international symposium on information theory. Publishing house of the Hungarian Academy of Sciences, Budapest, pp 268–281
Bigelow HG, Schroeder WC (1953) Sawfishes, guitarfishes, skates, and rays. In: Tee-Van J (ed) Fishes of the western North Atlantic. Mem. Sears Found. Mar. Res. Yale University, New Haven, pp 464–465
Campagna C, Short FT, Polidoro BA, McManus R, Collette BB, Pilcher NJ, Sadovy de Mitcheson Y, Stuart SN, Carpenter KE (2011) Gulf of Mexico oil blowout increases risks to globally threatened species. Bioscience 61:393–397
Campana SE (2001) Accuracy, precision and quality control in age determination, including a review of the use and abuse of age validation methods. J Fish Biol 59:197–242. doi:10.1111/j.1095-8649.2001.tb00127.x
Corcoran MJ, Gruber SH (1999) The use of photo-identification to study the social organization of the spotted eagle ray, Aetobatus narinari. Bahamas J Sci 7:21–27
Cuevas-Zimbrón E, Pérez-Jiménez JC, Méndez-Loeza I (2011) Spatial and seasonal variation in a target fishery for spotted eagle ray Aetobatus narinari in the southern gulf of Mexico. Fish Sci 77:723–730
Culter JK, Leverone JR (1993) Bay bottom habitat assessment: final report draft. Sarasota Bay National Estuary Program, Mote Marine Laboratory Technical Report No. 303
den Hartog J, Reijns R (2007) Interactive Individual identification system (I3S). Version 2.0. www.reijns.com/i3s.
Dubick JD (2000) Age and growth of the spotted eagle ray, Aetobatus narinari (Euphrasen, 1790), from southwest Puerto Rico with notes on its biology and life history. MS thesis, Univ. Puerto Rico, Mayaguez
Efron B, Tibshirani R (1986) Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Stat Sci 1:54–75
ESRI (2011) ArcGIS Desktop: Release 10. Environmental Systems Research Institute, Redlands
Euphrasen BA (1790) Raja (narinari). kongliga vetenskaps akademiens nya handlingar. Stockholm 11:217–219
Fabens AJ (1965) Properties and fitting of the von bertalanffy growth curve. Growth 29:265–289
Felder DL, Camp DK (eds) (2009) Biodiversity, Vol 1. In: Gulf of Mexico, Origin, Waters, and Biota. Texas A&M University Press
FFWCC (2013) Florida Fish and Wildlife Conservation Commission saltwater fishing regulations. www.eregulations.com/florida/fishing/saltwater/saltwater-fishing-regulations/. Accessed 1 August 2013
Flewelling LJ, Adams DH, Naar JP, Atwood KE, Granholm AA, O’Dea SN, Landsberg JH (2010) Brevetoxins in sharks and rays (chondrichthyes, elasmobranchii) from Florida coastal waters. Mar Biol 157:1937–1953
Francis RICC (1988) Maximum likelihood estimation of growth and growth variability from tagging data. New Zeal J Mar Freshw Res 22:42–51
Francis MP, Campana SE, Jones CM (2007) Age under-estimation in New Zealand porbeagle sharks (Lamna nasus): is there an upper limit to ages that can be determined from shark vertebrae? Mar Freshw Res 58:10–23
Gompertz B (1832) On the nature of the function expressive of the law of human mortality, and on a new mode of determining the value of life contingencies. Phil Trans R Soc Lond 123:513–583
Gray AE, Mulligan TJ, Hannah RW (1997) Food habits, occurrence, and population structure of the bat ray, Myliobatis californica, in Humboldt Bay, California. Environ Biol Fishes 49:227–238
Hoese HD, Moore RH (1977) Fishes of the Gulf of Mexico: Texas, Louisiana, and adjacent waters. Texas A&M University Press, College Station Texas, pp 107–123
Hope ACA (1968) A simplified monte Carlo significance test procedure. J Roy Statist Soc Ser B 30:582–598
IUCN (2013) IUCN Red List of Threatened Species. Version 2013.1. www.iucnredlist.org>. Downloaded on 29 July 2013
Keys AB (1928) The weight-length relationship in fishes. Proc Nat Acad Sci Wash 14:922–925
Kuhl H (1823) in van Hasselt, J.C. Uittreksel uit een’ brief van Dr. J. C. van Hasselt, aan den Heer C. J. Temminck. Algemen Konst- en Letter-bode I Deel 20:315–317
Kyne PM, Ishihara H, Dudley SFJ, White WT (2006) Aetobatus narinari. In: IUCN 2013. IUCN Red List of Threatened Species. Version 2013.1. www.iucnredlist.org. Accessed 29 July 2013
Last PR, Stevens JD (1994) Sharks and rays of Australia. CSIRO, Melbourne
Mahon J, Chua F, Newman P (2004) Successful spotted eagle ray (Aetobatus narinari) breeding program and details of an assisted birth. Drum and Croaker Special Edition No. 2, High Irreg J Pub Aqua 104–107
Martin LK, Cailliet GM (1988) Aspects of the reproduction of the bat ray, Myliobatis californica, in central California. Copeia 3:754–762
Martin TG, Wintle BA, Rhodes JR, Kuhnert PM, Field SA, Low-Choy SJ, Tyre AJ, Possingham HP (2005) Zero tolerance ecology: improving ecological inference by modelling the source of zero observations. Ecol Lett 8:1235–1246
Matern SA, Cech JJ Jr, Hopkins TE (2000) Diel movement of bat rays, Myliobatis californica, in Tomales Bay, California: evidence for behavioral thermoregulation? Environ Biol Fish 58:173–182
Matich P, Heithaus M (2012) Effects of an extreme temperature event on the behavior and age structure of an estuarine top predator Carcharhinus leucas. Mar Ecol Prog Ser 447:165–178
Naylor GJ, Caira JN, Jensen K, Rosana KAM, White WT, Last PR (2012) A DNA sequence-based approach to the identification of shark and ray species and its implications for global elasmobranch diversity and parasitology. Bull Amer Mus Nat Hist 367:1–262
Neer JA, Thompson BA (2005) Life history of the cownose ray, Rhinoptera bonasus, in the northern gulf of Mexico, with comments on geographic variability in life history traits. Environ Biol Fish 73:321–331
Parker FR, Bailey CM (1979) Massive aggregations of elasmobranchs near mustang and padre islands, Texas. Texas J Sci 31:255–256
Parsons GR, Hoffmayer ER (2005) Seasonal changes in the distribution and relative abundance of the Atlantic sharpnose shark Rhizoprionodon terraenovae in the north central gulf of Mexico. Copeia 4:914–920
Richards VP, Henning M, Witzell W, Shivji MS (2009) Species delineation and evolutionary history of the globally distributed spotted eagle ray (Aetobatus narinari). J Hered 100:273–283
Schluessel V (2008) Life history, population genetics and sensory biology of the white spotted eagle ray Aetobatus narinari (Euphrasen, 1790) with emphasis on the relative importance of olfaction. Dissertation, University of Queensland, Brisbane
Schluessel V, Bennett MB, Collin SP (2010) Diet and reproduction in the white-spotted eagle ray aetobatus narinari from Queensland, Australia and the Penghu islands, Taiwan. Mar Freshw Res 61:1278–1289
Sellas AB, Bassos-Hull K, Hueter RE, Feldheim KA (2011) Isolation and characterization of polymorphic microsatellite markers from the spotted eagle ray (Aetobatus narinari). Conserv Genet Resour 3:609–611
Shepard TD, Myers RA (2005) Direct and indirect fishery effects on small coastal elasmobranch in the northern gulf of Mexico. Ecol Lett 8:1095–1104
Silliman WR, Gruber SH (1999) Behavioral biology of the spotted eagle ray, Aetobatus narinari. Bahamas J Sci 7:13–20
Simon JL, Dauer DM (1972) A quantitative evaluation of red-tide induced mass mortalities of benthic invertebrates in Tampa Bay, Florida. Env Lett 4:229–234
Simpfendorfer CA, Heupel MR, White WT, Dulvy NK (2011) The importance of research and public opinion to conservation management of sharks and rays: a synthesis. Mar Freshw Res 62:518–527
Smale MJ, Jones RT, Correia JP, Henningsen AD, Crow GL, Garner R (2004) Research on elasmobranchs in public aquariums. In: Smith M, Warmolts D, Thoney D, Hueter R (eds) The elasmobranch husbandry manual: captive care elasmobranch husbandry manual: captive care of sharks, rays and their relatives. Special Publ of Ohio Biol Surv, Columbus, pp 533–541
Smith JW, Merriner JV (1987) Age and growth, movements and distribution of the cownose ray, Rhinoptera bonasus, in chesapeake bay. Estuaries 10:153–164
Sokal RR, Rohlf FJ (1998) Biometry: the principles and practice of statistics in biological research, 3rd edn. Freeman and Company, WH
Stevens JD, Bonfil R, Dulvy NK, Walker PA (2000) The effects of fishing on sharks, rays, and chimaeras (chondrichthyans), and the implications for marine ecosystems. ICES J Mar Sci 57:476–494
Tagliafico A, Rago N, Rangel S, Mendoza J (2012) Exploitation and reproduction of the spotted eagle ray (Aetobatus narinari) in the Los frailes archipelago, Venezuela. Fish Bull 110:307–316
Trent L, Parshley DE, Carlson JK (1997) Catch and bycatch in the shark drift gillnet fishery off Georgia and east Florida. Mar Fish Rev 59:19–28
Tricas TC (1980) Courtship and mating-related behaviors in myliobatid rays. Copeia 1980:553–556
Uchida S, Toda M, Kamei Y (1990) Reproduction of elasmobranchs in captivity. In: Pratt HL Jr, Gruber SH, Taniuchi T (eds) Elasmobranchs as living resources: advances in the biology, ecology, systematics, and the status of the fisheries. NOAA Technical Rep, NMFS 90:211–237
von Bertalanffy L (1938) A quantitative theory of organic growth: inquiries on growth laws II. Hum Biol 10:181–213
Vuong QH (1989) Likelihood ratio tests for model selection and non-nested hypotheses. Econometrica 57:307–333
Welch BL (1947) The generalization of “student’s” problem when several different population variances are involved. Biometrika 34:28–35
White WT, Dharmadi (2007) Species and size compositions and reproductive biology of rays (chondrichthyes, batoidea) caught in target and non- target fisheries in eastern Indonesia. J Fish Biol 70:1809–1837
White WT, Last PR, Naylor GJP, Jensen K, Caira JN (2010) Clarification of Aetobatus ocellatus (Kuhl, 1823) as a valid species, and a comparison with Aetobatus narinari (Euphrasen, 1790) (Rajiformes: Myliobatidae). In: Last PR, White WT, Pogonoski JJ (eds) Descriptions of new sharks and rays from Borneo. CSIRO 32:141–164
Wildt AR, Ahtola OT (1978) Analysis of covariance. Sage University paper series on quantitative applications in the social sciences, 12
Yamaguchi A, Kawahara I, Ito S (2005) Occurrence, growth and food of longheaded eagle ray, Aetobatus flagellum, in ariake sound, Kyushu, Japan. Environ Biol Fish 74:229–238
Acknowledgments
Our gratitude is extended to the Spotted Eagle Ray Conservation Program team of Mote staff, interns and collaborators, especially Capts. Greg Byrd and Charles (Chuck) Jelicks, Adam Lytton, Anna Sellas, Jen Newby, and Ashley Ross. We thank Jim Culter for his help with habitat and molluscan diversity in the eastern Gulf of Mexico and Henry Luciano for environmental temperature data. We also thank John Tyminski, Rachel Dryer and two anonymous reviewers for their helpful comments and constructive suggestions for improving the manuscript. We are grateful to William E. Pine III, University of Florida, for reviewing our methods and results for catch-per-unit-effort data and offering suggestions for graphic display of such data. Funding and in-kind support for this project were provided by the National Aquarium in Baltimore, Georgia Aquarium, Disney Worldwide Conservation Foundation, Save Our Seas Foundation, Mote Scientific Foundation, LightHawk, PADI Foundation, and anonymous donors. This study was conducted in accordance with Florida state laws and regulations for work on protected marine species (FWC SAL-13-1140-SRP) and IACUC protocols approved by Mote Marine Laboratory (Approval #13-02-PH1).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bassos-Hull, K., Wilkinson, K.A., Hull, P.T. et al. Life history and seasonal occurrence of the spotted eagle ray, Aetobatus narinari, in the eastern Gulf of Mexico. Environ Biol Fish 97, 1039–1056 (2014). https://doi.org/10.1007/s10641-014-0294-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-014-0294-z